Abstract
Background: Infections are a major cause of mortality in haemodialysis patients. The increasing antimicrobial resistance globally further exacerbates this concern. Procalcitonin has shown potential in aiding antimicrobial stewardship and reducing the mortality and morbidity associated with infections. This systematic review aims to synthesise the existing literature on the utility of procalcitonin as a diagnostic and monitoring tool for haemodialysis patients with suspected bacteraemia. Methods: Multiple electronic databases (EMBASE, MEDLINE, and the Cochrane Library) were systematically searched to identify primary studies evaluating procalcitonin use in haemodialysis patients with suspected bacteraemia. Using a narrative synthesis approach, along with other quality assessment tools, recommendations regarding procalcitonin usage in dialysis were formulated. Results: Eleven studies were identified. The review proposes a procalcitonin-guided antibiotic protocol aimed at facilitating antibiotic use in haemodialysis patients with suspected bacteraemia. Caution is, however, advised against relying solely on procalcitonin for diagnosis, emphasising the integration of procalcitonin with other clinical and biomarkers of infection. Conclusions: Procalcitonin shows promise as a valuable diagnostic and monitoring tool for suspected bacteraemia in haemodialysis patients. While caution is advised against relying solely on PCT for diagnosis, its integration with other clinical indicators can enhance infection management. To fully establish its clinical utility, more research is needed.
1. Introduction
Renal replacement therapy (RRT) for end-stage renal disease (ESRD) patients increases infection risk, particularly through vascular access sites like arteriovenous fistulas, grafts, and central venous catheters, serving as prominent conduits for the introduction of these infections, with bacterial infections accounting for up to 70% [1]. Prompt initiation of appropriate antibiotics is critical as each hour of delay is associated with an increased mortality risk in patients with septic shock and organ dysfunction, which are well-recognised complications of bacteraemia and sepsis [2,3].
Traditional infection markers like C-reactive protein (CRP), erythrocyte sedimentary rate (ESR), and ferritin are nonspecific and vary in sensitivity. Blood cultures, the gold standard for diagnosis, are limited by delays and collection accuracy [4,5]. Thus, there is a need for a sensitive, specific biomarker with rapid turnaround times.
Procalcitonin (PCT), produced by the thyroid and adipose tissues, is released during bacterial infections by neuroendocrine cells in the gut and lungs. Detectable within 2–4 h and peaking around 6 h post-infection, PCT levels remain stable for up to 24 h [6,7]. Multiple studies indicate PCT’s utility in early bacterial infection detection, antibiotic therapy guidance, and infection course prediction [8,9,10,11].
In chronic kidney disease, it is postulated that diminished renal function leads to an increase in the serum levels of proinflammatory metabolites (in association with uraemia), triggering immune system activation and inflammatory processes, consequently leading to an increased PCT release. In HD, the invasiveness of the dialysis process, presence of commensals associated with vascular access use, and immune reaction to the dialyser can trigger an inflammatory reaction, leading to higher levels of PCT [12,13,14].
It is also hypothesised that some of the serum PCT is removed by a combination of diffusive and convective transport processes across the dialysis membrane. Interestingly, this elimination process of PCT during haemodialysis occurs concurrently with the clearance of other substances, such as β-microglobulin and various other low-molecular-weight proteins. These substances, including PCT, can be transported across the dialysis membrane due to their small molecular size, allowing them to be removed from the bloodstream during the dialysis procedure [15,16]. These mechanisms can potentially create difficulties when using PCT as a clinical diagnostic or monitoring tool in ESRD patients on HD due to the inconsistency of results.
The use of PCT for diagnosing and treating bacterial infections in haemodialysis (HD) patients is not fully developed. Although many studies have explored PCT’s role in diagnosing and managing infections, few systematic reviews focus on its application for catheter-associated and non-catheter-associated bacteraemia in HD and non-HD settings [7,17]. Our review assessed PCT as a diagnostic marker and treatment guide for HD-related bacteraemia. We aimed to create a framework to assist clinicians in using PCT to determine when antibiotic therapy is necessary for HD patients.
2. Materials and Methods
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 (PRISMA 2020) statement [18]. The study protocol was developed following the PRISMA-P guidelines [19] and registered on PROSPERO (Registration Number: CRD42023422323).
2.1. Literature Search
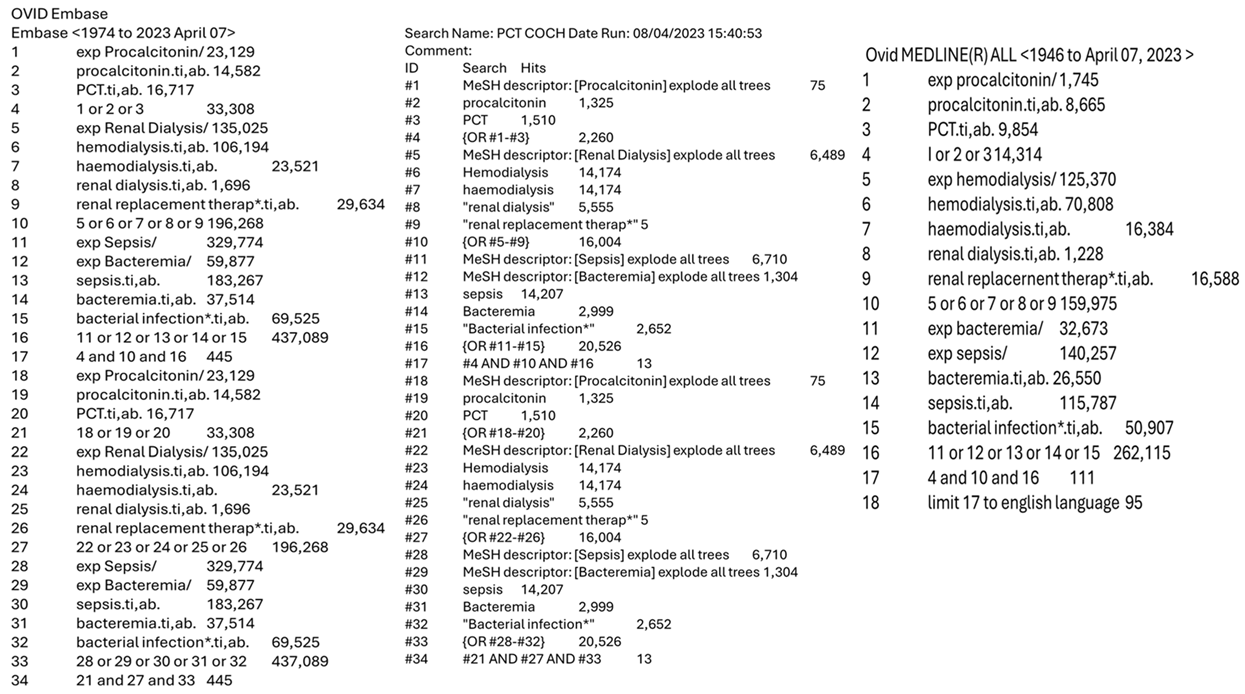
The literature search was conducted across the following databases: EMBASE, MEDLINE (via Ovid), and the Cochrane Library and was aimed at identifying studies that evaluated the use of procalcitonin in haemodialysis patients in multiple clinical settings. Using a predefined inclusion and exclusion criteria, the studies included met the following conditions: (1) published from the inception of the selected databases up to March 2023, (2) written in the English language, (3) observational or interventional studies, including randomised and non-randomised controlled trials, cohort studies, cross-sectional, and case-control studies, that evaluated the use of procalcitonin as a primary or secondary outcome in haemodialysis patients, (4) involved participants aged over 18 years, and (5) provided access to the full text and complete study data. Studies were excluded if they were (1) case reports, case series, reviews, or other forms of secondary research, or (2) published in a language other than English. Supplementary approaches to identify relevant studies were employed, such as a partial grey literature search using Google Scholar and searching the reference lists of included studies. The following keywords and Medical Subject Heading (MeSH) terms were combined to create a search algorithm and used to search the prementioned databases: (Procalcitonin OR PCT) AND (“Renal Dialysis” OR Haemodialysis OR Hemodialysis OR “Renal replacement therapy”) AND (Bacteraemia OR “Bacterial Infection” OR Sepsis OR Infections). The search protocol and results from the corresponding databases can be found in the supplementary data (Appendix A).
2.2. Study Selection and Eligibility Criteria
Following an extensive database search, duplicates were removed using the Mendeley reference manager. The title and abstract content of the remaining articles were independently assessed in an unblinded standardised manner by two reviewers to evaluate their relevance to the chosen topic and their suitability based on the inclusion and exclusion criteria. Following the screening process, unrelated and unsuitable articles were removed. Any disagreement in the selected articles between the reviewers was discussed until an agreement was reached.
A final review of the selected articles, by both reviewers, was conducted to assess their eligibility for use. Research involving PCT use in the diagnosis or monitoring of bacteraemia in haemodialysis patients was preferentially selected.
2.3. Data Extraction and Quality of Studies
Data were extracted by two independent reviewers using a refined data extraction sheet based on the Cochrane Consumers and Communication review group’s template [20]. One reviewer extracted the data while another assessed it independently. Disagreements were resolved through consensus.
To determine the methodological quality of non-randomised trials, the ROBIN-I tool assessed potential biases [21]. The Newcastle-Ottawa Scale (NOS) was employed for observational studies, with scores categorising quality as high (7–9), moderate (4–6), or low (0–3). A modified Newcastle-Ottawa Scale was used for cross-sectional studies, with quality categorised from high (6–7) to low (≤3) [22,23] (Appendix C and Appendix D).
Two independent reviewers evaluated the studies, resolving significant disagreements through discussion. To standardise the process and remove bias when using the NOS for cohort studies, survey questions were developed based on the original NOS covering each domain (Appendix B).
2.4. Data Analysis and Presentation
Given the presumed heterogeneity and limited number of relevant studies, a narrative synthesis approach was selected to address the study’s complex questions and avoid issues with calculating standardised effect sizes from unavailable and non-comparable data points. The initial step involved an inductive thematic analysis with the goal of systematically identifying, tabulating, and narratively describing recurring or pertinent themes derived from the data to address the review questions. By grouping and organising the studies into meaningful categories and mapping out key consistent trends, this allowed for the exploration of relationships within and between the included studies, creating a structured framework for the synthesis and reporting of the results. The data were presented graphically in tables and narrative texts, including important study characteristics and evidence that supported the results and degree of heterogeneity [24,25,26].
2.5. Analysis of Heterogeneity and Certainty of Evidence
Regarding heterogeneity, due to the limitations in using statistical assessments of measurements, heterogeneity was informally assessed by addressing the clinical and methodological diversity between the studies by assessing the variability in participants, intervention, outcome, study design, and risk of bias. Findings from this were summarised using the terms “high” or “low” heterogeneity [25]. After extracting and synthesising data from the included studies, the relevant evidence was combined to form good practice recommendations (GPR) pertaining to the use of procalcitonin in patients undergoing haemodialysis. The criteria for defining these GPRs required each statement to be a clear and actionable clinical suggestion specifically related to the clinical use of procalcitonin in haemodialysis patients and be supported by a rationale.
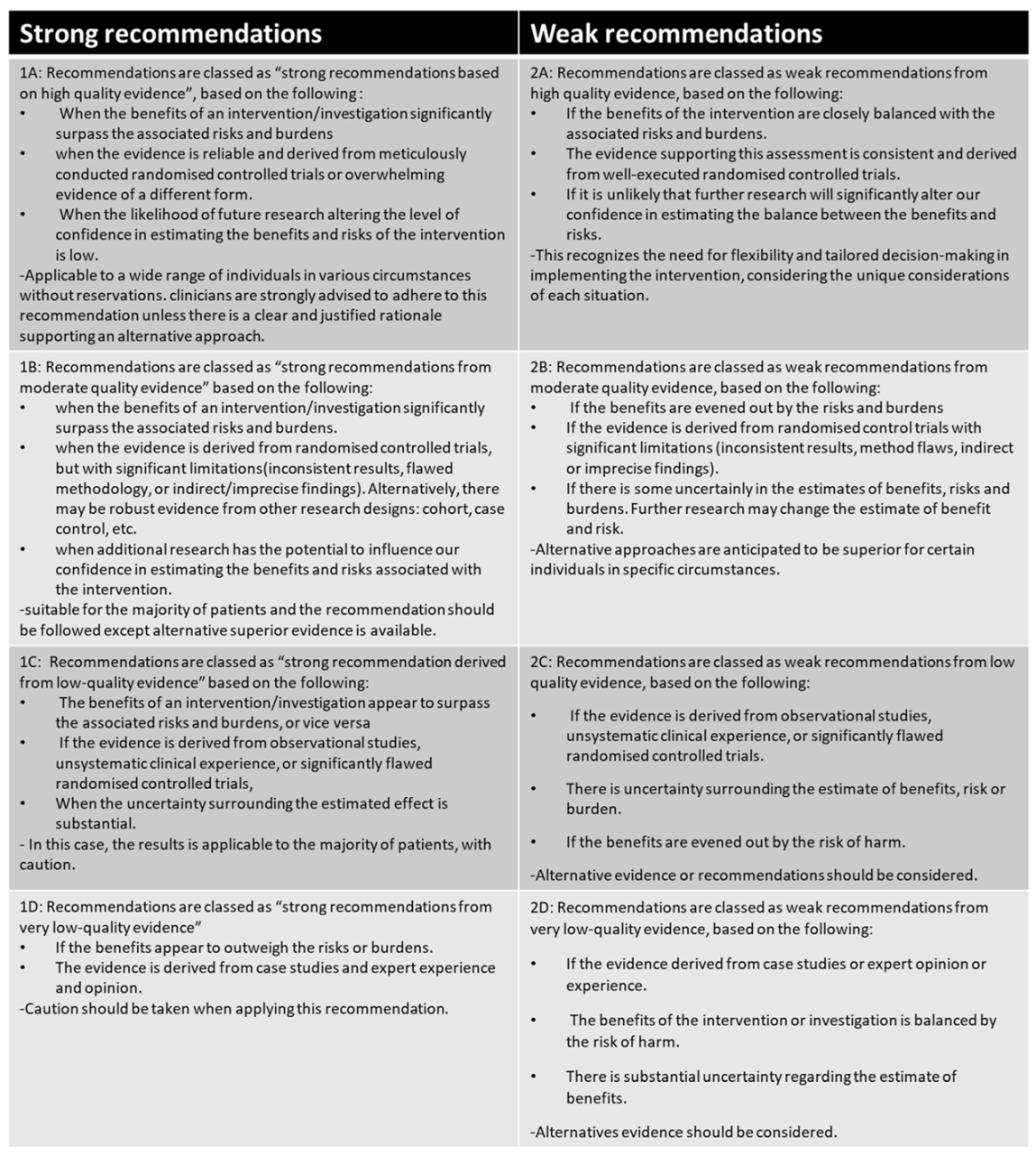
A modified GRADE system based on a British HIV association archetype [26] was used to evaluate the evidence’s certainty, strength, and quality for deriving GPRs. This modification addressed anticipated challenges with assessing specific GRADE domains due to different study methods and types. A copy of the modified GRADE scale can be found in the supplementary data (Appendix E).
2.6. Ethical Considerations
Informed consent from participants was not required due to the use of secondary data from other sources. The study protocol was reviewed and approved by the University of South Wales ethics committee.
3. Results
3.1. Study Selection
A total of 569 records were identified, with 432 remaining after removing duplicates and non-English articles. Following screening, 366 were excluded for being case reports/series, involving paediatric populations, being review papers, or being non-relevant. An additional 56 were excluded due to inability to retrieve the full text or containing non-useful data. Additional searches using Google Scholar and the reference lists identified eight more records, but only one met the inclusion criteria. In total, 11 articles were included, as shown in Figure 1.
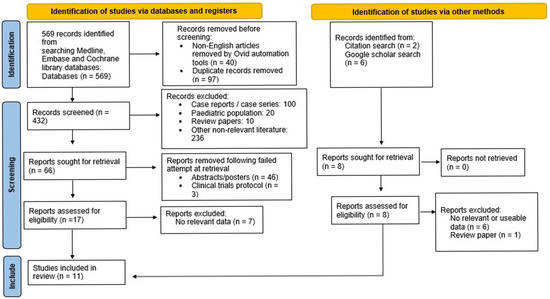
Figure 1.
PRISMA 2020 flow chart of study identification and inclusion.
3.2. Characteristics of Included Studies
Of the included studies, ten were observational, including cohort, case-control, and cross-sectional studies. The remaining study was a non-randomised controlled trial (NRCT). This diversity in design provided varied perspectives but potentially reduced the generalisation of results. Six studies were retrospective, while five were prospective. The total cohort included 3373 participants aged 18 and above, primarily diagnosed with chronic or acute kidney injury. These individuals were from various clinical environments, such as intensive care units, general wards, and dialysis centres. There was an active step to try and exclude data from patients not on haemodialysis.
3.3. Summary of Findings
After conducting a preliminary synthesis and in-depth analysis of the data extracted from the included studies, a framework was developed based on thematic deduction derived from each individual study. The resulting framework comprised two major themes: the use of procalcitonin as a means to identify haemodialysis (HD) patients with bacteraemia and the utility of PCT as a monitoring and prognostic tool for HD patients with bacteraemia. The findings of the review demonstrated that PCT levels were significantly elevated in HD patients with bacteraemia and that, in a suitable cohort of patients, PCT can be used as an accurate indicator of bacteraemia in HD patients.
A cutoff PCT level of >1.5 ng/mL exhibited a sensitivity level of 89% and 81% specificity in detecting severe bacteraemia [27]. A similar PCT cutoff of 1.65 ng/mL was associated with a sensitivity of 80.8% in one study [28] while a PCT cutoff level of 15.5 ng/mL possessed a sensitivity of 94% for detecting bacteraemia with 100% specificity in another study [29]. Conversely, PCT levels below 1 ng/mL were less likely to be associated with bacteraemia [27,30,31].
For haemodialysis patients, the level of procalcitonin was found to be elevated, even in the absence of infection. Our observations indicate that PCT levels equal to or below the range: 0.50–0.8 ng/mL could effectively exclude the presence of bacteraemia [27,30,32]. A single study within the review linked a PCT level of less than or equal to 1.1 ng/mL to the absence of bacteraemia [29].
Our review revealed contradictory evidence in relation to the identification of Gram-positive and Gram-negative bacteraemia. A single study [33] within the review reported a greater median procalcitonin level in haemodialysis patients with Gram-positive bacteraemia compared to Gram-negative bacteraemia, specifically 61.2 ng/mL (25–75th centile: 0.9–145) versus 21.2 ng/mL (25–75th centile: 4.3–114), while another included study reported a higher mean level of PCT in patients with Gram-negative bacteraemia, as opposed to Gram-positive bacteraemia: 21.9 ± 42.2 vs. 13.7 ± 24.8. However, this result was not statistically significant [34]. Patients with Gram-negative bacteraemia had a higher proportion of the complications ascribed to bacteraemia.
In the comparison of diagnostic markers, it was found that PCT outperformed C-reactive protein and white blood cell count in diagnosing bacteraemia with or without sepsis [28,30,31]. However, one study disagreed with the premise that PCT was a better diagnostic biomarker for detecting bacteraemia than CRP in HD patients, despite the higher cost. However, this was a cross-sectional study with a retrospective design and was at high risk of selection bias [32].
The synthesis of the data also revealed that a dynamic approach involving the measurement of PCT in HD patients with bacteraemia yielded better prognostic information compared to a single measurement of procalcitonin, regardless of the absolute level. A substantial reduction in PCT levels (>63%) after 72 h of treatment was associated with lower mortality rates [35]. Furthermore, it was observed that higher PCT levels were associated with an increased risk of bacterial sepsis-induced complications, such as bacterial metastasis, septic shock necessitating intensive care admission, and death. Notably, PCT levels ranging between 2 and 4 ng/mL were linked to a higher risk of hospitalisation and death [27,33,34,35].
Regarding monitoring treatment with PCT, a corresponding rise and fall in procalcitonin levels was observed alongside changes in other inflammatory markers and clinically observed signs of infection [33,35,36,37]. In some of the studies, there was no significant correlation between procalcitonin and other inflammatory markers [29]. Pre-dialysis measurements of PCT were found to be more reliable in terms of diagnostic or prognostic value compared to the measurements taken during or immediately after dialysis, as dialysis had an impact on the clearance of procalcitonin [27,34].
The studies included implemented exclusion criteria targeting certain medical conditions based on their potential to introduce confounding effects on the serum procalcitonin levels. These identified medical conditions comprise severe burns, recent major surgery, malignancy, immunodeficiencies, patients receiving immunosuppressants, pregnancy, and acute pancreatitis. Consequently, these conditions were intentionally excluded from the study population to ensure a more accurate and unbiased assessment of the relationship between procalcitonin levels and the investigated factors.
Based on the findings, it was discouraged to rely solely on procalcitonin as a diagnostic tool due to some overlap in the levels observed between patients with bacteraemia and those without. Instead, a comprehensive approach integrating multiple clinical and laboratory parameters was recommended for the accurate diagnosis and management of bacteraemia in HD patients [31,32,34]. Table 1 summarises the characteristics and findings from the included studies.

Table 1.
Summary of the characteristics and findings of the included studies.
3.4. Quality Assessment
The observational studies in this systematic review were of moderate-to-high quality based on the Newcastle-Ottawa Scale, as shown in Table 2. The main limitations were non-representative samples, inadequate selection controls, insufficient control for confounding variables, and lack of rigorous follow-up or comprehensive outcome assessment.

Table 2.
Quality assessment data using Newcastle-Ottawa (NOS) quality assessment tool for cohort and case control studies, and modified Newcastle-Ottawa tool (adapted from the original NOS tool) for cross-sectional studies.
The NRCT risk of bias assessment (Table 3) indicated a high potential for bias due to confounding and outcome measurement, with scores of three and four, respectively. The overall bias score of three reflects a moderate-to-high risk of bias. These findings necessitate cautious interpretation of the findings and highlight the need for future research to address these biases to enhance study quality.

Table 3.
Risk Of Bias in Non-randomised studies—of interventions (ROBINS-I) assessment tool—for Zhai 2021 [37].
3.5. Heterogeneity
The systematic review revealed significant heterogeneity among the included observational studies and non-randomised control trials in terms of participants, exposure, outcomes, and methodology. Participant differences such as diverse ages, genders, and comorbidities, along with varying types of renal failure, introduced confounding factors and limit generalisability. There was significant variation in exposures and interventions, including different diagnostic tests, treatments, and the types of organisms causing bacteraemia. The outcome measures varied, with studies focusing on diagnostic potential, prognostic indicators, or treatment efficacy. Methodological differences, such as study design, sample size, and statistical analyses, further contributed to heterogeneity, affecting the reliability of the results. Thus, the findings should be interpreted with caution, considering the context and specific study characteristics.
3.6. Grading Good Practice Recommendations
The objective of this review was to develop a framework for clinicians on using procalcitonin (PCT) for haemodialysis patients suspected of having bacteraemia. We have summarised the recommendations, with an attached modified GRADE score, below:
- (a)
- Check procalcitonin levels when there is clinical and biochemical evidence of bacteraemia. Our review found a correlation between procalcitonin and bacteraemia, both related to access and non-access related bacteraemia (1B).
- (b)
- In cases of uncertainty regarding a bacterial infection, it is advisable to consider initiating broad-spectrum antibiotics if the procalcitonin level is greater than or equal to 1.5 ng/mL and there are clinical or other biochemical signs of bacterial infections. Our review indicated that a procalcitonin cutoff value of 1.5 ng/mL had a sensitivity of 89% and an accuracy of 85%. A recent meta-analysis also supports this conclusion. Antibiotics can be started with a procalcitonin range greater than 0.5–1 ng/mL if there is a strong clinical suspicion of a possible bacterial infection (1B).
- (c)
- We recommend against using procalcitonin alone for diagnosing bacteraemia due to significant overlap between patients with and without bacteraemia (1B).
- (d)
- Dynamic measurements of procalcitonin are more useful for monitoring disease progression or recovery. We suggest measuring (PreHD) procalcitonin levels every 72 h, and a reduction of more than 60% in procalcitonin level indicates recovery (1C).
- (e)
- We recommend discontinuing antibiotics when the procalcitonin level is below 0.5 ng/mL and there is resolution of clinical symptoms (1B).
4. Discussion
We hypothesised that procalcitonin (PCT) is a valuable diagnostic and monitoring tool for haemodialysis patients with bacteraemia. The review consistently found elevated PCT levels in haemodialysis patients with bacteraemia. A PCT cutoff of 1.5 ng/mL effectively distinguished HD patients with severe bacteraemia, demonstrating a sensitivity and accuracy exceeding 80%. PCT was found to be more specific than C-reactive protein in this regard, while maintaining a comparable level of sensitivity to bacteraemia.
A meta-analysis [38] highlighted that raising the PCT cutoff from 0.68–0.85 ng/mL to 1.5–15 ng/mL decreased sensitivity from 96% to 86% but increased specificity from 77% to 89%. Our review supports a PCT cutoff of 1.5 ng/mL. While this meta-analysis focused primarily on diagnostic accuracy, our review takes a more clinically applied perspective by exploring how procalcitonin can be used to guide antimicrobial decision-making in practice.
Multiple studies found that procalcitonin levels below 1 ng/mL in HD patients usually indicate the absence of bacteraemia, with a reliable cutoff range of 0.5–0.8 ng/mL [27,30,31,32]. One excluded study agreed with a cutoff level of <0.5 ng/mL [16]. For access-related bacteraemia (ARB), PCT levels ranged from 0.9–1.1 ng/mL, with higher levels observed in more severe infections. The review highlighted that relying solely on PCT as a diagnostic marker is insufficient due to overlapping levels in patients with and without bacteraemia. Combining PCT with CRP improves diagnostic accuracy, as shown by one of the included studies with a combined cutoff of CRP > 100 mg/dl and PCT > 5 ng/mL, yielding a positive predictive value of 100% and a negative predictive value of 94% [39].
The ability of procalcitonin to distinguish between Gram-positive and Gram-negative bacteraemia yielded conflicting results. Agrawal et al. [35] found higher median PCT levels in HD patients with Gram-positive bacteraemia, while Yunus et al. [34] reported higher but not clinically significant PCT levels in Gram-negative bacteraemia. Despite this, Gram-negative bacteraemia consistently had worse outcomes. Li et al. [40] identified a PCT threshold of 2.44 ng/mL to differentiate between Gram-negative and Gram-positive bacteraemia, with a specificity of 77.4% and a sensitivity of 68.4%. This suggests that PCT could aid in detecting Gram-negative bacteraemia and guiding antibiotic therapy, but further research is needed to refine these thresholds for HD patients.
Our review found that PCT levels above 2–4 ng/mL correlated with higher risks of hospitalisation, bacterial complications, multi-organ failure, and death. Patients with PCT levels below 1.5 ng/mL had lower chances of needing intensive care or experiencing fatal outcomes.
Procalcitonin is a valuable predictor of infection severity and outcomes. A 2008 study showed that PCT was superior to CRP and white blood cell count in predicting survival and mortality, and it was as effective as CRB65 (confusion, respiratory rate > 30, hypotension, age > 65) for identifying low-risk pneumonia patients [41]. Similarly, a 2015 systematic review demonstrated that higher PCT levels and reduced PCT clearance were associated with increased all-cause mortality in sepsis [40]. A 2018 meta-analysis found that PCT-guided antibiotic treatment in intensive care significantly reduced mortality rates and treatment durations compared to control groups, with consistent benefits across various sepsis severity subgroups [42].
This review highlighted the connection between changes in PCT levels and other biological and clinical infection indicators. Increased or decreased PCT levels generally corresponded with similar changes in CRP, white blood cell count, lactate, and clinical markers like the Sequential Organ Failure Assessment (SOFA) score. Other studies have provided evidence of this correlation [41,42].
A single PCT level showed a weak correlation with survival, regardless of disease severity or initial PCT level [34]. A more accurate survival predictor was the percentage of PCT clearance after 72 h of treatment. Survivors showed a reduction in PCT levels exceeding 63% after 72 h of treatment initiation. Adopting a dynamic approach to monitor disease progression and treatment efficacy using PCT could guide therapeutic decision-making, but further high-level evidence is needed to support this approach [35].
Procalcitonin levels should be measured before a haemodialysis session to ensure optimal diagnostic and prognostic accuracy, as dialysis can reduce circulating PCT levels and compromise the reliability of the result [27].
Based on the synthesised recommendations from this review, a hypothetical procalcitonin-guided antibiotic framework was developed for haemodialysis patients presenting with symptoms suggestive of bacteraemia. This proposed approach is intended to support clinical decision-making around antibiotic use by incorporating procalcitonin levels. Presented as a flow chart in Figure 2, it outlines when to consider starting, stopping, re-evaluating, or escalating antibiotic therapy. It is important to note that this framework has not been clinically validated and should be viewed as a hypothesis generated from the current evidence base.
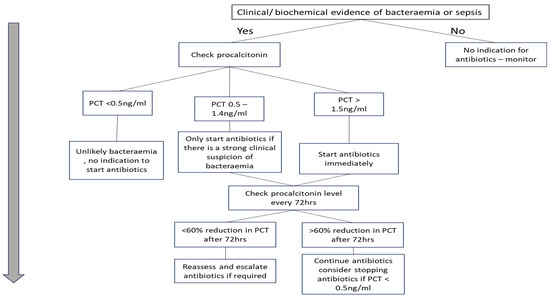
Figure 2.
Procalcitonin-guided antibiotic protocol for suspected bacteraemia in HD patients.
An important clinical trial evaluated procalcitonin-guided antibiotic therapy in ICU patients, using procalcitonin levels as the primary determinant for adjusting the antibiotic regimen. The study found no clinical benefit and, in fact, reported increased mortality and greater antibiotic use in the procalcitonin group [2]. In contrast, our protocol uses procalcitonin as a supportive adjunct alongside clinical judgment and other lab tests. It incorporates a 60% reduction in procalcitonin after 72 h as an indicator of treatment response and highlights the need for reassessment before an escalation of antibiotics if there is no clinical improvement. Unlike the critically ill ICU population studied in the trial, where factors such as fungal infections may interfere with procalcitonin accuracy, our protocol is designed for non-ICU inpatients, offering a more targeted and context-appropriate application. Further rigorous evaluation is crucial to assess its effectiveness.
This review synthesised the existing data on procalcitonin use in haemodialysis (HD) patients with bacteraemia using a rigorous systematic approach to develop evidence-based guidance. However, our study has several limitations. First, our search protocol and criteria might have excluded some relevant studies. Also, our inability to access the full papers of relevant studies may have limited our evaluation due to their exclusion, especially considering that most included studies were observational and susceptible to biases that could compromise our findings [43,44]. The lack of randomised controlled trials prevented us from establishing causal relationships. Finally, the significant heterogeneity among studies in design, methods, participant characteristics, and outcomes made comparisons difficult, leading to a qualitative, subjective approach that may not have fully addressed this variability and could introduce reviewer biases [45,46]. Unmeasured confounding factors, such as other treatments, fluctuations in procalcitonin levels due to timing of dialysis, chronic kidney disease, and other factors, for which no stratification was carried out, also increased the bias risk. These limitations highlight the need for more rigorous research, including well-designed RCTs, to better investigate procalcitonin use in HD patients with bacteraemia.
5. Conclusions
This review underscores the potential of PCT as a diagnostic and monitoring tool in haemodialysis patients with bacteraemia. Despite current limitations, the consistent association between elevated PCT levels and bacterial infection supports its clinical utility. Addressing knowledge gaps through well-designed RCTs, cost-effectiveness analyses, and clinical impact assessments will optimise PCT’s use in diagnosing and monitoring bacteraemia in haemodialysis patients. The proposed hypothetical procalcitonin-guided framework provides a foundation for future research and clinical implementation.
Author Contributions
Conceptualisation, A.-A.U.; methodology, A.-A.U.; formal analysis, A.-A.U.; investigation, A.-A.U.; data curation, A.-A.U.; writing—A.-A.U. and Y.Z.; writing—review and editing, A.-A.U. and Y.Z.; visualisation, A.-A.U. and Y.Z; supervision, A.-A.U. The first author, A.-A.U. conceptualised the systematic review and methodology, developed the protocol, assessed and interpreted the data, and wrote the discussion section. The second author, Y.Z. participated in writing part of the discussion and conclusion; also, drafting, editing, and proofreading the manuscript and summarising the content to meet publication requirements. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors wish to extend their gratitude to TR, who contributed to the data quality assessment based on the protocol established.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. A Search Protocol from the Corresponding Databases. (Asterisk (*) Tells the Database to Search for All Possible Word Endings That Begin with the Specified Root)
Appendix B. Original Newcastle–Ottawa Scale (NOS) for Cohort Studies (Asterisks (*/**/^) Are Awarded If Study Meets Each Criterion)

Appendix C. Modified Newcastle–Ottawa Scale (NOS) for Cross Sectional Study (Asterisks (*) Are Awarded If Study Meets Each Criterion)

Appendix D. Newcastle–Ottawa Scale (NOS) for Case Control Studies (Asterisks (*) Are Awarded If Study Meets Each Criterion)

Appendix E. Modified Grade System
References
- Nassar, G.M.; Ayus, J.C. Infectious complications of the hemodialysis access. Kidney Int. 2001, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.U.; Lundgren, B.; Hein, L.; Mohr, T.; Petersen, P.L.; Andersen, L.H.; Lauritsen, A.O.; Hougaard, S.; Mantoni, T.; Bømler, B.; et al. The Procalcitonin And Survival Study (PASS)—A randomised multi-center investigator-initiated trial to investigate whether daily measurements biomarker Procalcitonin and pro-active diagnostic and therapeutic responses to abnormal Procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infect. Dis. 2008, 8, 91. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, C.-H.; Hong, M.-Y.; Tang, H.-J.; Ko, W.-C. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit. Care 2017, 21, 119. [Google Scholar] [CrossRef]
- Lamy, B.; Dargère, S.; Arendrup, M.C.; Parienti, J.-J.; Tattevin, P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front. Microbiol. 2016, 7, 697. [Google Scholar] [CrossRef]
- Patient.info. Inflammation Blood Tests: ESR, CRP and PV Values. 2024. Available online: https://patient.info/treatment-medication/blood-tests/blood-tests-to-detect-inflammation (accessed on 26 May 2025).
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [CrossRef]
- Davies, J. Procalcitonin. J. Clin. Pathol. 2015, 68, 675–679. [Google Scholar] [CrossRef]
- Bassetti, M.; Russo, A.; Righi, E.; Dolso, E.; Merelli, M.; D’Aurizio, F.; Sartor, A.; Curcio, F. Role of procalcitonin in predicting etiology in bacteremic patients: Report from a large single-center experience. J. Infect. Public Health 2020, 13, 40–45. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Müller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Müller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Müller, B. Procalcitonin Guidance of Antibiotic Therapy in Community-acquired Pneumonia. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef]
- Nobre, V.; Harbarth, S.; Graf, J.-D.; Rohner, P.; Pugin, J. Use of Procalcitonin to Shorten Antibiotic Treatment Duration in Septic Patients. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Jungers, P.; Witko-Sarsat, V. Immune System Dysregulation in Uremia: Role of Oxidative Stress. Blood Purif. 2002, 20, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Lavín-Gómez, B.A.; Palomar-Fontanet, R.; Gago-Fraile, M.; Quintanar-Lartundo, J.A.; Gómez-Palomo, E.; González-Lamuño, D.; García-Unzueta, M.T.; Arias-Rodríguez, M.A.; Gómez-Gerique, J.A. Inflammation markers, chronic kidney disease, and renal replacement therapy. Adv. Perit. Dialysis. Conf. Perit. Dial. 2011, 27, 33–37. [Google Scholar]
- Meisner, M.; Lohs, T.; Huettemann, E.; Schmidt, J.; Hueller, M.; Reinhart, K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur. J. Anaesthesiol. 2001, 18, 79–87. [Google Scholar] [CrossRef]
- Rockel, A.; Abdelhamid, S.; Fliegel, P.; Walb, D. Elimination of Low Molecular Weight Proteins with High Flux Membranes1. In Contributions to Nephrology; Streicherm, E., Seyffart, G., Eds.; S. Karger AG: Basel, Switzerland, 1985; Volume 46, pp. 69–74. [Google Scholar] [CrossRef]
- Mori, K.-I.; Noguchi, M.; Sumino, Y.; Sato, F.; Mimata, H. Use of procalcitonin in patients on chronic hemodialysis: Procalcitonin is not related with increased serum calcitonin. ISRN Urol. 2012, 2012, 431859. [Google Scholar] [CrossRef][Green Version]
- Lu, X.-L.; Xiao, Z.-H.; Yang, M.-Y.; Zhu, Y.-M. Diagnostic value of serum procalcitonin in patients with chronic renal insufficiency: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2013, 28, 122–129. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Cochrane Consumers and Communication Review Group. Data Extraction Template for Included Studies (Revised 2015, Final June 20, 2016 (Revised Nov 29) [Microsoft Word File]. Cochrane. 2016. Available online: https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/uploads/det_2015_revised_final_june_20_2016_nov_29_revised.doc (accessed on 1 May 2023).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Gates, M.; Wingert, A.; Featherstone, R.; Samuels, C.; Simon, C.; Dyson, M.P. Impact of fatigue and insufficient sleep on physician and patient outcomes: A systematic review. BMJ Open 2018, 8, e021967. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Popay, J.; Sowden, A.; Arai, L.; Britten, N.; Duffy, S.; Robert, H.; Petticrew, M.; Rodgers, M.; Roen, K. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Lancaster University: Lancaster, UK, 2006. [Google Scholar] [CrossRef]
- Cochrane Collaboration. Cochrane Training. Part 2: Reason for Using ‘Narrative Synthesis’. Available online: https://youtu.be/KBUK47X6a6M (accessed on 1 May 2023).
- Waters, L.; Winston, A.; Reeves, L. BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022. HIV Med. 2022, 23, 3–115. [Google Scholar] [CrossRef] [PubMed]
- Herget-Rosenthal, S.; Marggraf, G.; Pietruck, F.; Husing, J.; Strupat, M.; Philipp, T.; Kribben, A. Procalcitonin for accurate detection of infection in haemodialysis. Nephrol. Dial. Transplant. 2001, 16, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shao, X.; Xing, W.; Sun, Y. Biomarkers and risk factors for sepsis in stage 5 chronic kidney disease: A retrospective case-control study. Int. Urol. Nephrol. 2019, 51, 691–698. [Google Scholar] [CrossRef]
- Hamada Imam, M.; Gamal, E. Procalcitonin level as a surrogate for catheter-related blood stream infection among hemodialysis patients. J. Vasc. Access 2017, 18, 498–502. [Google Scholar] [CrossRef]
- Demir, N.A.; Sumer, S.; Celik, G.; Afsar, R.E.; Demir, L.S.; Ural, O. How should procalcitonin and C-reactive protein levels be interpreted in haemodialysis patients? Intern. Med. J. 2018, 48, 1222–1228. [Google Scholar] [CrossRef]
- Schneider, R.; Cohen, M.J.; Benenson, S.; Duchin, O.; Haviv, Y.S.; Elhalel-Darnitski, M.; Levin, P.D. Procalcitonin in hemodialysis patients presenting with fever or chills to the emergency department. Intern. Emerg. Med. 2020, 15, 257–262. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.H.; Jang, H.R.; Kim, M.-J.; Jung, S.-H.; Lee, J.E.; Huh, W.; Kim, Y.-G.; Kim, D.J.; Oh, H.Y. Clinical relevance of procalcitonin and C-reactive protein as infection markers in renal impairment: A cross-sectional study. Crit. Care 2014, 18, 640. [Google Scholar] [CrossRef]
- Agrawal, V.; Valson, A.T.; Mohapatra, A.; David, V.G.; Alexander, S.; Jacob, S.; Bakthavatchalam, Y.D.; Prakash, J.A.J.; Balaji, V.; Varughese, S. Fast and furious: A retrospective study of catheter-associated bloodstream infections with internal jugular nontunneled hemodialysis catheters at a tropical center. Clin. Kidney J. 2019, 12, 737–744. [Google Scholar] [CrossRef]
- Yunus, I.; Fasih, A.; Wang, Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS ONE 2018, 13, e0206527. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Ye, B.M.; Kim, M.J.; Kim, S.R.; Lee, D.W.; Lee, S.B. Procalcitonin decrease predicts survival and recovery from dialysis at 28 days in patients with sepsis-induced acute kidney injury receiving continuous renal replacement therapy. PLoS ONE 2022, 17, e0279561. [Google Scholar] [CrossRef]
- Navas, A.; Ferrer, R.; Martinez, M.L.; Goma, G.; Gili, G.; Masip, J.; Suarez, D.; Artigas, A. Impact of hemoperfusion with polymyxin B added to hemofiltration in patients with endotoxic shock: A case-control study. Ann. Intensive Care 2018, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Pan, J.; Zhang, C. The application value of oXiris-Endotoxin adsorption in sepsis. Am. J. Transl. Res. 2021, 13, 3839–3844. [Google Scholar] [PubMed] [PubMed Central]
- Tao, M.; Zheng, D.; Liang, X.; He, Q.; Zhang, W. Diagnostic value of procalcitonin for bacterial infections in patients undergoing hemodialysis: A systematic review and meta-analysis. Ren. Fail. 2022, 44, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Su, L.; Han, G.; Yan, P.; Xie, L. Prognostic Value of Procalcitonin in Adult Patients with Sepsis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0129450. [Google Scholar] [CrossRef]
- Li, S.; Rong, H.; Guo, Q.; Chen, Y.; Zhang, G.; Yang, J. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J. Res. Med. Sci. 2016, 21, 39. [Google Scholar] [CrossRef]
- Kruger, S.; Ewig, S.; Marre, R.; Papassotiriou, J.; Richter, K.; von Baum, H.; Suttorp, N.; Welte, T. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur. Respir. J. 2008, 31, 349–355. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef]
- Siepmann, T.; Spieth, P.M.; Kubasch, A.S.; Penzlin, A.I.; Illigens, B.M.-W.; Barlinn, K. Randomized controlled trials—A matter of design. Neuropsychiatr. Dis. Treat. 2016, 2016, 1341–1349. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1716. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Moher, D.; Boon, H.; Beyene, J.; Bombardier, C. Investigating clinical heterogeneity in systematic reviews: A methodologic review of guidance in the literature. BMC Med. Res. Methodol. 2012, 12, 111. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).