Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study
Abstract
1. Introduction
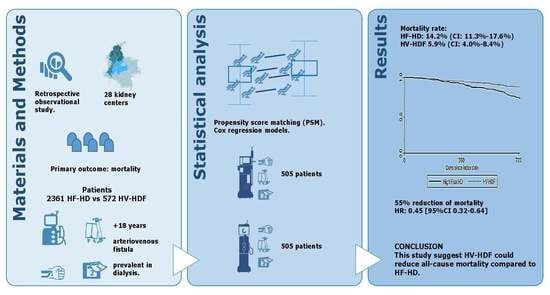
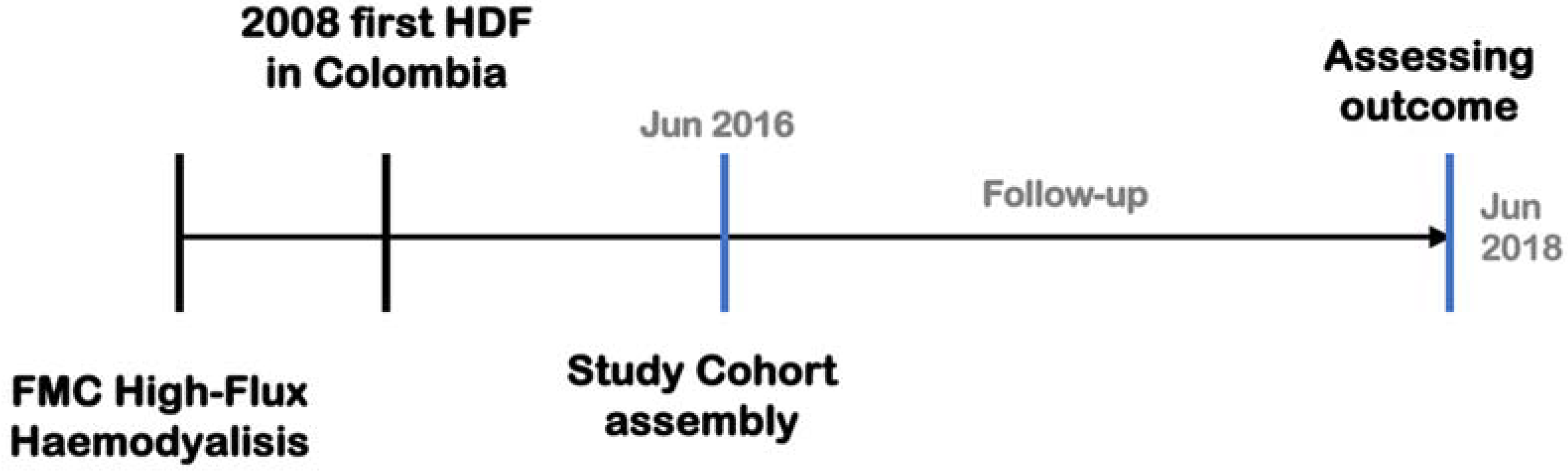
2. Materials and Methods
2.1. Patients
2.2. Dialysis Treatment
2.3. Data Collection
2.4. Statistical Analysis
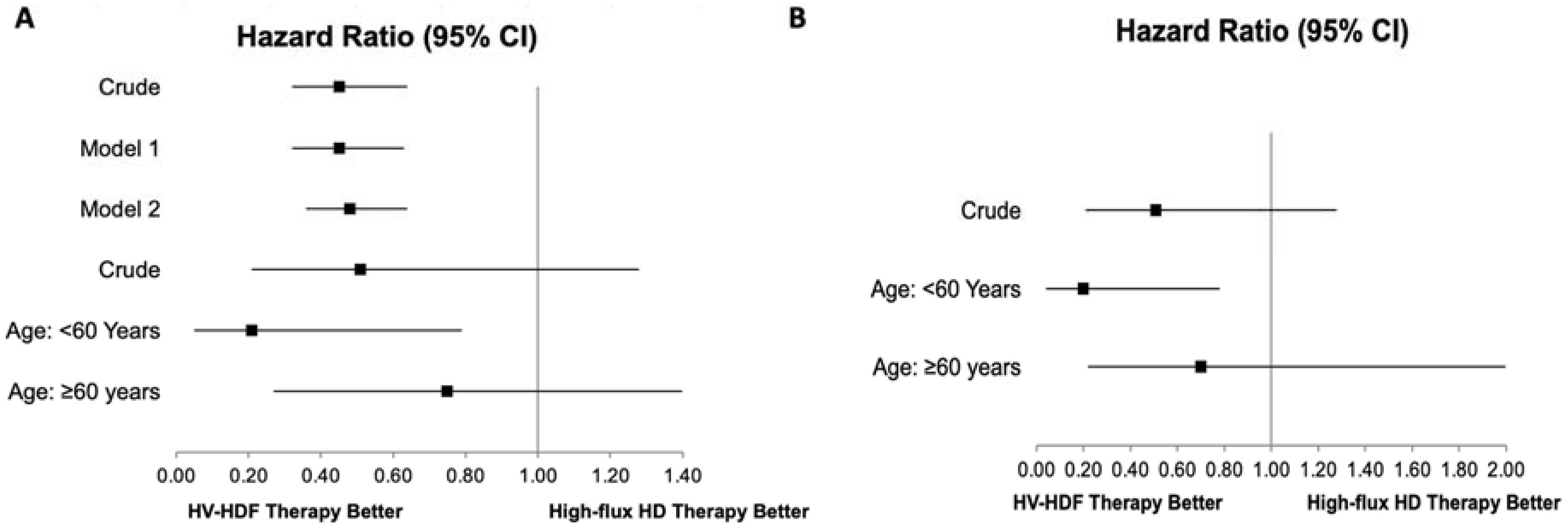
3. Results
Survival Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A.; Stevens, P.E.; Bilous, R.W.; Lamb, E.J.; Coresh, J. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3, 5–14. [Google Scholar]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Afentakis, N.; Ambühl, P.M.; Andrusev, A.M.; Fuster, E.A.; Arribas Monzón, F.E.; Åsberg, A.; et al. The European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: A Summary. Clin. Kidney J. 2018, 11, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, D.A.; Bragg-Gresham, J.L.; Koenig, K.G.; Wolfe, R.A.; Akiba, T.; Andreucci, V.E.; Saito, A.; Rayner, H.C.; Kurokawa, K.; Port, F.K.; et al. Association of Comorbid Conditions and Mortality in Hemodialysis Patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J. Am. Soc. Nephrol. 2003, 14, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, B.; Bolus, R.; Desai, A.A.; Zagar, P.; Parker, T.; Moran, J.; Solomon, M.D.; Khawar, O.; Gitlin, M.; Talley, J.; et al. Dialysis Practices That Distinguish Facilities with Below- versus above-Expected Mortality. Clin. J. Am. Soc. Nephrol. 2010, 5, 2024–2033. [Google Scholar] [CrossRef]
- Lornoy, W.; Becaus, I.; Billiouw, J.M.; Sierens, L.; Van Malderen, P.; D’Haenens, P. On-Line Haemodiafiltration. Remarkable Removal of Beta2-Microglobulin. Long-Term Clinical Observations. Nephrol. Dial. Transplant. 2000, 15 (Suppl. 1), 49–54. [Google Scholar] [CrossRef]
- Dessì, M.; Di Giovamberardino, G.; Pieri, M.; Noce, A.; Zenobi, R.; Di Daniele, N.; Pastore, A. Influence of Dialysis Techniques and Alternate Vitamin Supplementation on Homocysteine Levels in Patients with Known MTHFR Genotypes. Clin. Exp. Nephrol. 2015, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and Cardiovascular Events in Online Haemodiafiltration (OL-HDF) Compared with High-Flux Dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transplant. 2013, 28, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Jaussent, A.; Chalabi, L.; Leray-Moragues, H.; Chenine, L.; Debure, A.; Thibaudin, D.; Azzouz, L.; Patrier, L.; Maurice, F.; et al. Treatment Tolerance and Patient-Reported Outcomes Favor Online Hemodiafiltration Compared to High-Flux Hemodialysis in the Elderly. Kidney Int. 2017, 91, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Grooteman, M.P.C.; van den Dorpel, M.A.; Bots, M.L.; Penne, E.L.; van der Weerd, N.C.; Mazairac, A.H.A.; den Hoedt, C.H.; van der Tweel, I.; Lévesque, R.; Nubé, M.J.; et al. Effect of Online Hemodiafiltration on All-Cause Mortality and Cardiovascular Outcomes. J. Am. Soc. Nephrol. 2012, 23, 1087–1096. [Google Scholar] [CrossRef]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-Efficiency Postdilution Online Hemodiafiltration Reduces All-Cause Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.C.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and Mortality in End-Stage Kidney Disease Patients: A Pooled Individual Participant Data Analysis from Four Randomized Controlled Trials. Nephrol. Dial. Transplant. 2016, 31, 978–984. [Google Scholar] [CrossRef]
- Mercadal, L.; Franck, J.-E.; Metzger, M.; Urena Torres, P.; de Cornelissen, F.; Edet, S.; Béchade, C.; Vigneau, C.; Drüeke, T.; Jacquelinet, C.; et al. Hemodiafiltration Versus Hemodialysis and Survival in Patients with ESRD: The French Renal Epidemiology and Information Network (REIN) Registry. Am. J. Kidney Dis. 2016, 68, 247–255. [Google Scholar] [CrossRef]
- Canaud, B.; Köhler, K.; Sichart, J.-M.; Möller, S. Global Prevalent Use, Trends and Practices in Haemodiafiltration. Nephrol. Dial. Transplant. 2020, 35, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Gil, F.; Sanabria, M. Hemodialysis vs Peritoneal Dialysis: Comparison of Net Survival in Incident Patients on Chronic Dialysis in Colombia. Can. J. Kidney Health Dis. 2021, 8, 2054358120987055. [Google Scholar] [CrossRef]
- Pérez-García, R.; Palomares-Sancho, I.; Merello-Godino, J.I.; Aljama-García, P.; Bustamante-Bustamante, J.; Luño, J.; Maduell-Canals, F.; Martín-de Francisco, A.L.; Martín-Malo, A.; Mirapeix-i-Vicens, E.; et al. Epidemiological Study of 7316 Patients on Haemodialysis Treated in FME Clinics in Spain, Using Data from the EuCliD® Database: Results from Years 2009–2010. Nefrol. Publ. Of. Soc. Esp. Nefrol. 2012, 32, 743–753. [Google Scholar]
- Siriopol, D.; Canaud, B.; Stuard, S.; Mircescu, G.; Nistor, I.; Covic, A. New Insights into the Effect of Haemodiafiltration on Mortality: The Romanian Experience. Nephrol. Dial. Transplant. 2015, 30, 294–301. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y. Balance Diagnostics after Propensity Score Matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Fine, J.P. Practical Recommendations for Reporting Fine-Gray Model Analyses for Competing Risk Data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient Survival on Haemodiafiltration and Haemodialysis: A Cohort Study Using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol. Dial. Transplant. 2019, 34, 326–338. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution Online Hemodiafiltration Is Associated with Improved Survival Compared with Hemodialysis. Kidney Int. 2019, 95, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, I.M.; Blankestijn, P.J.; Bots, M.L.; Covic, A.; Davenport, A.; Grooteman, M.P.C.; Hegbrant, J.; Locatelli, F.; Vanholder, R.; Nubé, M.J. Clinical Evidence on Hemodiafiltration: A Systematic Review and a Meta-Analysis. Semin. Dial. 2014, 27, 119–127. [Google Scholar] [CrossRef]
- Locatelli, F.; Karaboyas, A.; Pisoni, R.L.; Robinson, B.M.; Fort, J.; Vanholder, R.; Rayner, H.C.; Kleophas, W.; Jacobson, S.H.; Combe, C.; et al. Mortality Risk in Patients on Hemodiafiltration versus Hemodialysis: A “real-World” Comparison from the DOPPS. Nephrol. Dial. Transplant. 2018, 33, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nistor, I.; Palmer, S.C.; Craig, J.C.; Saglimbene, V.; Vecchio, M.; Covic, A.; Strippoli, G.F.M. Convective versus Diffusive Dialysis Therapies for Chronic Kidney Failure: An Updated Systematic Review of Randomized Controlled Trials. Am. J. Kidney Dis. 2014, 63, 954–967. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, M.; Llorca, J. Bias. J. Epidemiol. Community Health 2004, 58, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Páll, A.; Czifra, Á.; Sebestyén, V.; Becs, G.; Kun, C.; Balla, J.; Paragh, G.; Lőrincz, I.; Páll, D.; Padra, T.J.; et al. Hemodiafiltration and Hemodialysis Differently Affect P Wave Duration and Dispersion on the Surface Electrocardiogram. Int. Urol. Nephrol. 2016, 48, 271–277. [Google Scholar] [CrossRef]
- Ramponi, F.; Ronco, C.; Mason, G.; Rettore, E.; Marcelli, D.; Martino, F.; Neri, M.; Martin-Malo, A.; Canaud, B.; Locatelli, F. Cost-Effectiveness Analysis of Online Hemodiafiltration versus High-Flux Hemodialysis. Clin. Outcomes Res. 2016, 8, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Schiffl, H. Online Hemodiafiltration and Mortality Risk in End-Stage Renal Disease Patients: A Critical Appraisal of Current Evidence. Kidney Res. Clin. Pract. 2019, 38, 159–168. [Google Scholar] [CrossRef]
- Montagud-Marrahi, E.; Broseta, J.; Rodriguez-Espinosa, D.; Lidia, R.; Hermida-Lama, E.; Xipell, M.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; Bedini, J.L.; et al. Optimization of Dialysate Bicarbonate in Patients Treated with Online Haemodiafiltration. Clin. Kidney J. 2021, 14, 1004–1013. [Google Scholar] [CrossRef]
- de Rooij, E.N.M.; Dekker, F.W.; le Cessie, S.; Hoorn, E.J.; de Fijter, J.W.; Hoogeveen, E.K.; Bijlsma, J.A.; Boekhout, M.; Boer, W.H.; van der Boog, P.J.M.; et al. Serum Potassium and Mortality Risk in Hemodialysis Patients: A Cohort Study. Kidney Med. 2022, 4, 100379. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Chou, C.-Y.; Hsiao, Y.-T.; Liang, C.-C.; Kuo, H.-L.; Chang, C.-T.; Liu, J.-H.; Wang, I.-K.; Huang, C.-C. Patients with Diabetes as the Primary Kidney Disease Have a Worse Survival than Patients with Comorbid Diabetes in Chronic Haemodialysis Patients. Nephrology 2015, 20, 155–160. [Google Scholar] [CrossRef] [PubMed]
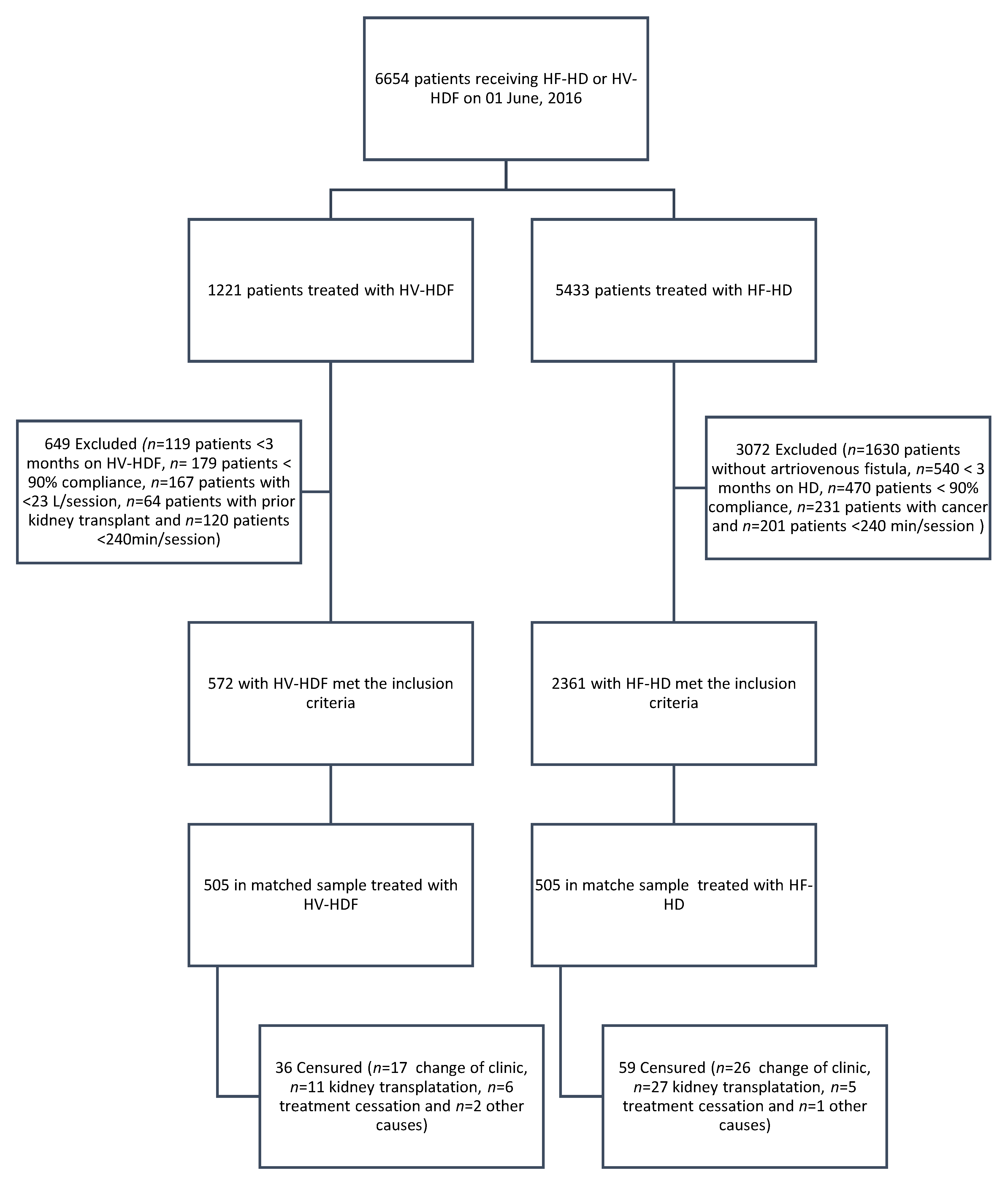

| Covariates | HF-HD (n = 2361) | HV-HDF (n = 572) | d a | p Value | HF-HD (n = 505) | HV-HDF (n = 505) | d a | p Value |
|---|---|---|---|---|---|---|---|---|
| Age, yr | ||||||||
| Median (IQR) | 58.0 (47.0–68.0) | 61.0 (50.0–70.0) | 0.112 | 0.002 | 60.0 (51.0–69.5) | 61.0 (50.0–69.0) | 0.023 | 0.897 |
| Sex, n (%) | ||||||||
| Female | 790 (33.5) | 188 (32.9) | 0.013 | 0.787 | 155 (30.7) | 156 (30.9) | 0.004 | 0.946 |
| Country Region | - | 0 | - | 0 | ||||
| Central | 733 (31.0) | 208 (36.4) | 134 (26.5) | 196 (38.8) | ||||
| North | 954 (40.4) | 74 (12.9) | 182 (36.0) | 74 (14.6) | ||||
| Sourth-west | 674 (28.6) | 290 (50.7) | 189 (37.4) | 235 (46.5) | ||||
| Comorbidities, n (%) | ||||||||
| HT | 1633 (30.8) | 352 (61.5) | 0.161 | 0.001 | 343 (67.9) | 327 (64.7) | 0.067 | 0.287 |
| DM | 884 (37.4) | 202 (35.3) | 0.044 | 0.345 | 183 (36.2) | 178 (35.2) | 0.021 | 0.743 |
| CVD | 386 (16.3) | 63 (11.0) | 0.156 | 0.001 | 59 (11.7) | 55 (10.9) | 0.025 | 0.766 |
| SBP, mmHg | ||||||||
| Median (IQR) | 149.0 (135.0–163.0) | 151.0 (136.0–163.0) | 0.008 | 0.357 | 149.0 (135.0–162.0) | 150.0 (136.0–162.0) | 0.019 | 0.546 |
| Dialysis Time, months | ||||||||
| Median (IQR) | 52.7 (25.0–90.2) | 61.2 (34.6–97.5) | 0.139 | 0.001 | 60.7 (32.3–103.9) | 61.9 (34.7–97.6) | 0.034 | 0.884 |
| Ted, min | ||||||||
| Median (IQR) | 240.0 (240.0–241.0) | 240.0 (240.0–242.5) | 0.222 | 0 | 240.0 (240.0–243.2) | 240 (240.0–242.0) | 0.041 | 0.02 |
| QB, mL/mto. | ||||||||
| Median (IQR) | 410.0 (383.0–427.0) | 426.0 (409.0–449.0) | 0.72 | 0 | 426.0 (408.0–441.0) | 423.0 (408.0–447.0) | 0.025 | 0.974 |
| Hb, g/dL | ||||||||
| Mean ± SD | 11.6 ± 1.6 | 11.5 ± 1.4 | 0.043 | 0.347 | 11.5 ± 1.5 | 11.6 ± 1.4 | 0.065 | 0.344 |
| Albumin, g/dL | ||||||||
| Median (IQR) | 4.1 (3.9–4.4) | 4.1 (3.9–4.3) | 0.06 | 0.049 | 4.1 (3.9–4.3) | 4.1 (3.9–4.3) | 0.018 | 0.768 |
| Posphorus, mg/dL | ||||||||
| Median (IQR) | 4.1 (3.3–5.0) | 3.9 (3.2–4.8) | 0.115 | 0.007 | 3.9 (3.2–4.9) | 3.9 (3.2–4.8) | 0.027 | 0.963 |
| eKt/v | ||||||||
| Median (IQR) | 1.7 (1.5–1.9) | 1.9 (1.7–2.2) | 0.728 | 0 | 1.9 (1.6–2.1) | 1.8 (1.7–2.1) | 0.025 | 0.992 |
| iPTH, ng/L | ||||||||
| Median (IQR) | 265.7 (134.3–484.3) | 298.0 (141.8–547.3) | 0.079 | 0.015 | 275.7 (144.4–538.9) | 306.6 (151.5–580.5) | 0.044 | 0.298 |
| Covariables | Univariate | Multivariate | p Value for Interaction with Treatments | ||
|---|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | ||
| HV-HDF, Ref: High-flux HD | 0.45 [0.32–0.64] | 0.000 | 0.47 [0.39–0.57] | <0.001 | - |
| Age, years | 1.03 [1.01–1.05] | 0.001 | 1.03 [1.01–1.05] | 0.012 | 0.000 |
| Sex, Ref: female | 1.52 [1.03–2.24] | 0.034 | 1.41 [0.93–2.14] | 0.101 | 0.908 |
| Country Region Central North South-west | 1.19 [0.72–1.99] Ref. 0.90 [0.55–1.47] | 0.488 - 0.683 | - | - | - |
| SBP | 1.01 [1.00–1.02] | 0.030 | 1.01 [1.00–1.02] | 0.002 | 0.655 |
| HT | 0.84 [0.49–1.43] | 0.519 | - | - | - |
| DM | 1.78 [1.48–2.12] | 0.000 | - | - | - |
| CVD | 0.85 [0.57–1.26] | 0.419 | - | - | - |
| Dialysis Time, months | 0.99 [0.99–1.00] | 0.157 | - | - | - |
| Ted, min | 0.94 [0.87–1.02] | 0.134 | - | - | - |
| QB, mL/mto. | 0.99 [0.98–0.99] | 0.018 | - | - | - |
| Hb, g/dL | 0.70 [0.65–0.77] | 0.000 | 0.76 [0.71–0.81] | 0.000 | 0.687 |
| Albumin, g/dL | 0.22 [0.15–0.31] | 0.000 | 0.31 [0.18–0.54] | 0.000 | 0.969 |
| Posphorus, mg/dL | 0.82 [0.76–0.88] | 0.000 | - | - | - |
| eKt/v | 1.62 [0.73–3.58] | 0.233 | - | - | - |
| iPTH, ng/L | 1.00 [0.99–1.00] | 0.222 | - | - | - |
| SHR [95% CI] | p Value | |
|---|---|---|
| HV-HDF, Ref: HF-HD | 0.44 [0.37 to 0.53] | 0.000 |
| Age, years | 1.03 [1.01 to 1.05] | 0.009 |
| Sex, Ref: female | 1.40 [0.88 to 2.21] | 0.154 |
| SBP | 1.01 [1.00 to 1.02] | 0.001 |
| Hb, g/dL | 0.77 [0.71 to 0.83] | 0.000 |
| Albumin, g/dL | 0.32 [0.18 to 0.57] | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valderrama, L.A.; Barrera, L.; Cantor, E.J.; Muñoz, J.; Arango, J.; Tobon, C.; Canaud, B. Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney Dial. 2022, 2, 209-220. https://doi.org/10.3390/kidneydial2020022
Valderrama LA, Barrera L, Cantor EJ, Muñoz J, Arango J, Tobon C, Canaud B. Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney and Dialysis. 2022; 2(2):209-220. https://doi.org/10.3390/kidneydial2020022
Chicago/Turabian StyleValderrama, Luis A., Lena Barrera, Erika J. Cantor, Jesús Muñoz, Javier Arango, Carlos Tobon, and Bernard Canaud. 2022. "Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study" Kidney and Dialysis 2, no. 2: 209-220. https://doi.org/10.3390/kidneydial2020022
APA StyleValderrama, L. A., Barrera, L., Cantor, E. J., Muñoz, J., Arango, J., Tobon, C., & Canaud, B. (2022). Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney and Dialysis, 2(2), 209-220. https://doi.org/10.3390/kidneydial2020022