Abstract
Objectives: Dialysis patients suffer from xerostomia based on hyposalivation even in stimulated saliva testing. Therefore, the aim of this study was (i) to evaluate an optimized oral hygiene system with an oral care gel reducing oral dryness, supporting dental plaque control and reducing the number of gingivitis affected teeth; (ii) to document the efficacy in a randomized controlled trial with dental indices; and (iii) to record the individual’s oral health related quality of life. Methods: After ethical approval (EC-UWH 103/2019), 44 dialysis patients provided signed informed consent and were divided into two groups followed up for four weeks. Subjects of the verum group (A) received daily oral care gel OROFAN® based on biopolymers with a mild antibacterial and saliva stimulating action. They were compared to the control group (B) who executed routine oral hygiene with conventional dentifrice and toothbrushes. At baseline (T0), the medical report and all dental indices (sialometry, DMF/S, plaque index, periodontal index, tongue coating and denture hygiene index) were assessed. After 2 weeks and 4 weeks, T1 and T2 dry mouth frequency and all dental indices were assessed. An Oral Health Impact Profile was documented at baseline (T0) and at the end of the study (T2). Results: Twenty-two subjects were allocated to group A and to group B. At baseline, there were no statistical differences concerning renal insufficiency (years on dialysis and complications), systemic diseases and dental background (caries experience, periodontal diseases, plaque index and number of gingivitis affected teeth). All subjects exhibited hyposalivation (<0.7–1.0 mL/min). The control group (B) showed no statistical differences from baseline (T0) to the end of the study (T2) in dry mouth parameters, in all dental plaque and inflammation indices and in their Oral Health Impact Profile. In contrast, the verum group (A) exhibited a highly significant (p < 0.001) decrease in xerostomia frequency, in the number of gingivitis affected teeth and in their plaque index. Furthermore, their Oral Health Impact Profile improved considerably (p < 0.01). Significance of Results: Terminally ill patients may gain better oral health and, consequently, an improved quality of life by rather simple oral care using long-lasting, edible anti-inflammatory oral hygiene gel and a dentist’s devotion.
1. Introduction
Xerostomia is the subjective sensation of dry mouth. The objective sign of dry mouth is the hypofunction of the salivary glands with measurably decreased saliva production [1]. Salivary flow plays a critical role in maintaining physiological balance in the oral cavity. Loss of saliva volume means loss of antibacterial properties, favored plaque formation, acceleration of tooth demineralization and caries development. Microorganisms in the oral cavity are a component of eubiotic plaque, contributing to the prevention of infections and performing a protective function. If this physiological biofilm with eubiotic conditions becomes unbalanced, a pathogenic plaque with dysbiotic conditions forms and increases the risk of caries and gingivitis development [2].
The reduction in salivary flow means the patient is at increased risk of developing caries, having problems wearing dentures or developing infectious diseases [3]. In addition, the consequences and diseases resulting from xerostomia can lead to a decrease in quality of life and even to a decrease in patients’ psychological stability.
Xerostomia is relatively common in Europe and is found in more than 20 percent of the population, with women and the elderly being more frequently affected [4,5,6]. In those over 65 years of age, it is reported to occur in about 30% [7].
A recent study in a population of 65-year-old Oslo residents showed that 8% of the population had unstimulated salivary flow below 0.1 mL/min, and 3% of the subjects studied had xerostomia and hyposalivation (related to unstimulated salivary flow). Xerostomia was significantly correlated with medication, rheumatic diseases, radiotherapy of head and neck tumors and type 2 diabetes [8].
Xerostomia is a common accompanying symptom in patients with chronic kidney disease or end-stage renal failure with required dialysis. Changes in the amount and composition of saliva lead to hyposalivation or xerostomia [9,10,11]. Xerostomia in renal patients is favored by other chronic diseases such as diabetes mellitus or hypertension and taking necessary medications.
The aim of this study was to examine whether the subjectively perceived xerostomia is associated with reduced saliva production in patients with renal failure requiring dialysis and whether oral health can be favorably influenced by means of intensified oral care. In particular, it should be examined whether this is more successful with a special edible oral care gel and a nursing toothbrush than with measures previously used by the patients.
2. Methods
The study was performed with 44 patients (18 women and 26 men out of 139 invited patients) between 30 and 85 years of age (mean age 57.5 years) under ambulatory dialysis therapy (43 hemodialysis patients and 1 peritoneal dialysis patient) as a randomized controlled trial at the PHV Institute, Nephrology Outpatient Clinic/Dialysis Hildesheim, Germany. Ethical approval was obtained from the Ethical Committee of the University of Witten/Herdecke (No. 103/2019; German Clinical Trials Register DRKS00029636, 20 July 2022). The inclusion criteria comprised a dialysis period of more than four years, the subjective feeling of dry mouth and provision of written consent after clinical and procedural information provided by the study dentist (LE).
The subjects were randomly divided into two groups: The verum group (A) used a new oral care gel OROFAN® containing xylitol, ChitoClear, aloe vera, hydroxyethyl cellulose and O-cymen-5-Ol. (Dr. Hinz Group, Herne, Germany) and the OROFAN® nursing toothbrush, designed and clinically tested for patients with disabilities [12]. The subjects of the control group (B) continued their usual oral hygiene at home with conventional dentifrice and manual toothbrushes (16 patients) or powered toothbrushes (6 patients). The study period was 28 days. The present report complies with the CONSORT 2010 Statement.
At the start of the study, after information, education and informed consent, a baseline examination (T0) was performed; after two weeks, an intermediate examination (T1), and after four weeks, the final examination (T2). At the same time, all subjects in both groups were trained in the correct performance of twice-daily oral hygiene. Subjects of the verum group were instructed how to use the gel and the nursing toothbrush.
At the baseline examination, a general survey of personal data, underlying diseases, secondary diseases, other diseases and drug medications, performance of dialysis and oral hygiene was carried out. At the intermediate examination (T1), subjects were questioned regarding their subjective oral hygiene and dry mouth feeling. In case of problems and difficulties concerning toothbrushing, plaque control and bleeding due to gingivitis, the individual oral care was optimized by re-instruction. The final anamnestic questionnaires were taken after four weeks (T2).
At baseline, the stimulated total salivary flow rate was measured for evidence of hyposalivation. Oral findings were recorded at the baseline examination. The assessment of plaque accumulation on the dorsum of the tongue was performed at three severity levels. Dental plaque was assessed using the Silness and Löe plaque index SLI [13] in five codes, and gingivitis and periodontitis experience was assessed using the GPM/T index [14]. counting the number of teeth affected by gingivitis and periodontitis after six-point probing around all single teeth. Caries prevalence was assessed using the DMF/S index [15]. Denture hygiene for the maxilla and mandible was documented with the denture hygiene index (DHI) according to Wefers [16] and indicated as a percentage of the coating (plaque)-free areas.
To investigate subjective perceptions and record individual oral health-related quality of life, the Oral Health Impact Profile (OHIP-G14) [17] was asked at baseline and at the end of the study. This German validated version is a partly self-administered questionnaire that focuses on seven items (functional limitation, pain, psychological discomfort, physical disability, psychological disability and social disability). The 14 questions in the hands of subjects were commented and finally self-documented in 5 codes (0—never, 1—hardly ever, 2—occasionally, 3—fairly often, 4—very often). Therefore, the total code number ranges from 0 to 56.
At the intermediate examination, a special control belief questionnaire was completed to assess the patient’s own attitude toward oral hygiene. At the final examination, the subjective success of oral hygiene measures was also assessed by means of a questionnaire.
All clinical methods, assessments of indexes and self-reported questionnaires were carried out in accordance with the Helsinki Declaration and GCP guidelines. The study dentist LE was calibrated for all relevant clinical methods.
The output parameter of the G*Power 3.1 analysis resulted in p = 0.823 and the sample size per group n = 19. Statistical analysis was performed with the statistical program system IBM SPSS Professional Statistics Premium, release 26, 64-bit version, using the Kolmogorov–Smirnov test, the t-test, the Wilcoxon–Mann–Whitney U test, the Wilcoxon signed-rank test, Pearson’s chi-square test and the McNemar test. The significance level was set using p < 0.05, p < 0.01 and p < 0.001.
3. Results
There were 22 subjects in each group, and no differences were found between the two groups in terms of years on dialysis (A 6.2 vs. B 4.9), number of complications under dialysis (A 11.5 vs. B 10.3) and medications (continuous therapy, medications for dialysis or taken on-demand; A 11.5 vs. B 12.1), as well as the need for care and number of diagnoses. The most common disease in both groups was hypertension (A 19 vs. B 21). There was no difference in the number of diabetes mellitus subjects (A 6 vs. B 9; HbA1c 6.9%), and nicotine consumption was similar in both groups, with the majority of subjects describing themselves as nonsmokers. No statistical differences could be documented with regard to the number of teeth per patient, the last visit to the dentist and/or a dental hygienist.
At baseline, a statistically significant difference between the two groups was seen only in the frequency of dry mouth (p < 0.05), with the greater frequency in the verum group. Dry mouth during dialysis was present in 13 subjects in the verum group and 11 in the control group.
The stimulated salivary flow rate was similar in both groups. Normal saliva production under stimulation (>1.0 mL/min) could not be documented in any patient. A significantly decreased salivary flow rate below 0.7 mL/min was detected in 86.4% of patients in the control group and in 81.8% of patients in the verum group. A salivary flow rate of 0.7–1.0 mL per minute was additionally found in three patients in the control group, and in four patients in the verum group.
Significant differences in dental diagnostic parameters were not found between the groups at baseline. Tongue plaque was present in 63.6% of the control group and 59.0% of the verum group. The mean modified plaque index (SLI), as well as the GPM/T (gingivitis affected teeth, periodontitis affected teeth, missing teeth and total index) and denture hygiene index (DHI) were similar, as was oral health-related quality of life (OHIP-14) (Table 1).

Table 1.
Oral hygiene, Oral Health Impact Profile parameters and statistical differentiation at baseline (T0) and final examination (T2) in the control and verum groups of dialysis patients.
Dry mouth during dialysis was present in 59.1% of patients in the verum group initially, 42.9% at the intermediate examination and only 19.0% at the end of the study. Furthermore, 66.7% of those who complained of dry mouth during dialysis at the baseline examination did not report dry mouth at the final examination. In contrast, the dry mouth symptoms did not change in the control group (50.0% of subjects at baseline examination and 52.4% at the end of the study).
At the intermediate examination, the verum group showed a decrease in the frequency of dry mouth (p < 0.001), the SLI dental plaque index (p < 0.001) and number of gingivitis affected teeth using the GPM/T index (p < 0.001). The maxillary denture hygiene index DHI (p < 0.05) and the mandibular denture hygiene index DHI (p < 0.05) documented increased percentages of plaque-free denture areas. The mean value of the SLI dental plaque index improved from 1.44 to 1.16, the number of gingivitis affected teeth decreased from 7.56 to 5.82, the maxillary denture hygiene index DHI increased from 33.89% to 56.00% and the mandibular DHI from 32.71% to 49.71% of plaque-free areas (Table 1).
The trend of improving oral health continued in the verum group at the final examination, and some pronounced differences could be assessed: the frequency of dry mouth and dry mouth during dialysis both improved significantly, the SLI plaque index decreased (Figure 1) and the number of gingivitis affected teeth and periodontitis affected teeth decreased (p < 0.05) (Figure 2).
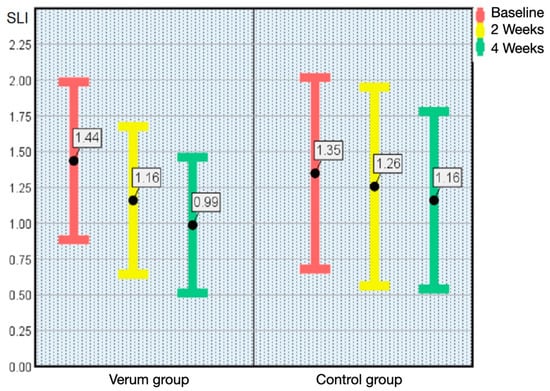
Figure 1.
Plaque index according to Silness and Löe (SLI; x ± SD) from baseline (T0) to the end of the study (T2) in the verum group and control group. The Error Bars of mean values and standard deviations demonstrate highly significant (p < 0.001) lower dental plaque accumulation on all teeth in whole mouth assessment as an indicator of improved oral hygiene.
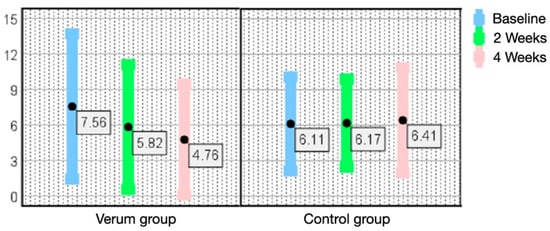
Figure 2.
Number of teeth exhibiting gingivitis according to the GPM/T index (x ± SD) from baseline (T0) to the end of the study (T2) in the verum group and control group. Error bars of mean values and standard deviations show the highly significant (p < 0.001) decrease in the number of gingivitis affected teeth per subject in the verum group compared to an insignificant increase in the number of gingivitis affected teeth in the control group. Fewer bleeding gums around the teeth are due to effective dental plaque control.
Individual oral health-related quality of life assessed using OHIP-14 started with a rather low dental impact on quality of life in both groups with a mean code at 6.25 in the control group and 6.62 in the verum group. At the final examination, a statistical improvement with a mean code at 4.75 in the verum group was documented. Taking all patients together, the OHIP-14 code ranged from 0 to 23 at baseline and from 0 to 18 at the end of the study. Comparing the mean OHIP-14 code 0–9 of all patients (no impact and very little impact of oral health on quality of life) with mean code 10–28 (occasionally to fairly often), the slight improvement is significantly different between the two groups. This is supported by four verum group patients at baseline versus seven patients at the end of the study having never experienced any dental/oral impact on quality of life during the previous month (OHIP-14 code 0).
In contrast to the low OHIP-14 impact, the questionnaires concerning the subjective value of oral hygiene in the verum group documented positive perceptions. Subjects rated the taste of the edible oral care gel as good and expressed satisfaction with the new oral hygiene recommendation. The moisturizing effect on the lips and oral mucosa was rated particularly suitable, as was the refreshing taste of lemon to palliate the dry mouth feeling. The special nursing toothbrush was rated as congenial for proper oral hygiene and particularly for cleaning of complex dentures.
When comparing the results of the baseline and the end of the study, there were no significant differences in the parameters measured in the control group (Table 1).
4. Discussion
Dialysis patients are often found to have less saliva and alterations in its composition, often resulting in hyposalivation or xerostomia [9,10]. Under dialysis, not only are systemic infections favored, but also oral diseases such as caries, gingivitis and periodontitis [18]. At the onset of dialysis, dental and periodontal status is comparable to systemically healthy individuals but worsens with increasing durations of dialysis [19]. Periodontal dis [eases and poor oral hygiene status are found more frequently than in healthy controls [20]. In hemodialysis patients, saliva production is decreased with and without salivary stimulation [10]. A recent meta-analysis shows that chronic kidney disease patients presented lower salivary flow rate, and it was also verified that hemodialysis can increase the salivary flow rate in these patients; it is concluded that there is a need for customized clinical planning of dental care [21].
All patients we studied reported xerostomia, and the sialometry did not measure normal saliva secretion in a single patient. Out of 139 patients initially interviewed, 53 patients (38.1%) reported that they suffered from dry mouth, which is very consistent with data from the literature [22]. Of these, 44 patients gave informed consent to participate in this study. On the one hand, this rather low number of subjects was due to the inclusion criterion of xerostomia and a duration of hemodialysis for more than 4 years, but on the other hand, some patients did not show an interest in taking part in an oral hygiene study.
The overall dental health of dialysis patients is rather poor and requires greater attention. Common findings include gingivitis and increased dental plaque accumulation [23].
Sufficient dentures of the maxilla were present in 39.5%, and of the mandible, in 23.8% of the patients in both examined groups. All of them exhibited poor denture hygiene.
Mouth dryness favors the occurrence of other problems in the oral cavity. In both peritoneal dialysis and hemodialysis, an increased dental plaque index is found [24,25], which worsens with duration of dialysis [26]. Under hemodialysis, increased tongue coating is observed in addition to dry mouth, often associated with taste changes [27].
A high frequency of periodontitis affected teeth with high severity has been documented in hemodialysis patients [24]. Patients with chronic periodontitis during hemodialysis showed a generally higher mortality than those without this concomitant disease, although this was not confirmed in multivariate statistical analysis, possibly due to insufficient patient numbers [28,29,30].
In any case, we observed a regression of inflammatory changes only in the verum group, which may be due to the long-lasting coating effect of the oral hygiene gel on mucosal irritation and gingival inflammation. The number of teeth exhibiting gingivitis and shallow periodontitis dropped from 7.56 to 4.76 (gingivitis) and from 9.00 to 8.35 (P1 shallow pocketing) because of the improved plaque control by the subjects and the mechanism of action (MOA) of the gel lasting for hours on tooth surfaces and on oropharyngeal mucosal surfaces as a protecting bio-layer.
In long-term hemodialysis over 6.7 ± 5.6 years, gingival and periodontal inflammatory reactions correlate with quality of life, both from physical and psychological aspects. Oral health-related quality of life, assessed with a short form 36-item health survey, could be improved by targeted intervention with periodontal treatment [31,32].
Hemodialysis seems to discourage patients from visiting their dentist, resulting in a higher need for treatment [33]. The majority of patients only visit their dentist when they have complaints, and not all dentists are aware of the oral health related needs of dialysis patients [34].
Patients on dialysis due to chronic renal failure with the symptom of dry mouth often present with other oral diseases. Therefore, a detailed medical and dental history is required. The measurement of the saliva flow rate is useful and corrective measures of hyposalivation may prevent oral diseases [35].
Strategies for treating xerostomia are aimed at alleviating symptoms and their consequences. They include stimulant pharmaceuticals and saliva replacement therapy with oral moistening agents [36,37]. Rinses with licorice water [37] or aloe vera [38] were also reported to be beneficial in hemodialysis patients.
The patients in the present study showed a very beneficial effect from the oral care gel application as the dry mouth that initially existed in more than half of the patients during dialysis was significantly reduced after four weeks. The study showed that consistent dental care and oral hygiene with suitable products lead to a highly significant reduction in xerostomia, plaque accumulation and the number of gingivitis affected teeth. Not only are objective parameters such as the SLI dental plaque index, the number of gingivitis affected teeth and shallow pocketing according to the GPM/T index, denture hygiene index DHI or tongue coating significantly improved, but also subjective perceptions such as frequency of dry mouth, dry mouth on dialysis or oral health-related quality of life (OHIP-14). Thus, even in chronic and terminally ill patients, dental care and oropharyngeal gel improve oral health-related quality of life.
Xerostomia is a common problem in end stage renal disease. It has also been demonstrated in the present study that this subjective feeling of dry mouth is often associated with hyposalivation in dialysis patients. Factors that are important for the development but cannot be influenced are gender, age and type of dialysis. Medication—dry mouth is a major side effect of many medications—can also only be adjusted within a narrow range. The performance of dialysis is associated with considerable psychological problems for the patient and requires an adjustment of the entire lifestyle, including a restriction in the amount of drinking. The problems associated with xerostomia are usually not considered as significant by the dialysis team and are also not considered a priority by the patient. Therefore, the willingness to consistently perform and optimize oral hygiene is also lacking. Daily oral care should also be better monitored by the dialysis team, with a specially organized collaboration between the dialysis facility and dental institutions producing favorable results [39].
The rather low impact of oral health on quality of life according to the OHP-14 data with 7 items and 14 questions is mainly due to the psychological status of patients under hemodialysis. They are under permanent supervision of nephrologists, and, consequently, their health behavior is dominated by their chronic kidney disease. However, a professional oral hygiene program is contributing to a slight increase of OHIP-14 related quality of life. In addition, and even more importantly, the motivation of taking more self-care using a long-lasting, saliva stimulating and fluoride containing oral hygiene gel under the umbrella of dental professionals’ devotion is contributing to psychological comfort and to the prevention of gingival and mucosal inflammation and dental caries progression.
It was the intention of this study to improve the oral hygiene status of all patients. However, the instruction and training of oral hygiene alone resulted in a limited improvement of the dental and psychological parameters. Using alternative oral care products such as a gel with long mucosal adhesion and, therefore, prolonged bioavailability and a special nursing toothbrush, it was demonstrated for the first time that a significant influence on xerostomia, plaque accumulation and the number of gingivitis affected teeth can be achieved within a short period of time in a vulnerable group of long-term patients requiring intensive care and without the need for additional measures. The results of this study may also apply to other vulnerable patient groups with oropharyngeal inflammatory reactions.
5. Conclusions
The complex dental examination including all relevant clinical parameters and the xerostomia status at baseline confirms the poor oral health in dialysis patients.
The primary goal of dental and medical care is to palliate the xerostomia in-between and during dialysis. The patients try to drink as little as possible, therefore, an oral edible gel may reduce the dry mouth feeling with a maximum of 15 mL of liquid per 24 h.
Dental plaque control after motivation, training and re-training of adequate oral hygiene with a multifunctional nursing manual toothbrush and gingivitis/mucositis control using a long-lasting gel based on biopolymers contributes to the improvement of oral health within a short period of four weeks while using rather limited personal and material resources.
The Oral Health Impact Profile, assessed by the OHIP-14 short questionnaire, documented a rather low impact because of overwhelming medical, physiological and psychological impacts of terminal renal insufficiency and dialysis. However, questionnaires on new experiences of oral hygiene with alternative consumer goods (toothbrushes) and cosmetics (oral edible gel) documented the contrary. The patients liked the attention of a dental professional, were satisfied with the toothbrushing training and appreciated the additional home use of the gel whenever the xerostomia feeling was disturbing. Most important was the dentist’s or dental hygienist’s devotion, followed by the appreciation of the patients.
Author Contributions
L.C.E. was the study dentist and wrote the manuscript; T.L. contributed to the study protocol and prepared the table and figures; B.K. was the responsible nephrologist contributing to the study protocol; P.G. was the Principal Investigator and wrote the manuscript; K.W.W. passed away in February 2022. All authors gave their consent to the final manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approval by Ethics Committee of University of Witten/Herdecke was given 19 September 2019, registration No. 103/2019.
Informed Consent Statement
All 44 patients gave written informed consent to participate. They also gave their consent for anonymous publication.
Data Availability Statement
The datasets generated and analyzed during the study are available at www.ormed.net (accessed on 5 March 2023). Further test materials are available from the corresponding author on reasonable request.
Acknowledgments
Part of this work was presented as an interactive talk presentation in the 99th General Session of the International Association for Dental Research, 21–24 July 2021; Journal of Dental Research Vol. 100, Spec. Issue B, Abstract 1691. We are thankful to Rainer Blaesius for critically reading the manuscript. Karl W. Weich passed away in February 2022; he was an outstanding statistician and for many years, a creative member of the ORMED team.
Conflicts of Interest
The authors declare no conflict of interest directly applicable to this research.
Abbreviations
| DHI | denture hygiene index |
| GPM/T | index assessing the number of gingivitis affected teeth (G), periodontitis affected teeth (P) and missing teeth (M) |
| HbA1c | glycated hemoglobin |
| MOA | mechanism of action |
| OHIP-G 14 | Oral Health Impact Profile, German version, 7 Items with 14 Questions |
| SLI | Silness–Löe dental plaque index |
References
- Ying Joanna, N.D.; Thomson, M.W. Dry mouth—An overview. Singap. Dent. J. 2015, 36, 12–17. [Google Scholar] [CrossRef]
- Gaengler, P.; Arnold, W.H.; Steinberg, D. Ätiologie und Pathogenese. In Konservierende Zahnheilkunde und Parodontologie; Gängler, P., Hoffmann, T., Willershausen, B., Schwenzer, N., Ehrenfeld, M., Eds.; Georg Thieme Verlag Stuttgart: New York, NY, USA, 2010; pp. 112–138. [Google Scholar]
- Neuhaus, K.W.; Strömberg, S. Zahn- und Mundpflege bei Mundtrockenheit. In Speichel; Filippi, A., Waltimo, T., Eds.; Quintessenz Verlag: Berlin, Germany, 2020; pp. 259–269. [Google Scholar]
- Billings, R.J.; Proskin, H.M.; Moss, M.E. Xerostomia and associated factors in a community-dwelling adult population. Commun. Dent. Oral Epidemiol. 1996, 24, 312–316. [Google Scholar] [CrossRef]
- Nederfors, T.; Isaksson, R.; Mörnstad, H.; Dahlöf, C. Prevalence of perceived symptoms od dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Commun. Dent. Oral Epidemiol. 1997, 25, 211–216. [Google Scholar] [CrossRef]
- Yeh, C.K.; Johnson, D.A.; Dodds, M.W.J. Impact of aging on human salivary gland function: A community-based study. Aging Clin. Exp. Res. 1998, 10, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef]
- Diep, M.T.; Jensen, J.L.; Skudutyte-Rysstad, R.; Young, A.; Sødal, A.T.T.; Petrovski, B.É.; Hove, L.H. Xerostomia and hyposalivation among a 65-yr-old population living in Oslo, Norway. Eur. J. Oral Sci. 2021, 129, e12757. [Google Scholar] [CrossRef] [PubMed]
- Jover Cerveró, A.; Bagán, J.V.; Soriano, Y.J.; Roda, R.P. Dental management in renal failure: Patients on dialysis. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, 419–426. [Google Scholar]
- Kaushik, A.; Reddy, S.; Umesh, L.; Devi, B.K.Y.; Santana, N.; Rakesh, N. Oral and salivary changes among renal patients undergoing hemodialysis: A cross-sectional study. Indian J. Nephrol. 2013, 23, 125–129. [Google Scholar] [CrossRef]
- Swapna, L.A.; Reddy, R.S.; Ramesh, T.; Reddy, R.L.; Vijayalaxmi, N.; Karmakar, P.; Pradeep, K. Oral health status in haemodialysis patients. J. Clin. Diagnos. Res. 2013, 7, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, J. Auswirkungen Professioneller Pflege auf die Mundgesundheit und Lebensqualität von Bewohnern eines Städtischen Pflegeheims. Ph.D. Dissertation, University of Witten/Herdecke, Witten, Germany, 2020. [Google Scholar]
- Silness, J.; Löe, H. Periodontal disease in Pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Gaengler, P. Prevalence and distribution of gingivitis, periodontitis and missing teeth in adolescents and adults according to GPM/T index. Commun. Dent. Oral Epidemiol. 1984, 12, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.; Palmer, C. Studies on dental caries. Public Health Rep. 1938, 53, 1353–1364. [Google Scholar] [CrossRef]
- Wefers, K.P. Der “Denture Hygiene Index” (DHI). Dent. Forum 1999, 1, 13–15. [Google Scholar]
- John, M.T.; Patrick, D.L.; Slade, G.D. The German version of the Oral Health Impact Profile—Translation and psychometric properties. Eur. J. Oral. Sci. 2002, 110, 425–433. [Google Scholar] [CrossRef]
- Ariyamuthu, V.K.; Nolph, K.D.; Ringdahl, B.E. Periodontal disease in chronic kidney disease and end-stage renal disease patients: A review. Cardiorenal Med. 2013, 3, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, G.; Kurtulus, I.; Duraduryan, A.; Cintan, S.; Kazancıoğlu, R.T.; Yildiz, A.; Bural, C.; Bozfakioglu, S.; Besler, M.; Trablus, S.; et al. Dental and periodontal findings in hemodialysis patients. Oral Dis. 2007, 13, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Bhatsange, A.; Patil, S.R. Assessment of periotontal health status in patients undergoing renal dialysis: A descriptive, cross-sectional study. J. Indian Soc. Periodontol. 2012, 16, 37–42. [Google Scholar] [CrossRef]
- Rodrigues, R.P.C.B.; Vidigal, M.T.C.; Vieira, W.A.; Nascimento, G.G.; Sabino-Silva, R.; Blumenberg, C.; Siqueira, M.F.; Siqueira, W.L.; Paranhos, L.R. Salivary changes in chronic kidney disease and in patients undergoing hemodialysis: A systematic review and meta-analysis. J. Nephrol. 2022, 35, 1339–1367. [Google Scholar] [CrossRef]
- Cunha, F.L.; Tagliaferro, E.P.S.; Pereira, A.C.; Meneghim, M.C.; Hebling, E. Oral health of a Brazilian population on renal dialysis. Spec. Care Dent. 2007, 27, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Klassen, J.T.; Krasko, B.M. The dental health status of dialysis patients. J. Canad. Dent. Assoc. 2002, 68, 34–38. [Google Scholar]
- Altamimi, A.; AlBakr, S.; Alanazi, T.; Alshahrani, F.; Chalisserry, E.; Anil, S. Prevalence of periodontitis in patients undergoing hemodialysis: A case control study. Mater. Sociomed. 2018, 30, 58–61. [Google Scholar] [CrossRef]
- Bayraktar, G.; Kurtulus, I.; Kazancıoğlu, R.T.; Bayramgurler, I.; Cintan, S.; Bural, C.; Bozfakıoğlu, S.; Besler, M.; Trablus, S.; Issever, H.; et al. Evaluation of periodontal parameters in patients undergoing peritoneal dialysis or hemodialysis. Oral Dis. 2008, 14, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Atassi, F. Oral home care and the reasons for seeking dental care by individuals on renal dialysis. J. Contemp. Dent. Pract. 2002, 3, 31–41. [Google Scholar] [CrossRef]
- Kho, H.-S.; Lee, S.-W.; Chung, S.-C.; Kim, Y.-K. Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. Oral Surg. Oral Med. Oral Radiol. Endodont. 1999, 88, 316–319. [Google Scholar] [CrossRef]
- Almeida, S.; Figueredo, C.M.; Lemos, C.; Bregman, R.; Fischer, R.G. Periodontal treatment in patients with chronic kidney disease: A pilot study. J. Periodont. Res. 2017, 52, 262–267. [Google Scholar] [CrossRef]
- de Souza, C.M.; Braosi, A.P.; Luczyszyn, S.M.; Olandoski, M.; Kotanko, P.; Craig, R.G.; Trevilatto, P.C.; Pecoits-Filho, R. Association among oral health parameters, periodontitis, and its treatment and mortality in patients undergoing hemodialysis. J. Periodontol. 2014, 85, 169–178. [Google Scholar] [CrossRef]
- Palmer, S.C.; Ruospo, M.; Wong, G.; Craig, J.; Petruzzi, M.; De Benedittis, M.; Ford, P.; Johnson, D.W.; Tonelli, M.; Natale, P.; et al. Dental health and mortality in people with end-stage kidney disease treated with hemodialysis: A multinational cohort study. Am. J. Kidney Dis. 2015, 66, 666–676. [Google Scholar] [CrossRef]
- Veisa, G.; Tasmoc, A.; Nistor, I.; Segall, L.; Siriopol, D.; Solomon, S.M.; Donciu, M.D.; Voroneanu, L.; Nastasa, A.; Covic, A. The impact of periodontal disease on physical and psychological domains in long-term hemodialysis patients: A cross-sectional study. Intern. Urol. Nephrol. 2017, 49, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Veisa, G.; Tasmoc, A.; Nistor, I.; Segall, L.; Siriopol, D.; Solomon, S.M.; Donciu, M.D.; Voroneanu, L.; Nastasa, A.; Covic, A. Lack of correlation between periodontitis and renal dysfunction in systemically healthy patients. Eur. J. Dent. 2011, 5, 8–18. [Google Scholar]
- Xie, T.; Yang, Z.; Dai, G.; Yan, K.; Tian, Y.; Zhao, D.; Zou, H.; Deng, F.; Chen, X.; Yuan, Q. Evaluation of the oral health status in Chinese hemodialysis patients. Hemodial. Intern. 2014, 18, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ziebolz, D.; Fischer, P.; Hornecker, E.; Mausberg, R.F. Oral health of hemodialysis patients: A cross-sectional study at two German dialysis centers. Hemodial. Int. 2012, 16, 69–75. [Google Scholar] [CrossRef]
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing xerostomia and salivary gland hypofunction. J. Am. Dent. Assoc. 2014, 145, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Dalodom, S.; Lam-Ubol, A.; Jeanmaneechotechai, S.; Takamfoo, L.; Intachai, W.; Duangchada, K.; Hongsachum, B.; Kanjanatiwat, P.; Vacharotayangul, P.; Trachootham, D. Influence of oral moisturizing jelly as a saliva substitute for the relief of xerostomia in elderly patients with hypertension and diabetes mellitus. Geriatr. Nurs. 2016, 37, 101–109. [Google Scholar] [CrossRef]
- Yu, I.-C.; Tsai, Y.-F.; Fang, J.-T.; Yeh, M.-M.; Fang, J.-Y.; Liu, C.-Y. Effects of mouthwash interventions on xerostomia and unstimulated whole saliva flow rate among hemodialysis patients: A randomized controlled study. Int. J. Nurs. Stud. 2016, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bin Mohsin, A.H.; Reddy, S.V.; Kumar, M.P.; Samee, S. Aloe vera for dry mouth denture patients—Palliative therapy. J. Clin. Diagnos. Res. 2017, 11, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Shirayama, Y.; Imoto, I.; Hinode, D.; Yanagisawa, S.; Takeuchi, Y. Current status of collaborative relationships between dialysis facilities and dental facilities in Japan: Results of a nationwide survey. BMC Nephrol. 2015, 16, 17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).