Salt Reduction Using a Smartphone Application Based on an Artificial Intelligence System for Dietary Assessment in Patients with Chronic Kidney Disease: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
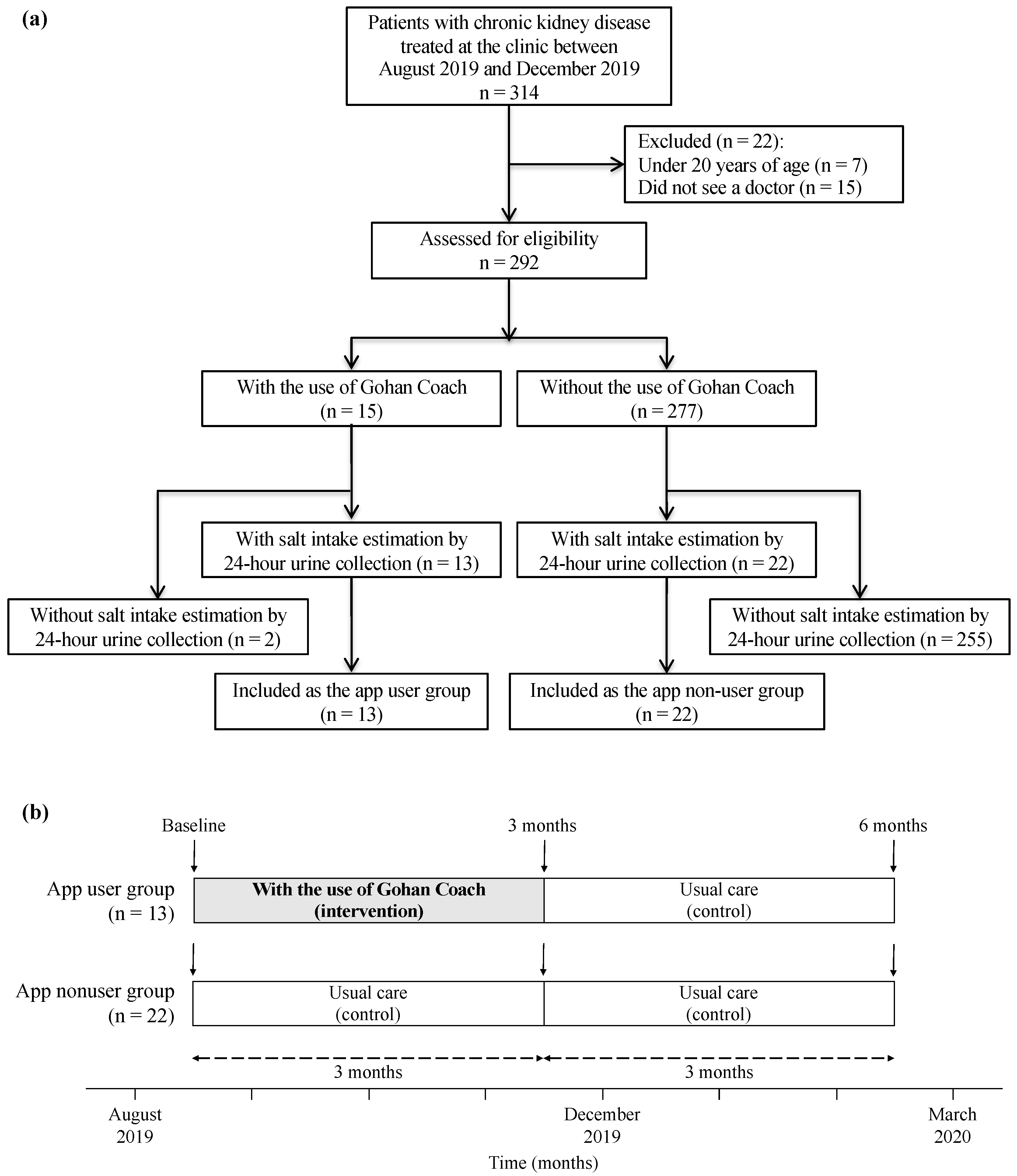
2.1. Study Design and Population
2.2. Outcome Measures and Data Collection
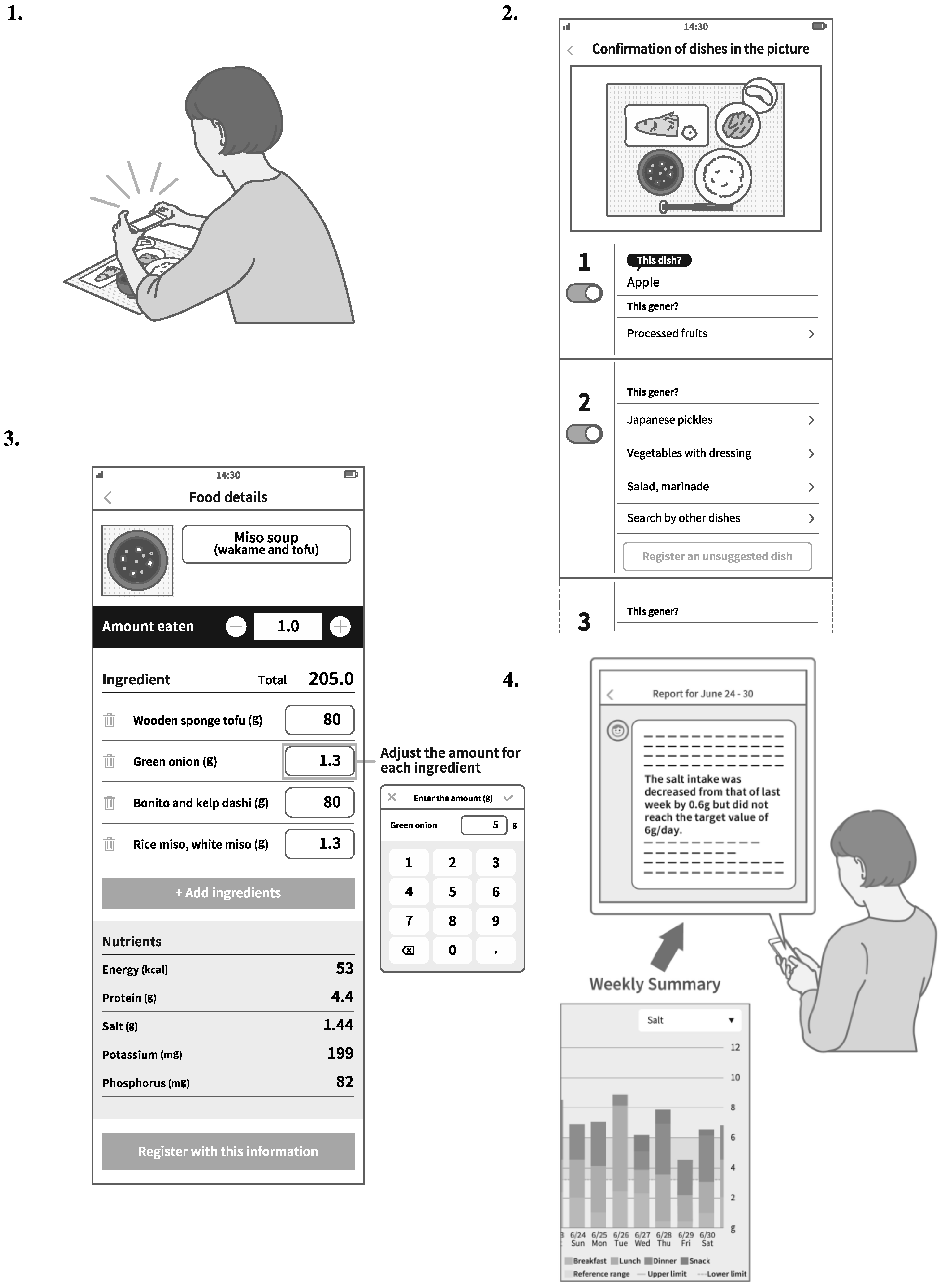
2.3. Intervention and Follow-Up
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
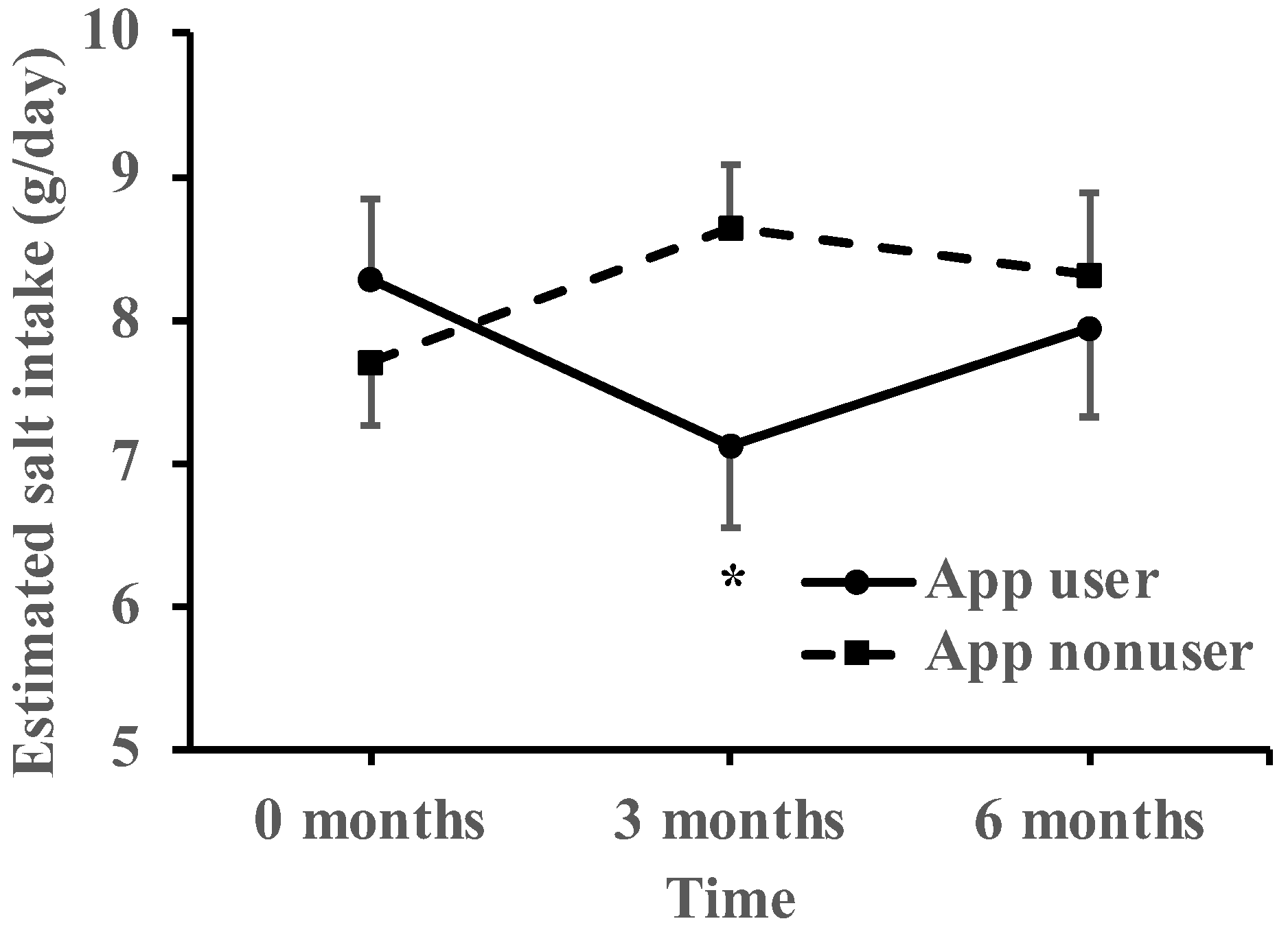
3.2. Within-Group Comparison on Salt Reduction
3.3. Within-Group Comparisons on Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Urinary sodium and potassium excretion and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin. Exp. Nephrol. 2019, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Tsuchihashi, T.; Ueno, M.; Kajioka, T.; Onaka, U.; Tominaga, M.; Eto, K. Relationship between the awareness of salt restriction and the actual salt intake in hypertensive patients. Hypertens. Res. 2004, 27, 243–246. [Google Scholar] [CrossRef]
- Ohta, Y.; Tsuchihashi, T.; Onaka, U.; Eto, K.; Tominaga, M.; Ueno, M. Long-term compliance with salt restriction in Japanese hypertensive patients. Hypertens. Res. 2005, 28, 953–957. [Google Scholar] [CrossRef]
- Uchiyama, K.; Yanai, A.; Ishibashi, Y. Spot urine-guided salt reduction in chronic kidney disease patients. J. Ren. Nutr. 2017, 27, 311–316. [Google Scholar] [CrossRef]
- Hirota, S.; Sadanaga, T.; Mitamura, H.; Fukuda, K. Spot urine-guided salt reduction is effective in Japanese cardiology outpatients. Hypertens. Res. 2012, 35, 1069–1071. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Stathopoulou, T.; Vasiloglou, M.F.; Pinault, L.F.; Kiley, C.; Spanakis, E.K.; Mougiakakou, S. goFOODTM: An Artificial Intelligence System for Dietary Assessment. Sensors 2020, 20, 4283. [Google Scholar] [CrossRef]
- Tsuchihashi, T.; Kai, H.; Kusaka, M.; Kawamura, M.; Matsuura, H.; Miura, K.; Ando, K.; Maruyama, S.; Hayabuchi, H.; Takagi, Y.; et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension (3) Assessment and application of salt intake in the management of hypertension. Hypertens. Res. 2013, 36, 1026–1031. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Kitayama, C.; Yanai, A.; Ishibashi, Y. The effect of trichlormethiazide in autosomal dominant polycystic kidney disease patients receiving tolvaptan: A randomized crossover controlled trial. Sci. Rep. 2021, 11, 17666. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated gfr from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Meuleman, Y.; Hoekstra, T.; Dekker, F.W.; Navis, G.; Vogt, L.; van der Boog, P.J.M.; Bos, W.J.W.; van Montfrans, G.A.; van Dijk, S.; ESMO Study Group. Sodium restriction in patients with CKD: A randomized controlled trial of self-management support. Am. J. Kidney Dis. 2017, 69, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Humalda, J.K.; Klaassen, G.; de Vries, H.; Meuleman, Y.; Verschuur, L.C.; Straathof, E.J.M.; Laverman, G.D.; Bos, W.J.W.; van der Boog, P.J.M.; Vermeulen, K.M.; et al. A self-management approach for dietary sodium restriction in patients with CKD: A randomized controlled trial. Am. J. Kidney Dis. 2020, 75, 847–856. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J.; et al. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Cirillo, M.; Bilancio, G.; Cavallo, P.; Palladino, R.; Terradura-Vagnarelli, O.; Laurenzi, M. Sodium intake and kidney function in the general population: An observational, population-based study. Clin. Kidney. J. 2020, 14, 647–655. [Google Scholar] [CrossRef]
- Cook, N.R.; Kumanyika, S.K.; Cutler, J.A.; Whelton, P.K.; Trials of Hypertension Prevention Collaborative Research Group. Dose–response of sodium excretion and blood pressure change among overweight, nonhypertensive adults in a 3-year dietary intervention study. J. Hum. Hypertens. 2005, 19, 47–54. [Google Scholar] [CrossRef][Green Version]
- de Vries, L.V.; Dobrowolski, L.C.; van den Bosch, J.J.; Riphagen, I.J.; Krediet, C.T.; Bemelman, F.J.; Bakker, S.J.; Navis, G. Effects of dietary sodium restriction in kidney transplant recipients treated with renin-angiotensin-aldosterone system blockade: A randomized clinical trial. Am. J. Kidney Dis. 2016, 67, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Shahar, D.R.; Froom, P.; Harari, G.; Yerushalmi, N.; Lubin, F.; Kristal-Boneh, E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur. J. Clin. Nutr. 1999, 53, 395–400. [Google Scholar] [CrossRef]
- Wu, Z.; Lan, S.; Chen, C.; Zhang, X.; Zhang, Y.; Chen, S. Seasonal Variation: A Non-negligible Factor Associated with Blood Pressure in Patients Undergoing Hemodialysis. Front. Cardiovasc. Med. 2022, 9, 820483. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, C.; Luo, B.; Zang, J.; Wang, Z.; Guo, C.; Jia, X.; Wang, W.; Shen, X.; Lu, Y.; et al. The Dietary Intake and Its Features across Four Seasons in the Metropolis of China. J. Nutr. Sci. Vitaminol. 2019, 65, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Mihara, Y.; Kado, H.; Yokota, I.; Shiotsu, Y.; Sonomura, K.; Kusaba, T.; Hatta, T.; Matoba, S.; Tamagaki, K. Rapid weight loss with dietary salt restriction in hospitalized patients with chronic kidney disease. Sci. rep. 2019, 9, 8787. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, L.; Carnethon, M.; Fortmann, S.P. Association Between microalbuminuria and the metabolic syndrome: NHANES III. Am. J. Hypertens. 2003, 16, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Chagnac, A.; Weinstein, T.; Korzets, A.; Ramadan, E.; Hirsch, J.; Gafter, U. Glomerular hemodynamics in severe obesity. Am. J. Physiol. Ren. Physiol. 2000, 278, F817–F822. [Google Scholar] [CrossRef]
- Singh, K.; Diamantidis, C.J.; Ramani, S.; Bhavsar, N.A.; Mara, P.; Warner, J.; Rodriguez, J.; Wang, T.; Wright-Nunes, J. Patients’ and nephrologists’ evaluation of patient-facing smartphone apps for CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 523–529. [Google Scholar] [CrossRef]
- Ong, S.W.; Jassal, S.V.; Miller, J.A.; Porter, E.C.; Cafazzo, J.A.; Seto, E.; Thorpe, K.E.; Logan, A.G. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1054–1062. [Google Scholar] [CrossRef]
- Chang, A.R.; Bailey-Davis, L.; Hetherington, V.; Ziegler, A.; Yule, C.; Kwiecen, S.; Graboski, E.; Melough, M.M.; Collins, C.; Anderson, C. Remote dietary counseling using smartphone applications in patients with stages 1–3a chronic kidney disease: A mixed methods feasibility study. J. Ren. Nutr. 2020, 30, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vasiloglou, M.F.; van der Horst, K.; Stathopoulou, T.; Jaeggi, M.P.; Tedde, G.S.; Lu, Y.; Mougiakakou, S. The Human Factor in Automated Image-Based Nutrition Apps: Analysis of Common Mistakes Using the goFOOD Lite App. JMIR Mhealth Uhealth 2021, 9, e24467. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 35) | App Nonuser Group (n = 22) | App User Group (n = 13) | p Value | t Value |
|---|---|---|---|---|---|
| Age (years) | 60.5 ± 15.0 | 63.3 ± 15.9 | 55.8 ± 12.7 | 0.15 | 1.47 |
| Male/female | 21/14 (60/40%) | 13/9 (59/41%) | 8/5 (62/38%) | 1 | – |
| Body weight (kg) | 65.6 ± 15.0 | 64.2 ± 15.7 | 68.1 ± 13.9 | 0.46 | −0.75 |
| Body mass index (kg/m2) | 24.0 ± 3.8 | 23.6 ± 3.8 | 24.8 ± 3.8 | 0.35 | −0.95 |
| CKD stages | |||||
| 2 | 11 (31%) | 8 (36%) | 3 (23%) | 0.43 | – |
| 3a | 11 (31%) | 6 (27%) | 5 (38%) | 0.71 | – |
| 3b | 3 (9%) | 1 (5%) | 2 (15%) | 0.54 | – |
| 4 | 10 (29%) | 7 (32%) | 3 (23%) | 0.71 | – |
| Comorbidity | |||||
| Hypertension | 26 (74%) | 16 (73%) | 10 (77%) | 1 | – |
| Diabetes | 13 (37%) | 9 (41%) | 4 (31%) | 0.72 | – |
| Cardiovascular disease | 3 (9%) | 2 (9%) | 1 (8%) | 1 | – |
| Arrythmia | 5 (14%) | 4 (18%) | 1 (8%) | 0.63 | – |
| Cerebrovascular disease | 0 (0%) | 0 (0%) | 0 (0%) | 1 | – |
| Malignancy | 0 (0%) | 0 (0%) | 0 (0%) | 1 | – |
| Use of antihypertensive drugs | |||||
| Calcium channel blocker | 13 (37%) | 7 (32%) | 6 (46%) | 0.48 | – |
| RAS inhibitor | 18 (51%) | 11 (50%) | 7 (54%) | 1 | – |
| Diuretics | 2 (6%) | 0 (0%) | 2 (15%) | 0.13 | – |
| Number of prescribed antihypertensive drugs | |||||
| 0 | 15 (42%) | 10 (45%) | 5 (38%) | 0.74 | – |
| 1 | 8 (23%) | 6 (27%) | 2 (13%) | 0.68 | – |
| 2 | 11 (31%) | 6 (27%) | 5 (38%) | 0.71 | – |
| 3 | 1 (3%) | 0 (0%) | 1 (7%) | 0.37 | – |
| Systolic blood pressure (mmHg) | 132.0 ± 14.6 | 132.4 ± 12.6 | 131.4 ± 18.1 | 0.85 | 0.19 |
| Diastolic blood pressure (mmHg) | 76.9 ± 12.0 | 75.7 ± 9.5 | 78.8 ± 15.6 | 0.47 | −0.74 |
| Mean blood pressure (mmHg) | 95.3 ± 11.0 | 94.6 ± 8.2 | 96.4 ± 15.0 | 0.66 | −0.45 |
| Total protein (g/L) | 7.2 ± 0.4 | 7.1 ± 0.4 | 7.3 ± 0.4 | 0.08 | −1.81 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.4 ± 0.2 | 0.07 | −1.90 |
| Hemoglobin (g/dL) | 13.2 ± 1.7 | 13.2 ± 1.9 | 13.2 ± 1.4 | 1 | 0.0027 |
| Sodium (mEq/L) | 141.3 ± 1.9 | 141.6 ± 1.6 | 140.8 ± 2.3 | 0.26 | 1.14 |
| Potassium (mEq/L) | 4.1 ± 0.5 | 4.0 ± 0.5 | 4.2 ± 0.4 | 0.46 | −0.74 |
| Urea nitrogen (mg/dL) | 22.0 ± 13.8 | 22.2 ± 13.2 | 21.7 ± 15.2 | 0.92 | 0.10 |
| Creatinine (mg/dL) | 1.36 ± 0.75 | 1.35 ± 0.76 | 1.38 ± 0.76 | 0.91 | −0.12 |
| eGFR (mL/min/1.73 m2) | 50.9 ± 23.4 | 52.3 ± 24.4 | 48.7 ± 22.3 | 0.67 | 0.43 |
| Low-density lipoprotein cholesterol (mg/dL) | 99.5 ± 29.4 | 98.9 ± 26.8 | 100.5 ± 34.6 | 0.88 | −0.16 |
| High-density lipoprotein cholesterol (mg/dL) | 62.9 ± 20.3 | 64.9 ± 22.5 | 60.8 ± 18.5 | 0.62 | 0.50 |
| Triglyceride (mg/dL) | 131.7 ± 56.6 | 128.9 ± 59.3 | 136.5 ± 53.8 | 0.71 | −0.38 |
| Estimated salt intake (g/day) | 7.86 ± 2.70 | 7.26 ± 2.57 | 8.87 ± 2.71 | 0.09 | −1.76 |
| Urinary protein (g/day) | 0.54 ± 0.83 | 0.48 ± 0.67 | 0.63 ± 1.06 | 0.61 | −0.52 |
| App User Group | App Nonuser Group | |||||
|---|---|---|---|---|---|---|
| Baseline (0 Months) | 3 Months | p Value | 0 Months | 3 Months | p Value | |
| Estimated salt intake (g/day) | 8.87 ± 2.71 | 7.71 ± 2.81 | 0.09 | 7.26 ± 2.57 | 8.21 ± 3.21 | 0.1 |
| Urinary protein (g/day) | 0.63 ± 1.06 | 0.41 ± 0.81 | 0.07 | 0.47 ± 0.67 | 0.49 ± 0.65 | 0.83 |
| Systolic blood pressure (mmHg) | 131.9 ± 18.1 | 127.2 ± 14.8 | 0.23 | 132.4 ± 12.6 | 135.6 ± 9.2 | 0.18 |
| Diastolic blood pressure (mmHg) | 78.8 ± 15.6 | 73.9 ± 14.4 | 0.03 | 75.7 ± 9.5 | 78.4 ± 10.5 | 0.23 |
| Mean blood pressure (mmHg) | 96.4 ± 15.0 | 91.7 ± 13.5 | 0.07 | 94.6 ± 8.2 | 97.5 ± 7.8 | 0.18 |
| Body weight (kg) | 68.1 ± 13.9 | 67.3 ± 14.4 | 0.08 | 64.2 ± 15.7 | 64.4 ± 15.2 | 0.51 |
| Body mass index (kg/m2) | 24.8 ± 3.8 | 24.5 ± 4.0 | 0.07 | 23.6 ± 3.8 | 23.7 ± 3.7 | 0.4 |
| eGFR (mL/min/1.73 m2) | 48.7 ± 22.2 | 48.1 ± 22.2 | 0.71 | 52.3 ± 24.4 | 52.2 ± 24.7 | 0.43 |
| App User Group * | App Nonuser Group * | Effect of Intervention † | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 Months | 3 Months | 6 Months | 0 Months | 3 Months | 6 Months | Δ0−3 mo | p Value | Δ0−6 mo | p Value | |
| Estimated salt intake (g/day) | 8.29 ± 0.56 | 7.12 ± 0.56 | 7.95 ± 0.63 | 7.70 ± 0.43 | 8.65 ± 0.43 | 8.32 ± 0.57 | −2.12 ± 0.96 | 0.03 | −0.96 ± 1.08 | 0.38 |
| Urinary protein (g/day) | 0.56 ± 0.08 | 0.34 ± 0.08 | 0.26 ± 0.09 | 0.57 ± 0.06 | 0.58 ± 0.06 | 0.55 ± 0.08 | −0.23 ± 0.12 | 0.05 | −0.27 ± 0.14 | 0.05 |
| Systolic blood pressure (mmHg) | 132.0 ± 3.2 | 127.7 ± 3.2 | 131.1 ± 4.1 | 132.0 ± 2.5 | 135.3 ± 2.5 | 132.7 ± 3.3 | −7.5 ± 5.4 | 0.17 | −1.6 ± 6.3 | 0.8 |
| Diastolic blood pressure (mmHg) | 78.1 ± 2.3 | 73.2 ± 2.3 | 76.8 ± 2.9 | 76.5 ± 1.8 | 79.2 ± 1.8 | 81.2 ± 2.4 | −7.6 ± 4.0 | 0.06 | −6.0 ± 4.7 | 0.21 |
| Mean blood pressure (mmHg) | 96.1 ± 2.3 | 91.4 ± 2.3 | 94.9 ± 2.9 | 95.0 ± 1.8 | 97.8 ± 1.8 | 98.3 ± 2.4 | −7.6 ± 3.9 | 0.06 | −4.5 ± 4.6 | 0.33 |
| Body weight (kg) | 63.9 ± 1.4 | 63.0 ± 1.4 | 63.3 ± 1.4 | 64.9 ± 1.1 | 65.1 ± 1.1 | 66.0 ± 1.2 | −1.1 ± 0.5 | 0.04 | −1.8 ± 0.6 | 0.003 |
| Body mass index (kg/m2) | 24.1 ± 0.1 | 23.8 ± 0.1 | 23.9 ± 0.1 | 24.1 ± 0.1 | 24.2 ± 0.1 | 24.5 ± 0.1 | −0.4 ± 0.2 | 0.03 | −0.7 ± 0.2 | 0.002 |
| eGFR (mL/min/1.73 m2) | 49.4 ± 0.9 | 48.8 ± 0.9 | 47.6 ± 0.9 | 49.5 ± 0.7 | 49.0 ± 0.7 | 46.8 ± 0.9 | −0.1 ± 1.6 | 0.96 | 0.8 ± 1.4 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanai, A.; Uchiyama, K.; Suganuma, S. Salt Reduction Using a Smartphone Application Based on an Artificial Intelligence System for Dietary Assessment in Patients with Chronic Kidney Disease: A Single-Center Retrospective Cohort Study. Kidney Dial. 2023, 3, 139-151. https://doi.org/10.3390/kidneydial3010012
Yanai A, Uchiyama K, Suganuma S. Salt Reduction Using a Smartphone Application Based on an Artificial Intelligence System for Dietary Assessment in Patients with Chronic Kidney Disease: A Single-Center Retrospective Cohort Study. Kidney and Dialysis. 2023; 3(1):139-151. https://doi.org/10.3390/kidneydial3010012
Chicago/Turabian StyleYanai, Akane, Kiyotaka Uchiyama, and Shinya Suganuma. 2023. "Salt Reduction Using a Smartphone Application Based on an Artificial Intelligence System for Dietary Assessment in Patients with Chronic Kidney Disease: A Single-Center Retrospective Cohort Study" Kidney and Dialysis 3, no. 1: 139-151. https://doi.org/10.3390/kidneydial3010012
APA StyleYanai, A., Uchiyama, K., & Suganuma, S. (2023). Salt Reduction Using a Smartphone Application Based on an Artificial Intelligence System for Dietary Assessment in Patients with Chronic Kidney Disease: A Single-Center Retrospective Cohort Study. Kidney and Dialysis, 3(1), 139-151. https://doi.org/10.3390/kidneydial3010012







