Cardiorenal Crosstalk in Patients with Heart Failure
Abstract
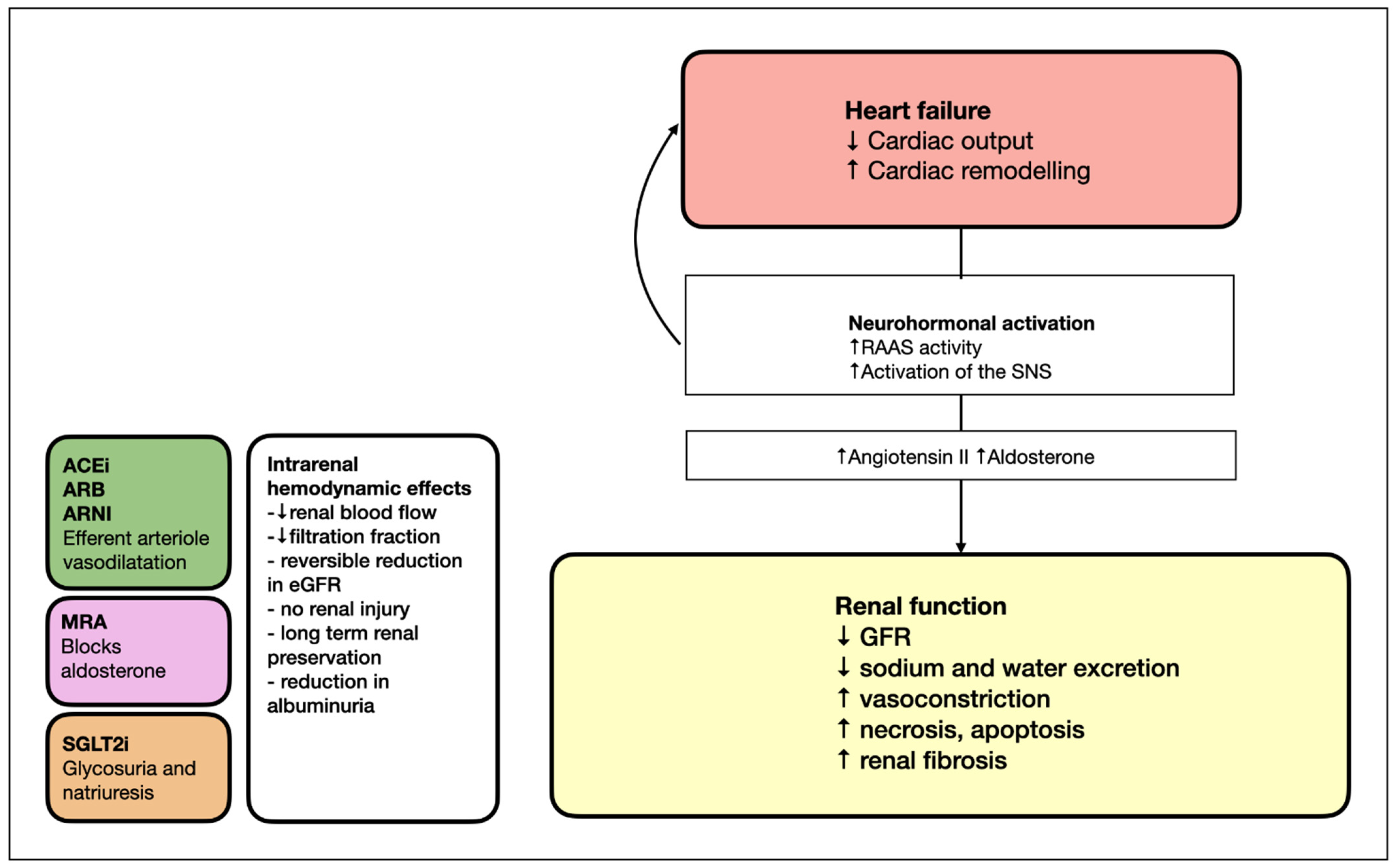
:1. Introduction
2. Chronic Kidney Disease in Heart Failure—The Real-World Data
3. Definition of Worsening Kidney Function
4. Antagonists of the Renin–Angiotensin–Aldosterone System (RAAS)
4.1. Angiotensin Receptor Blockers (ARBs)
4.1.1. ARB in HFrEF with Kidney Dysfunction
4.1.2. ARB in HFpEF with Kidney Dysfunction
4.2. Angiotensin-Converting Enzyme Inhibitors (ACEIs)
4.2.1. ACEI in HFrEF with Kidney Dysfunction
4.2.2. ACEI in HFpEF with Kidney Dysfunction
4.2.3. ACEI in Diabetic Kidney Disease
4.3. Angiotensin Receptor–Neprilysin Inhibitor (ARNI)
4.3.1. ARNI in HFrEF with Kidney Dysfunction
4.3.2. ARNI in HFpEF with Kidney Dysfunction
4.3.3. ARNI in Diabetic Kidney Dysfunction
4.4. Mineralocorticoid Receptor Antagonist (MRA)
4.4.1. MRA in HFrEF with Kidney Dysfunction
4.4.2. MRA in HFpEF with Kidney Dysfunction
4.4.3. MRA in Diabetic Kidney Dysfunction
5. Overall Consideration of RAAS Antagonists
6. Beta-Blockers in HFrEF with Kidney Dysfunction
7. Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2)
7.1. SGLT2 Inhibition in HFrEF Patients with Kidney Dysfunction
7.2. SGLT2 Inhibition in HFpEF Patients with Kidney Dysfunction
7.3. SGLT2 Inhibition in Patients with Diabetic Kidney Dysfunction
8. Non-Pharmacological Treatment Options
8.1. Ultrafiltration
8.2. MitraClip Placement in HFrEF Patients with Kidney Dysfunction
8.3. Tricuspid Clip
9. Long-Term Mechanical Circulatory Systems (LT-MCSs)
10. Orthotopic Heart Transplantation
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- de Silva, R.; Nikitin, N.P.; Witte, K.K.; Rigby, A.S.; Goode, K.; Bhandari, S.; Clark, A.L.; Cleland, J.G. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: Contributing factors and relationship to prognosis. Eur. Heart J. 2006, 27, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, C.A.; Seidu, S.; Zaccardi, F.; McCann, G.; Kadam, U.T.; Davies, M.J.; Lam, C.S.; Heerspink, H.L.; Khunti, K. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine 2021, 32, 100739. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Kimmel, S.E.; Dries, D.L.; Coca, S.G. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ. Heart Fail. 2011, 4, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesogor, A.; Cohn, J.N.; Latini, R.; Tognoni, G.; Krum, H.; Massie, B.; Zalewski, A.; Kandra, A.; Hua, T.A.; Gimpelewicz, C. Interaction between baseline and early worsening of renal function and efficacy of renin-angiotensin-aldosterone system blockade in patients with heart failure: Insights from the Val-HeFT study. Eur. J. Heart Fail. 2013, 15, 1236–1244. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Clark, H.; Krum, H.; Hopper, I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur. J. Heart Fail. 2014, 16, 41–48. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Streng, K.W.; Ter Maaten, J.M.; Voors, A.A.; van der Meer, P.; Rossignol, P.; McMurray, J.J.; Damman, K. Renin-Angiotensin System Inhibition, Worsening Renal Function, and Outcome in Heart Failure Patients With Reduced and Preserved Ejection Fraction: A Meta-Analysis of Published Study Data. Circ. Heart Fail. 2017, 10, e003588. [Google Scholar] [CrossRef]
- Hullin, R.; Meyer, P.; Yerly, P.; Kirsch, M. Cardiac Surgery in Advanced Heart Failure. J. Clin. Med. 2022, 11, 773. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Urso, R.; Laroche, C.; Damman, K.; Dahlström, U.; Tavazzi, L.; Maggioni, A.P.; Voors, A.A. Co-morbidities in patients with heart failure: An analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014, 16, 103–111. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Damman, K.; van der Meer, P.; Wijkstra, P.J.; Luijckx, G.J.; van Beek, A.; van Veldhuisen, D.J.; Voors, A.A. Co-morbidities in heart failure. Heart Fail. Rev. 2014, 19, 163–172. [Google Scholar] [CrossRef]
- Liu, P.P. Cardiorenal syndrome in heart failure: A cardiologist’s perspective. Can. J. Cardiol. 2008, 24 (Suppl. B), 25b–29b. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Fonarow, G.C.; DeVore, A.D.; Sharma, P.P.; Vaduganathan, M.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; McCague, K.; Patterson, J.H.; et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 2365–2383. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; Ter Maaten, J.M.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef]
- Patel, R.B.; Fonarow, G.C.; Greene, S.J.; Zhang, S.; Alhanti, B.; DeVore, A.D.; Butler, J.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Kidney Function and Outcomes in Patients Hospitalized With Heart Failure. J. Am. Coll. Cardiol. 2021, 78, 330–343. [Google Scholar] [CrossRef]
- Wirtz, H.S.; Sheer, R.; Honarpour, N.; Casebeer, A.W.; Simmons, J.D.; Kurtz, C.E.; Pasquale, M.K.; Globe, G. Real-World Analysis of Guideline-Based Therapy After Hospitalization for Heart Failure. J. Am. Heart Assoc. 2020, 9, e015042. [Google Scholar] [CrossRef]
- Krumholz, H.M.; Chen, Y.T.; Vaccarino, V.; Wang, Y.; Radford, M.J.; Bradford, W.D.; Horwitz, R.I. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am. J. Cardiol. 2000, 85, 1110–1113. [Google Scholar] [CrossRef]
- Jose, P.; Skali, H.; Anavekar, N.; Tomson, C.; Krumholz, H.M.; Rouleau, J.L.; Moye, L.; Pfeffer, M.A.; Solomon, S.D. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J. Am. Soc. Nephrol. 2006, 17, 2886–2891. [Google Scholar] [CrossRef]
- Cohn, J.N.; Tognoni, G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef]
- Granger, C.B.; McMurray, J.J.; Yusuf, S.; Held, P.; Michelson, E.L.; Olofsson, B.; Ostergren, J.; Pfeffer, M.A.; Swedberg, K. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet 2003, 362, 772–776. [Google Scholar] [CrossRef]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; Michelson, E.L.; Olofsson, B.; Ostergren, J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damman, K.; Perez, A.C.; Anand, I.S.; Komajda, M.; McKelvie, R.S.; Zile, M.R.; Massie, B.; Carson, P.E.; McMurray, J.J. Worsening renal function and outcome in heart failure patients with preserved ejection fraction and the impact of angiotensin receptor blocker treatment. J. Am. Coll. Cardiol. 2014, 64, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Dei Cas, A.; Khan, S.S.; Butler, J.; Mentz, R.J.; Bonow, R.O.; Avogaro, A.; Tschoepe, D.; Doehner, W.; Greene, S.J.; Senni, M.; et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015, 3, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Ma, I.; Thompson, C.R.; Humphries, K.; Salem, D.N.; Sarnak, M.J.; Levin, A. Kidney function and mortality among patients with left ventricular systolic dysfunction. J. Am. Soc. Nephrol. 2006, 17, 244–253. [Google Scholar] [CrossRef] [Green Version]
- CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 1987, 316, 1429–1435. [Google Scholar] [CrossRef]
- Ljungman, S.; Kjekshus, J.; Swedberg, K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial). Am. J. Cardiol. 1992, 70, 479–487. [Google Scholar] [CrossRef]
- Cleland, J.G.; Tendera, M.; Adamus, J.; Freemantle, N.; Polonski, L.; Taylor, J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J. 2006, 27, 2338–2345. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Packer, M.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Rouleau, J.L.; Shi, V.; et al. Angiotensin-neprilysin inhibition and renal outcomes across the spectrum of ejection fraction in heart failure. Eur. J. Heart Fail. 2022. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Gori, M.; Claggett, B.; Jhund, P.S.; Senni, M.; Lefkowitz, M.P.; Prescott, M.F.; Shi, V.C.; Rouleau, J.L.; Swedberg, K.; et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018, 6, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Mc Causland, F.R.; Lefkowitz, M.P.; Claggett, B.; Anavekar, N.S.; Senni, M.; Gori, M.; Jhund, P.S.; McGrath, M.M.; Packer, M.; Shi, V.; et al. Angiotensin-Neprilysin Inhibition and Renal Outcomes in Heart Failure With Preserved Ejection Fraction. Circulation 2020, 142, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Jering, K.S.; Zannad, F.; Claggett, B.; Mc Causland, F.R.; Ferreira, J.P.; Desai, A.; Barkoudah, E.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Cardiovascular and Renal Outcomes of Mineralocorticoid Receptor Antagonist Use in PARAGON-HF. JACC Heart Fail. 2021, 9, 13–24. [Google Scholar] [CrossRef]
- Seferovic, J.P.; Solomon, S.D.; Seely, E.W. Potential mechanisms of beneficial effect of sacubitril/valsartan on glycemic control. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820970444. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Vardeny, O.; Wu, D.H.; Desai, A.; Rossignol, P.; Zannad, F.; Pitt, B.; Solomon, S.D. Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: Insights from RALES (Randomized Aldactone Evaluation Study). J. Am. Coll. Cardiol. 2012, 60, 2082–2089. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.Y.; Ruospo, M.; Natale, P.; Bolignano, D.; Navaneethan, S.D.; Palmer, S.C.; Strippoli, G.F. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2020, 10, CD007004. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Anker, S.D.; Siddiqi, T.J.; Coats, A.J.S.; Dorigotti, F.; Filippatos, G.; Friede, T.; Göhring, U.M.; Kosiborod, M.N.; Lund, L.H.; et al. Patiromer for the management of hyperkalaemia in patients receiving renin-angiotensin-aldosterone system inhibitors for heart failure: Design and rationale of the DIAMOND trial. Eur. J. Heart Fail. 2022, 24, 230–238. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Myhre, P.L.; Bristow, M.; Claggett, B.; Damman, K.; Fang, J.C.; Fleg, J.L.; McKinlay, S.; Lewis, E.F.; OMeara, E.; et al. Spironolactone in Patients With Heart Failure, Preserved Ejection Fraction, and Worsening Renal Function. J. Am. Coll. Cardiol. 2021, 77, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Takashi, Y.; Muta, Y.; Oda, N.; Nagata, D.; Takahashi, H.; Tanabe, M. Mineralocorticoid Receptor Antagonists in Diabetic Kidney Disease. Front. Pharmacol. 2021, 12, 754239. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Uijl, A.; Savarese, G.; Vaartjes, I.; Dahlström, U.; Brugts, J.J.; Linssen, G.C.M.; van Empel, V.; Brunner-La Rocca, H.P.; Asselbergs, F.W.; Lund, L.H.; et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 973–982. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kotecha, D.; Gill, S.K.; Flather, M.D.; Holmes, J.; Packer, M.; Rosano, G.; Böhm, M.; McMurray, J.J.V.; Wikstrand, J.; Anker, S.D.; et al. Impact of Renal Impairment on Beta-Blocker Efficacy in Patients with Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2893–2904. [Google Scholar] [CrossRef]
- Ghali, J.K.; Wikstrand, J.; Van Veldhuisen, D.J.; Fagerberg, B.; Goldstein, S.; Hjalmarson, A.; Johansson, P.; Kjekshus, J.; Ohlsson, L.; Samuelsson, O.; et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: Insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF). J. Card. Fail. 2009, 15, 310–318. [Google Scholar] [CrossRef]
- Carrero, E.L.F.; Alicia, U.; Friedo, W.D.; Lars, H.L.; Gianluigi, S.; Juan, J. Association Between β-Blocker Use and Mortality/Morbidity in Patients With Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction and Advanced Chronic Kidney Disease. Circ. Heart Fail. 2020, 13, e007180. [Google Scholar] [CrossRef]
- Jhund, P.S.; Solomon, S.D.; Docherty, K.F.; Heerspink, H.J.L.; Anand, I.S.; Böhm, M.; Chopra, V.; de Boer, R.A.; Desai, A.S.; Ge, J.; et al. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction: Results of DAPA-HF. Circulation 2021, 143, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Zannad, F.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Brueckmann, M.; Zeller, C.; Hauske, S.; Anker, S.D. Influence of endpoint definitions on the effect of empagliflozin on major renal outcomes in the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2021, 23, 1798–1799. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Geisberg, C.; Howser, R.; Portner, P.M.; Rogers, J.G.; Deng, M.C.; Pierson, R.N., 3rd. Relationship between renal function and left ventricular assist device use. Ann. Thorac. Surg. 2006, 81, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [Green Version]
- Dekkers, C.C.J.; Wheeler, D.C.; Sjöström, C.D.; Stefansson, B.V.; Cain, V.; Heerspink, H.J.L. Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 2005–2011. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Guglin, M.E.; Saltzberg, M.T.; Jessup, M.L.; Bart, B.A.; Teerlink, J.R.; Jaski, B.E.; Fang, J.C.; Feller, E.D.; Haas, G.J.; et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol. 2007, 49, 675–683. [Google Scholar] [CrossRef]
- Kar, S.; Mack, M.J.; Lindenfeld, J.; Abraham, W.T.; Asch, F.M.; Weissman, N.J.; Enriquez-Sarano, M.; Lim, D.S.; Mishell, J.M.; Whisenant, B.K.; et al. Relationship Between Residual Mitral Regurgitation and Clinical and Quality-of-Life Outcomes After Transcatheter and Medical Treatments in Heart Failure: COAPT Trial. Circulation 2021, 144, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Sivak, J.A.; Phelan, M.; Schulte, P.J.; Patel, U.; Velazquez, E.J. Prevalence and Outcomes of Left-Sided Valvular Heart Disease Associated With Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e006044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Pellerin, D.; Gaze, D.C.; Mehta, R.L.; Gregson, H.; Streather, C.P.; Collinson, P.O.; Brecker, S.J. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis 2007, 191, 348–354. [Google Scholar] [CrossRef]
- Raheja, H.; Ahuja, K.R.; Nazir, S.; Saad, A.M.; Gad, M.M.; Chatterjee, S.; Abdelfattah, O.M.; Hassanein, M.; Harb, S.; Kapadia, S.R. Association of baseline kidney disease with outcomes of transcatheter mitral valve repair by MitraClip. Catheter. Cardiovasc. Interv. 2021, 97, E857–E867. [Google Scholar] [CrossRef]
- Shah, B.; Villablanca, P.A.; Vemulapalli, S.; Manandhar, P.; Amoroso, N.S.; Saric, M.; Staniloae, C.; Williams, M.R. Outcomes After Transcatheter Mitral Valve Repair in Patients With Renal Disease. Circ. Cardiovasc. Interv. 2019, 12, e007552. [Google Scholar] [CrossRef]
- Lurz, P.; Stephan von Bardeleben, R.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.N.; Nabauer, M.; Tang, G.H.L.; et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef]
- Adams, K.F., Jr.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.; Horton, D.P. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef]
- Nohria, A.; Hasselblad, V.; Stebbins, A.; Pauly, D.F.; Fonarow, G.C.; Shah, M.; Yancy, C.W.; Califf, R.M.; Stevenson, L.W.; Hill, J.A. Cardiorenal interactions: Insights from the ESCAPE trial. J. Am. Coll. Cardiol. 2008, 51, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443. [Google Scholar] [CrossRef]
- Miller, L.W.; Pagani, F.D.; Russell, S.D.; John, R.; Boyle, A.J.; Aaronson, K.D.; Conte, J.V.; Naka, Y.; Mancini, D.; Delgado, R.M.; et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 2007, 357, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., 3rd; Long, J.W.; et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, D.; Sakaguchi, T.; Saito, S.; Miyagawa, S.; Nishi, H.; Yoshikawa, Y.; Fukushima, S.; Saito, T.; Daimon, T.; Ueno, T.; et al. Predictor of early mortality for severe heart failure patients with left ventricular assist device implantation: Significance of INTERMACS level and renal function. Circ. J. 2012, 76, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Sandner, S.E.; Zimpfer, D.; Zrunek, P.; Rajek, A.; Schima, H.; Dunkler, D.; Grimm, M.; Wolner, E.; Wieselthaler, G.M. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann. Thorac. Surg. 2009, 87, 1072–1078. [Google Scholar] [CrossRef]
- Bansal, N.; Hailpern, S.M.; Katz, R.; Hall, Y.N.; Kurella Tamura, M.; Kreuter, W.; O’Hare, A.M. Outcomes Associated With Left Ventricular Assist Devices Among Recipients With and Without End-stage Renal Disease. JAMA Intern. Med. 2018, 178, 204–209. [Google Scholar] [CrossRef]
- Thomas, H.L.; Banner, N.R.; Murphy, C.L.; Steenkamp, R.; Birch, R.; Fogarty, D.G.; Bonser, A.R. Incidence, determinants, and outcome of chronic kidney disease after adult heart transplantation in the United Kingdom. Transplantation 2012, 93, 1151–1157. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Roest, S.; Hesselink, D.A.; Klimczak-Tomaniak, D.; Kardys, I.; Caliskan, K.; Brugts, J.J.; Maat, A.; Ciszek, M.; Constantinescu, A.A.; Manintveld, O.C. Incidence of end-stage renal disease after heart transplantation and effect of its treatment on survival. ESC Heart Fail. 2020, 7, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Habib, P.J.; Patel, P.C.; Hodge, D.; Chimato, N.; Yip, D.S.; Hosenpud, J.D.; Wadei, H.M. Pre-orthotopic heart transplant estimated glomerular filtration rate predicts post-transplant mortality and renal outcomes: An analysis of the UNOS database. J. Heart Lung Transplant. 2016, 35, 1471–1479. [Google Scholar] [CrossRef]
| Drug Class | Medical Therapy | Target Daily Dose in Heart Failure Clinical Trials | Drug Dose Recommendations in Advanced CKD |
|---|---|---|---|
| ACE inhibitors (ACEis) | Enalapril | 20 mg BD | Maximum dose of 5 mg D for enalapril |
| Lisinopril | 50 mg D | Maximum dose of 5 mg D for lisinopril | |
| Captopril | 75 mg BD | Maximum dose of 6.25 mg D for captopril | |
| Angiotensin II Receptor Blockers (ARBs) | Candesartan | 32 mg D | Candesartan untested in eGFR < 15 mL/min |
| Valsartan | 160 mg BD | Valsartan untested in eGFR < 10 mL/min | |
| Angiotensin Receptor–Neprilysin Inhibitor (ARNI) | Sacubritril/Valsartan | 200 mg BD | No adjustment recommended |
| Steroidal Mineralocorticoid receptor Antagonist (MRA) | Spironolactone | 50 mg D | Contraindicated with eGFR < 30 mL/min |
| Eplerenone | 50 mg D | Contraindicated with eGFR < 30 mL/min | |
| Nonsteroidal Mineralocorticoid Antagonist | Finerenon | 10 mg D | Not recommended with eGFR < 25 mL/min |
| Beta-blockers | Carvedilol | 50 mg BD | No adjustment recommended for carvedilol, cardvedilol, or bisoprolol |
| Bisoprolol | 10 mg D | ||
| Metoprolol | 200 mg D | ||
| Sodium–glucose cotransporter-2 inhibitor (SGLT2i) | Dapaglifozin | 10 mg D | No adjustment recommended |
| Empaglifozin | 10 mg D | Not recommended with eGFR < 20 mL/min (results of the EMPA-Kidney pending) | |
| Practice guidelines recommend up-titration of evidence-based medications at trial doses for all HF patients, as tolerated. Close monitoring of blood pressure, serum potassium and kidney function is recommended | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schukraft, S.; Hullin, R. Cardiorenal Crosstalk in Patients with Heart Failure. Kidney Dial. 2022, 2, 369-385. https://doi.org/10.3390/kidneydial2030033
Schukraft S, Hullin R. Cardiorenal Crosstalk in Patients with Heart Failure. Kidney and Dialysis. 2022; 2(3):369-385. https://doi.org/10.3390/kidneydial2030033
Chicago/Turabian StyleSchukraft, Sara, and Roger Hullin. 2022. "Cardiorenal Crosstalk in Patients with Heart Failure" Kidney and Dialysis 2, no. 3: 369-385. https://doi.org/10.3390/kidneydial2030033
APA StyleSchukraft, S., & Hullin, R. (2022). Cardiorenal Crosstalk in Patients with Heart Failure. Kidney and Dialysis, 2(3), 369-385. https://doi.org/10.3390/kidneydial2030033





