Tumor Microenvironment: Current Understanding and Therapeutic Implications in Adult and Pediatric Cancers
Simple Summary
Abstract
1. Introduction
2. Key Components of TME
2.1. Cellular Components
2.1.1. Immune Cells
2.1.2. Cancer-Associated Fibroblasts (CAFs)
2.1.3. Vasculature and Angiogenesis
2.2. Extracellular Matrix (ECM) and ECM Remodeling
3. Pediatric Versus Adult: Differences in TME
4. Role of TME in Various Pediatric Malignancies
4.1. Acute Lymphoblastic Leukemia (All)
4.1.1. Role of Tumor Microenvironment
4.1.2. Potential Targets
4.2. Acute Myeloid Leukemia
4.2.1. Role of Tumor Microenvironment
4.2.2. Potential Targets
4.3. B Cell Lymphomas
4.3.1. Role of Tumor Microenvironment
4.3.2. Potential Targets
4.4. Hodgkin Lymphoma
4.4.1. Role of Tumor Microenvironment
4.4.2. Potential Targets
4.5. Neuroblastoma
4.5.1. Role of Tumor Microenvironment
4.5.2. Potential Targets
4.6. Pediatric Brain Tumors
4.6.1. Role of Tumor Microenvironment
4.6.2. Potential Targets
4.7. Wilms Tumor and Other Pediatric Renal Tumors
4.7.1. Role of Tumor Microenvironment
4.7.2. Potential Targets
4.8. Osteosarcoma
4.9. Other Sarcomas
4.9.1. Role of Tumor Microenvironment
4.9.2. Potential Targets
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, C.E.; Kuo, P.C. The tumor microenvironment. Surg. Oncol. 2012, 21, 172–177. [Google Scholar] [CrossRef]
- Pathania, A.S. Immune microenvironment in childhood cancers: Characteristics and therapeutic challenges. Cancers 2024, 16, 2201. [Google Scholar] [CrossRef]
- Belgiovine, C.; Mebelli, K.; Raffaele, A.; De Cicco, M.; Rotella, J.; Pedrazzoli, P.; Zecca, M.; Riccipetitoni, G.; Comoli, P. Pediatric Solid Cancers: Dissecting the Tumor Microenvironment to Improve the Results of Clinical Immunotherapy. Int. J. Mol. Sci. 2024, 25, 3225. [Google Scholar] [CrossRef]
- World Health Organization. Cancer in Children; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/fact-sheets/detail/cancer-in-children (accessed on 16 October 2025).
- Sultan, I.; Alfaar, A.S.; Sultan, Y.; Salman, Z.; Qaddoumi, I. Trends in childhood cancer: Incidence and survival analysis over 45 years of SEER data. PLoS ONE 2025, 20, e0314592. [Google Scholar] [CrossRef]
- Messiaen, J.; Jacobs, S.A.; De Smet, F. The tumor micro-environment in pediatric glioma: Friend or foe? Front. Immunol. 2023, 14, 1227126. [Google Scholar] [CrossRef]
- Elaskalani, O.; Abbas, Z.; Malinge, S.; Wouters, M.A.; Truong, J.; Johnson, I.M.; Kuster, J.; Nassar, A.; Wan, A.; Smolders, H.; et al. Age-dependent tumor-immune interactions underlie immunotherapy response in pediatric cancer. bioRxiv 2025. [Google Scholar] [CrossRef]
- Riaz, N.; Morris, L.; Havel, J.J.; Makarov, V.; Desrichard, A.; Chan, T.A. The role of neoantigens in response to immune checkpoint blockade. Int. Immunol. 2016, 28, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Bai, X.; Huang, X.; Wang, Q. Tumor microenvironment as a complex milieu driving cancer progression: A mini review. Clin. Transl. Oncol. 2025, 27, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qin, S.; Si, W.; Wang, A.; Xing, B.; Gao, R.; Ren, X.; Wang, L.; Wu, X.; Zhang, J.; et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 2021, 374, abe6474. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-infiltrating B cells: Immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 2022, 22, 414–430. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef] [PubMed]
- Syrimi, E.; Khan, N.; Murray, P.; Willcox, C.; Haigh, T.; Willcox, B.; Masand, N.; Bowen, C.; Dimakou, D.B.; Zuo, J.; et al. Defects in NK cell immunity of pediatric cancer patients revealed by deep immune profiling. iScience 2024, 27, 110837. [Google Scholar] [CrossRef]
- Flaadt, T.; Ladenstein, R.L.; Ebinger, M.; Lode, H.N.; Arnardóttir, H.B.; Poetschger, U.; Schwinger, W.; Meisel, R.; Schuster, F.R.; Döring, M.; et al. Anti-GD2 antibody dinutuximab beta and low-dose interleukin 2 after haploidentical stem-cell transplantation in patients with relapsed neuroblastoma: A multicenter, phase I/II trial. J. Clin. Oncol. 2023, 41, 3135–3148. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front. Immunol. 2023, 13, 1063711. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.-L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Brichkina, A.; Polo, P.; Sharma, S.D.; Visestamkul, N.; Lauth, M. A Quick Guide to CAF Subtypes in Pancreatic Cancer. Cancers 2023, 15, 2614. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 1–30. [Google Scholar] [CrossRef]
- Maia, A.; Schöllhorn, A.; Schuhmacher, J.; Gouttefangeas, C. CAF-immune cell crosstalk and its impact in immunotherapy. Semin. Immunopathol. 2023, 45, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef] [PubMed]
- Siminzar, P.; Tohidkia, M.R.; Eppard, E.; Vahidfar, N.; Tarighatnia, A.; Aghanejad, A. Recent trends in diagnostic biomarkers of tumor microenvironment. Mol. Imaging Biol. 2023, 25, 464–482. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in car-cainogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Cates, J.M.; Cole, H.A.; Slosky, D.A.; Haro, H.; Ando, T.; Schwartz, H.S.; Schoenecker, J.G. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol. Cancer Res. 2014, 12, 1100–1111. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Vlenterie, M.; van der Graaf, W.T.A. Recent advances on anti-angiogenic multi-receptor tyrosine kinase inhibitors in osteosarcoma and Ewing sarcoma. Front. Oncol. 2023, 13, 1013359. [Google Scholar] [CrossRef]
- Wang, Y.-A.; Li, X.-L.; Mo, Y.-Z.; Fan, C.-M.; Tang, L.; Xiong, F.; Guo, C.; Xiang, B.; Zhou, M.; Ma, J.; et al. Effects of tumor metabolic microen-vironment on regulatory T cells. Mol. Cancer 2018, 17, 168. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular matrix stiffness: Mechanisms in tumor progression and therapeutic potential in cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef]
- Sergi, C.M. Pediatric cancer—Pathology and microenvironment influence: A perspective into osteosarcoma and non-osteogenic mesenchymal malignant neoplasms. Discov. Oncol. 2024, 15, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ahn, K.J.; Biyik-Sit, R.; Chen, C.; Thadi, A.; Chen, C.-H.; Molina, W.; Lockhart, B.; Laetsch, T.; Surrey, L.; et al. Dissecting pediatric sarcoma microenvironment using single-cell and spatial multi-omics. Cancer Res. 2024, 84, B061. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Long, A.H.; Morgenstern, D.A.; Leruste, A.; Bourdeaut, F.; Davis, K.L. Checkpoint immunotherapy in pediatrics: Here, gone, and back again. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 781–794. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, M.; Kośmider, K.; Bednarz, K.; Ostapińska, K.; Lejman, M.; Zawitkowska, J. Recent advances in treatment options for childhood acute lymphoblastic leukemia. Cancers 2022, 14, 2021. [Google Scholar] [CrossRef]
- Tiwari, A.; Trivedi, R.; Lin, S.-Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Savino, A.M.; Izraeli, S. Interleukin-7 signaling as a therapeutic target in acute lymphoblastic leukemia. Expert Rev. Hematol. 2017, 10, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Watanabe, T.; Saito, Y.; Kuroki, Y.; Hijikata, A.; Takagi, M.; Tomizawa, D.; Eguchi, M.; Eguchi-Ishimae, M.; Kaneko, A.; et al. Identification of CD34+ and CD34− leukemia-initiating cells in MLL-rearranged human acute lymphoblastic leukemia. Blood 2015, 125, 967–980. [Google Scholar] [CrossRef]
- Peled, A.; Klein, S.; Beider, K.; Burger, J.A.; Abraham, M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malig-nancies. Cytokine 2018, 109, 11–16. [Google Scholar] [CrossRef]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The varied roles of Notch in cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 245–275. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Radtke, F. Notch as a tumour suppressor. Nat. Rev. Cancer 2017, 17, 145–159. [Google Scholar] [CrossRef]
- Vadillo, E.; Dorantes-Acosta, E.; Pelayo, R.; Schnoor, M. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018, 32, 36–51. [Google Scholar] [CrossRef]
- Passaro, D.; Di Tullio, A.; Abarrategi, A.; Rouault-Pierre, K.; Foster, K.; Ariza-McNaughton, L.; Montaner, B.; Chakravarty, P.; Bhaw, L.; Diana, G.; et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell 2017, 32, 324–341.e6. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, J.; Lu, T.; Ma, D.; Wei, D.; Guo, Y.; Cheng, B.; Wang, W.; Fang, Q. Overexpression of heme oxygenase-1 in microenvi-ronment mediates vincristine resistance of B-cell acute lymphoblastic leukemia by promoting vascular endothelial growth factor secretion. J. Cell. Biochem. 2019, 120, 17791–17810. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Manesh, D.M.; El-Hoss, J.; Evans, K.; Richmond, J.; Toscan, C.E.; Bracken, L.S.; Hedrick, A.; Sutton, R.; Marshall, G.M.; Wilson, W.R.; et al. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood 2015, 126, 1193–1202. [Google Scholar] [CrossRef]
- Bui, B.P.; Nguyen, P.L.; Lee, K.; Cho, J. Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1a, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- August, K.J.; Guest, E.M.; Lewing, K.; Hays, J.A.; Gamis, A.S. Treatment of children with relapsed and refractory acute lymphoblastic leukemia with mitoxantrone, vincristine, pegaspargase, dexamethasone, and bortezomib. Pediatr. Blood Cancer 2020, 67, e28062. [Google Scholar] [CrossRef]
- Mehrpouri, M.; Safaroghli-Azar, A.; Pourbagheri-Sigaroodi, A.; Momeny, M.; Bashash, D. Anti-leukemic effects of histone deacetylase (HDAC) inhibition in acute lymphoblastic leukemia (ALL) cells: Shedding light on mitigating effects of NF-kappaB and autophagy on panobinostat cytotoxicity. Eur. J. Pharmacol. 2020, 875, 173050. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Inukai, T.; Imamura, T.; Yano, M.; Tomoyasu, C.; Lucas, D.M.; Nemoto, A.; Sato, H.; Huang, M.; Abe, M.; et al. Anti-leukemic activity of bortezomib and carfilzomib on B-cell precursor ALL cell lines. PLoS ONE 2017, 12, e0188680. [Google Scholar] [CrossRef]
- Tseng, S.; Lee, M.-E.; Lin, P.-C. A review of childhood acute myeloid leukemia: Diagnosis and novel treatment. Pharmaceuticals 2023, 16, 1614. [Google Scholar] [CrossRef]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A Phase 3 Study of Gemtuzumab Ozogamicin during Induction and Postconsolidation Therapy in Younger Patients with Acute Myeloid Leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Hino, C.; Pham, B.; Park, D.; Yang, C.; Nguyen, M.H.; Kaur, S.; Reeves, M.E.; Xu, Y.; Nishino, K.; Pu, L.; et al. Targeting the tumor mi-croenvironment in acute myeloid leukemia: The future of immunotherapy and natural products. Biomedicines 2022, 10, 1410. [Google Scholar] [CrossRef]
- Liao, D.; Wang, M.; Liao, Y.; Li, J.; Niu, T. A review of efficacy and safety of checkpoint inhibitor for the treatment of acute myeloid leukemia. Front. Pharmacol. 2019, 10, 609. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Costello, C.; Herrera, A.F.; Locke, F.L.; Maegawa, R.O.; Savell, A.; Mazzeo, M.; Anderson, A.; Boardman, A.P.; et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood 2020, 135, 2182–2191. [Google Scholar] [CrossRef]
- Mussai, F.; De Santo, C.; Abu-Dayyeh, I.; Booth, S.; Quek, L.; McEwen-Smith, R.M.; Qureshi, A.; Dazzi, F.; Vyas, P.; Cerundolo, V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 2013, 122, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Sendker, S.; Reinhardt, D.; Niktoreh, N. Redirecting the immune microenvironment in acute myeloid leukemia. Cancers 2021, 13, 1423. [Google Scholar] [CrossRef] [PubMed]
- Stringaris, K.; Sekine, T.; Khoder, A.; Alsuliman, A.; Razzaghi, B.; Sargeant, R.; Pavlu, J.; Brisley, G.; de Lavallade, H.; Sarvaria, A.; et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014, 99, 836–847. [Google Scholar] [CrossRef]
- Aru, B.; Pehlivanoğlu, C.; Dal, Z.; Dereli-Çalışkan, N.N.; Gürlü, E.; Yanıkkaya-Demirel, G. A potential area of use for immune checkpoint inhibitors: Targeting bone marrow microenvironment in acute myeloid leukemia. Front. Immunol. 2023, 14, 1108200. [Google Scholar] [CrossRef]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113. [Google Scholar] [CrossRef]
- He, S.Z.; Busfield, S.; Ritchie, D.S.; Hertzberg, M.S.; Durrant, S.; Lewis, I.D.; Marlton, P.; McLachlan, A.J.; Kerridge, I.; Bradstock, K.F.; et al. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk. Lymphoma 2015, 56, 1406–1415. [Google Scholar] [CrossRef]
- Ma, H.; Padmanabhan, I.S.; Parmar, S.; Gong, Y. Targeting CLL-1 for acute myeloid leukemia therapy. J. Hematol. Oncol. 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, Y.; Yao, H.; Zhan, R.; Chen, S.; Miao, W.; Ma, S.; Xu, X.; Li, Y.; Yu, M.; et al. CAR-NK cells for acute myeloid leukemia immunotherapy: Past, present and future. Am. J. Cancer Res. 2023, 13, 5559–5576. [Google Scholar]
- Bachanova, V.; Sarhan, D.; DeFor, T.E.; Cooley, S.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Curtsinger, J.M.; Burns, L.; Weisdorf, D.J.; Miller, J.S. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol. Immunother. 2018, 67, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Interactions of IDO and the Kynurenine Pathway with Cell Transduction Systems and Metabolism at the Inflammation-Cancer Interface. Cancers 2023, 15, 2895. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Stravokefalou, V.; Stellas, D.; Karaliota, S.; Felber, B.K.; Pavlakis, G.N. Heterodimeric IL-15 in Cancer Immunotherapy. Cancers 2021, 13, 837. [Google Scholar] [CrossRef]
- Mosna, F. The Immunotherapy of Acute Myeloid Leukemia: A Clinical Point of View. Cancers 2024, 16, 2359. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.; Barth, M.; Armenian, S.; Audino, A.N.; Barnette, P.; Cuglievan, B.; Ding, H.; Ford, J.B.; Galardy, P.J.; Gardner, R.; et al. Pediatric Aggressive Mature B-Cell Lymphomas, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1105–1123. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Wang, X. Targeting the tumor microenvironment in B-cell lymphoma: Challenges and opportunities. J. Hematol. Oncol. 2021, 14, 125. [Google Scholar] [CrossRef]
- Azzaoui, I.; Uhel, F.; Rossille, D.; Pangault, C.; Dulong, J.; Le Priol, J.; Lamy, T.; Houot, R.; Le Gouill, S.; Cartron, G.; et al. T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood 2016, 128, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Manfroi, B.; Moreaux, J.; Righini, C.; Ghiringhelli, F.; Sturm, N.; Huard, B. Tumor-associated neutrophils correlate with poor prognosis in diffuse large B-cell lymphoma patients. Blood Cancer J. 2018, 8, 66. [Google Scholar] [CrossRef]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Papin, A.; Tessoulin, B.; Bellanger, C.; Moreau, A.; Le Bris, Y.; Maisonneuve, H.; Moreau, P.; Touzeau, C.; Amiot, M.; Pellat-Deceunynck, C.; et al. CSF1R and BTK inhibitions as novel strategies to disrupt the dialog between mantle cell lymphoma and macrophages. Leukemia 2019, 33, 2442–2453. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, J.; Xu, J.; Li, D.; Shi, G.; Liu, F.; Ding, L.; Ren, J.; Dou, H.; Wang, T.; et al. MiR-30a increases MDSC differentiation and immu-nosuppressive function by targeting SOCS3 in mice with B-cell lymphoma. FEBS J. 2017, 284, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.-P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Thielens, A.; Marabelle, A.; Sagiv-Barfi, I.; Sola, C.; Chanuc, F.; Fuseri, N.; Bonnafous, C.; Czerwinski, D.; Rajapaksa, A.; et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014, 123, 678–686. [Google Scholar] [CrossRef]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016, 539, 112–117. [Google Scholar] [CrossRef]
- Patel, S.S.; Weirather, J.L.; Lipschitz, M.; Lako, A.; Chen, P.-H.; Griffin, G.K.; Armand, P.; Shipp, M.A.; Rodig, S.J. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood 2019, 134, 2059–2069. [Google Scholar] [CrossRef]
- Caruana, I.; Diaconu, I.; Dotti, G. From monoclonal antibodies to chimeric antigen receptors for the treatment of human malignancies. Semin. Oncol. 2014, 41, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Mauz-Korholz, C.; Hernandez, T.; Milgrom, S.A.; Castellino, S.M. Pediatric and Adolescent Hodgkin Lymphoma: Paving the Way for Standards of Care and Shared Decision Making. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432420. [Google Scholar] [CrossRef]
- Rusconi, C.; Ciavarella, S.; Fabbri, A.; Flenghi, L.; Puccini, B.; Re, A.; Sorio, M.; Vanazzi, A.; Zanni, M. Treatment of very high-risk classical Hodgkin Lymphoma: Cases’ selection from real life and critical review of the literature. Acta Biomed. 2020, 91, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Descalzi-Montoya, D.B.; Lodhi, N. The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: Cellular composition, cytokine profile, EBV, and exosomes. Cancer Rep. 2021, 4, e1311. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, V.; Papoudou-Bai, A.; Makis, A.; Kanavaros, P.; Hatzimichael, E. Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications. Biology 2023, 12, 862. [Google Scholar] [CrossRef]
- Greaves, P.; Clear, A.; Owen, A.; Iqbal, S.; Lee, A.; Matthews, J.; Wilson, A.; Calaminici, M.; Gribben, J.G. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 2013, 122, 2856–2863. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Putty, K.; Rendon, B.E.; Lamont, G.J.; Faughn, J.D.; Satoskar, A.; Lasnik, A.; Eaton, J.W.; Mitchell, R.A. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J. Immunol. 2013, 190, 2984–2993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guidetti, A.; Mazzocchi, A.; Miceli, R.; Paterno’, E.; Taverna, F.; Spina, F.; Crippa, F.; Farina, L.; Corradini, P.; Gianni, A.; et al. Early reduction of serum TARC levels may predict for success of ABVD as frontline treatment in patients with Hodgkin Lymphoma. Leuk. Res. 2017, 62, 91–97. [Google Scholar] [CrossRef]
- SoRelle, E.D.; Haynes, L.E.; Willard, K.A.; Chang, B.; Ch’NG, J.; Christofk, H.; Luftig, M.A. Epstein-Barr virus reactivation induces di-vergent abortive, reprogrammed, and host shutoff states by lytic progression. PLoS Pathog. 2024, 20, e1012341. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.G.; Wilson, J.B.; Anderson, S.J.; Longnecker, R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 1998, 9, 405–411. [Google Scholar] [CrossRef]
- Bosch-Schips, J.; Granai, M.; Quintanilla-Martinez, L.; Fend, F. The Grey Zones of Classic Hodgkin Lymphoma. Cancers 2022, 14, 742. [Google Scholar] [CrossRef]
- Lin, L.Y.; Du, L.M.; Cao, K.; Huang, Y.; Yu, P.F.; Zhang, L.Y.; Li, F.Y.; Wang, Y.; Shi, Y.F. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene 2016, 35, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.P.; Engels, H.M.; Dams, M.; Paes Leme, A.F.; Pauletti, B.A.; Simhadri, V.L.; Dürkop, H.; Reiners, K.S.; Barnert, S.; Engert, A.; et al. Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the micro-environment of Hodgkin’s lymphoma. J. Pathol. 2014, 232, 485–487. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. KEYNOTE-204 investigators. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524, Erratum in Lancet Oncol. 2021, 22, e184. https://doi.org/10.1016/S1470-2045(21)00193-5. [Google Scholar] [CrossRef] [PubMed]
- Ramchandren, R.; Domingo-Domènech, E.; Rueda, A.; Trněný, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J. Clin. Oncol. 2019, 37, 1997–2007. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Bühnen, I.; Meissner, J.; Trautmann-Grill, K.; Herhaus, P.; Halbsguth, T.V.; Schaub, V.; Kerkhoff, A.; Mathas, S.; Bormann, M.; et al. Nivolumab and doxorubicin, vinblastine, and dacarbazine in early-stage unfavorable Hodgkin lymphoma: Final analysis of the randomized German Hodgkin Study Group phase II NIVAHL trial. J. Clin. Oncol. 2023, 41, 1193–1199. [Google Scholar] [CrossRef]
- Herrera, A.F.; LeBlanc, M.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Parsons, S.K.; Ahmed, S.; et al. Nivolumab + AVD in advanced-stage classic Hodg-kin’s lymphoma. New Engl. J. Med. 2024, 391, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, C.S.; Hong, F.; Ambinder, R.F.; Cohen, J.B.; Robertson, M.J.; David, K.A.; Advani, R.H.; Fenske, T.S.; Barta, S.K.; Palmisiano, N.D.; et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: Phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020, 7, e660–e670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Collins, G.P. Checkpoint inhibitors and the changing face of the relapsed/refractory classical Hodgkin lymphoma pathway. Curr. Oncol. Rep. 2022, 24, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.; Foster, J.H. Treatment of high-risk neuroblastoma. Children 2023, 10, 1302. [Google Scholar] [CrossRef]
- Joshi, S. Targeting the tumor microenvironment in neuroblastoma: Recent advances and future directions. Cancers 2020, 12, 2057. [Google Scholar] [CrossRef]
- Louault, K.; De Clerck, Y.A.; Janoueix-Lerosey, I. The neuroblastoma tumor microenvironment: From an in-depth characterization towards novel therapies. EJC Paediatr. Oncol. 2024, 3, 100161. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, E.; Jiang, C.; Cheng, C. B cell infiltration is highly associated with prognosis and an immune-infiltrated tumor mi-croenvironment in neuroblastoma. J. Cancer Metastasis Treat. 2021, 7. [Google Scholar] [CrossRef]
- Feng, C.; Li, T.; Xiao, J.; Wang, J.; Meng, X.; Niu, H.; Jiang, B.; Huang, L.; Deng, X.; Yan, X.; et al. Tumor Microenvironment Profiling Identifies Prognostic Signatures and Suggests Immunotherapeutic Benefits in Neuroblastoma. Front. Cell Dev. Biol. 2022, 10, 814836. [Google Scholar] [CrossRef]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verhoeven, B.M.; Mei, S.; Olsen, T.K.; Gustafsson, K.; Valind, A.; Lindström, A.; Gisselsson, D.; Fard, S.S.; Hagerling, C.; Kharchenko, P.V.; et al. The immune cell atlas of human neuroblastoma. Cell Rep. Med. 2022, 3, 100657. [Google Scholar] [CrossRef]
- Blavier, L.; Yang, R.-M.; DeClerck, Y.A. The tumor microenvironment in neuroblastoma: New players, new mechanisms of inter-action and new perspectives. Cancers 2020, 12, 2912. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef]
- Wieczorek, A.; Śladowska, K.; Lode, H.N. Efficacy and Safety of Anti-GD2 Immunotherapy with Dinutuximab Beta in the Treatment of Relapsed/Refractory High-Risk Neuroblastoma. Target. Oncol. 2025, 20, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Prathipati, P.; Murakonda, S.P.; Murakonda, A.B.; Srivastava, A.; Avadhesh; Byrareddy, S.N.; Coulter, D.W.; Gupta, S.C.; Challagundla, K.B. Immune checkpoint molecules in neuroblastoma: A clinical perspective. Semin. Cancer Biol. 2022, 86, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Fox, E.; Isikwei, E.; Reid, J.M.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; Mackall, C.L. A Phase I/II Trial of Nivolumab plus Ipilimumab in Children and Young Adults with Relapsed/Refractory Solid Tumors: A Children’s Oncology Group Study ADVL1412. Clin. Cancer Res. 2022, 28, 5088–5097. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webb, M.W.; Sun, J.; Sheard, M.A.; Liu, W.; Wu, H.; Jackson, J.R.; Malvar, J.; Sposto, R.; Daniel, D.; Seeger, R.C. Colony stimulating factor 1 receptor blockade improves the efficacy of chemotherapy against human neuroblastoma in the absence of T lymphocytes. Int. J. Cancer 2018, 143, 1483–1493. [Google Scholar] [CrossRef]
- Rohila, D.; Park, I.H.; Pham, T.V.; Jones, R.; Tapia, E.; Liu, K.X.; Tamayo, P.; Yu, A.; Sharabi, A.B.; Joshi, S. Targeting macrophage Syk en-hances responses to immune checkpoint blockade and radiotherapy in high-risk neuroblastoma. Front. Immunol. 2023, 14, 1148317. [Google Scholar] [CrossRef]
- Yu, L.; Huang, L.; Lin, D.; Lai, X.; Wu, L.; Liao, X.; Liu, J.; Zeng, Y.; Liang, L.; Zhang, G.; et al. GD2-specific chimeric antigen recep-tor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J. Cancer Res. Clin. Oncol. 2022, 148, 2643–2652. [Google Scholar] [CrossRef]
- Straathof, K.; Flutter, B.; Wallace, R.; Jain, N.; Loka, T.; Depani, S.; Wright, G.; Thomas, S.; Cheung, G.W.; Gileadi, T.; et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 2020, 12, eabd6169. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, E.; Keffes, N.; Vorri, S.; Tsitouras, V.; Gkantsinikoudis, N.; Tsitsopoulos, P.; Magras, J. The molecular basis of pediatric brain tumors: A review with clinical implications. Cancers 2025, 17, 1566. [Google Scholar] [CrossRef]
- Levine, A.B.; Nobre, L.; Das, A.; Milos, S.; Bianchi, V.; Johnson, M.; Fernandez, N.R.; Stengs, L.; Ryall, S.; Ku, M.; et al. Immuno-oncologic profiling of pediatric brain tumors reveals major clinical significance of the tumor immune microenvironment. Nat. Commun. 2024, 15, 5790. [Google Scholar] [CrossRef]
- Haberthur, K.; Brennan, K.; Hoglund, V.; Balcaitis, S.; Chinn, H.; Davis, A.; Kreuser, S.; Winter, C.; Leary, S.E.; Deutsch, G.H.; et al. NKG2D ligand expression in pediatric brain tumors. Cancer Biol. Ther. 2016, 17, 1253–1265. [Google Scholar] [CrossRef]
- Powell, A.B.; Yadavilli, S.; Saunders, D.; Van Pelt, S.; Chorvinsky, E.; Burga, R.A.; Albihani, S.; Hanley, P.J.; Xu, Z.; Pei, Y.; et al. Medullo-blastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J. Transl. Med. 2019, 17, 321. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Yoshioka, K.; Nakata, K.; Sato, T.; Kasahara, Y.; Nakao, S. Human microRNA-1245 down-regulates the NKG2D receptor in natural killer cells and impairs NKG2D-mediated functions. Haematologica 2012, 97, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Mineharu, Y.; Arakawa, Y.; Liu, B.; Tanji, M.; Yamaguchi, M.; Fujimoto, K.-I.; Fukui, N.; Terada, Y.; Yokogawa, R.; et al. High programmed cell death 1 ligand-1 expression: Association with CD8+ T-cell infiltration and poor prognosis in human meduloblastoma. J. Neurosurg. 2018, 128, 710–716. [Google Scholar] [CrossRef]
- Martin, A.M.; Nirschl, C.J.; Polanczyk, M.J.; Bell, W.R.; Nirschl, T.R.; Harris-Bookman, S.; Phallen, J.; Hicks, J.; Martinez, D.; Ogurtsova, A.; et al. PD-L1 expression in medulloblastoma: An evaluation by subgroup. Oncotarget 2018, 9, 19136–19146. [Google Scholar] [CrossRef] [PubMed]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47–SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef]
- Aldaregia, J.; Errarte, P.; Olazagoitia-Garmendia, A.; Gimeno, M.; Uriz, J.J.; Gershon, T.R.; Garcia, I.; Matheu, A. Erbb4 Is Required for Cerebellar Developmentand Malignant Phenotype of Medulloblastoma. Cancers 2020, 12, 997. [Google Scholar] [CrossRef]
- Khuong-Quang, D.-A.; Buczkowicz, P.; Rakopoulos, P.; Liu, X.-Y.; Fontebasso, A.M.; Bouffet, E.; Bartels, U.; Albrecht, S.; Schwartzentruber, J.; Letourneau, L.; et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting from Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef]
- Nabbi, A.; Beck, P.; Delaidelli, A.; Oldridge, D.A.; Sudhaman, S.; Zhu, K.; Yang, S.Y.C.; Mulder, D.T.; Bruce, J.P.; Paulson, J.N.; et al. Tran-scriptional immunogenomic analysis reveals distinct immunological clusters in paediatric nervous system tumours. Genome Med. 2023, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Gorsi, H.S.; Malicki, D.M.; Barsan, V.; Tumblin, M.; Yeh-Nayre, L.; Milburn, M.; Elster, J.D.; Crawford, J.R. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J. Pediatr. Hematol. 2019, 41, e235–e241. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.T.; Yalon, M.; Vainer, G.W.; Lossos, A.; Yust, S.; Tzach, L.; Cagnano, E.; Limon, D.; Bokstein, F. Pembrolizumab: First experience with recurrent primary central nervous system (CNS) tumors. J. Neuro-Oncol. 2016, 129, 453–460. [Google Scholar] [CrossRef]
- Tan, I.-L.; Arifa, R.D.N.; Rallapalli, H.; Kana, V.; Lao, Z.; Sanghrajka, R.M.; Bayin, N.S.; Tanne, A.; Wojcinski, A.; Korshunov, A.; et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedge-hog-Medulloblastoma model. Oncogene 2021, 40, 396–407. [Google Scholar] [CrossRef]
- Cachia, D.; Eskandari, R.; McDonald, D.G.; Infinger, L.K.; Vandergrift, W.A., III; Varma, A.K.; Patel, S.J.; Zukas, A.M.; Lindhorst, S.M.; Das, A. Low-dose radiation followed by on-target inhibition of Galectin-3 in combination with anti-4-1BB monoclonal antibody regu-lates immune responses in Group 3 and Group 4 medulloblastoma mouse model (P11-13.001). Neurology 2023, 100, 3857. [Google Scholar] [CrossRef]
- Johnson, T.S.; Mcgaha, T.; Munn, D.H. Chemo-immunotherapy: Role of indoleamine 2,3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 91–104. [Google Scholar] [CrossRef]
- Sharma, M.D.; Pacholczyk, R.; Shi, H.; Berrong, Z.J.; Zakharia, Y.; Greco, A.; Chang, C.-S.S.; Eathiraj, S.; Kennedy, E.; Cash, T.; et al. Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity. Immunity 2021, 54, 2354–2371.e8. [Google Scholar] [CrossRef]
- Johnson, T.S.; MacDonald, T.J.; Pacholczyk, R.; Aguilera, D.; Al-Basheer, A.; Bajaj, M.; Bandopadhayay, P.; Berrong, Z.; Bouffet, E.; Castellino, R.C.; et al. Indoximod-based chemo-immunotherapy for pediatric brain tumors: A first-in-children phase I trial. Neuro-Oncology 2024, 26, 348–361. [Google Scholar] [CrossRef]
- Thompson, E.; Ashley, D.M.; Ayasoufi, K.; Norberg, P.; Archer, G.E.; Buckley, E.; Herndon, J.E., II; Walter, A.; Archambault, B.; Flahiff, C.; et al. Outcomes and immune response after peptide vaccination targeting human cytomegalovirus antigen pp65 in children and young adults with recurrent high-grade glioma and medulloblastoma. J. Clin. Oncol. 2024, 42, 2039. [Google Scholar] [CrossRef]
- Mueller, S.; Taitt, J.M.; Villanueva-Meyer, J.E.; Bonner, E.R.; Nejo, T.; Lulla, R.R.; Goldman, S.; Banerjee, A.; Chi, S.N.; Whipple, N.S.; et al. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J. Clin. Investig. 2020, 130, 6325–6337. [Google Scholar] [CrossRef] [PubMed]
- Fenstermaker, R.A.; Ciesielski, M.J. Challenges in the development of a survivin vaccine (SurVaxM) for malignant glioma. Expert Rev. Vaccines 2014, 13, 377–385. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Ronsley, R.; Choe, M.; Henson, C.; Breedt, M.; Barrios-Anderson, A.; Wein, A.; Brown, C.; Beebe, A.; Kong, A.; et al. Locoregional CAR T cells for children with CNS tumors: Clinical procedure and catheter safety. Neoplasia 2023, 36, 100870. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Galopin, N.; Bonérandi, E.; Clémenceau, B.; Fougeray, S.; Birklé, S. CAR T cell therapy’s potential for pediatric brain tumors. Cancers 2021, 13, 5445. [Google Scholar] [CrossRef]
- Wu, W.-T.; Lin, W.-Y.; Chen, Y.-W.; Lin, C.-F.; Wang, H.-H.; Wu, S.-H.; Lee, Y.-Y. New era of immunotherapy in pediatric brain tumors: Chimeric antigen receptor T-cell therapy. Int. J. Mol. Sci. 2021, 22, 2404. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3–K27M+ diffuse midline gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood–brain barrier. Expert Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef]
- Salzillo, C.; Cazzato, G.; Serio, G.; Marzullo, A. Paediatric renal tumors: A state-of-the-art review. Curr. Oncol. Rep. 2025, 27, 211–224. [Google Scholar] [CrossRef]
- Hont, A.B.; Dumont, B.; Sutton, K.S.; Anderson, J.; Kentsis, A.; Drost, J.; Hong, A.L.; Verschuur, A. The tumor microenviron-ment and immune targeting therapy in pediatric renal tumors. Pediatr. Blood Cancer 2023, 70, e30110. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.; Wu, Y.; Wu, X.; Du, G.; Liu, W.; Wu, R.; Zhao, F.; Zhang, H.; Li, Y.; et al. Relationship of tumour-associated macrophages with poor prognosis in Wilms’ tumour. J. Pediatr. Urol. 2020, 16, 376.e1–376.e8. [Google Scholar] [CrossRef] [PubMed]
- Maturu, P.; Jones, D.; Ruteshouser, E.C.; Barasch, J.; Yang, Y.; Wang, T.; Ghosh, A.; Guevara, A.; Donehower, L.A.; Mendoza, G.; et al. Role of cyclooxygenase-2 pathway in creating an immunosuppressive microenvironment and in initiation and pro-gression of Wilms’ tumor. Neoplasia 2017, 19, 237–249. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 distribution and perspective for cancer immunotherapy—Blockade, knockdown, or inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Stewart, C.; Carter, S.L.; Ambrogio, L.; Cibulskis, K.; Sougnez, C.; Lawrence, M.S.; Lichtenstein, L.; Getz, G.; Wu, C.-L.; et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Investig. 2012, 122, 2983–2988. [Google Scholar] [CrossRef]
- Chun, H.J.E.; Johann, P.D.; Milne, K.; Zappia, K.J.; Malu, S.; Shah, M.; Yip, S.; Afzal, S.; Mayoh, C.; Byrnes, A.; et al. Identi-fication and analyses of extracranial and cranial rhabdoid tumor molecular subgroups reveal tumors with cytotoxic T cell in-filtration. Cell Rep. 2019, 29, 2338–2354.e7. [Google Scholar] [CrossRef]
- Wong, M.K.; Ng, C.C.Y.; Kuick, C.H.; Poonepalli, A.; Lau, C.C.; Chan, J.Y.; Foo, R.S.; Koay, E.S.-C.; Lim, C.C.T.; Petersson, F.; et al. Clear cell sarcomas of the kidney are characterised by BCOR gene abnormalities. Histopathology 2018, 72, 320–329. [Google Scholar] [CrossRef]
- Roelands, J.; Hendrickx, W.; Zoppoli, G.; Mall, R.; Saad, M.; Halliwill, K.; Curigliano, G.; Bedognetti, D.; Thorsson, V.; Mokrani, A.; et al. Oncogenic states dictate prognostic and predictive connotations. J. Immunother. Cancer 2020, 8, e000617. [Google Scholar] [CrossRef]
- Sherif, S.; Roelands, J.; Mifsud, W.; Lapuente-Sanchez, B.; Rinchai, D.; Richard, J.; Decalf, J.; Bedognetti, D. The immune landscape of solid pediatric tumors. J. Exp. Clin. Cancer Res. 2022, 41, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, H.; Shen, M.; Liu, W.; Li, Z.; Lin, J. Clinical significance of tumoral PD-L1 expression in Wilms tumors. J. Pediatr. Urol. 2022, 18, 14.e1–14.e8. [Google Scholar] [CrossRef]
- Pinto, N.; Park, J.R.; Murphy, E.; Hawkins, D.S.; Rudzinski, E.; Garrison, M.M.; Wharton, K.; Ziegler, D.S.; Gill, R.; Angiolillo, A.; et al. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr. Blood Cancer 2017, 64, e26613. [Google Scholar] [CrossRef]
- Yarmarkovich, M.; Maris, J.M. When cold is hot: Immune checkpoint inhibition therapy for rhabdoid tumors. Cancer Cell 2019, 36, 575–576. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. B7-H3 as a promoter of metastasis and promising therapeutic target. Front. Oncol. 2018, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T cells targeting B7-H3 demonstrate potent preclinical activity. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Navale, D.D.; Huynh, A.; Zhang, S.; Kan, K.; Tatman, K.; Allen, S.M.; Taft, S.L.; et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.E. Recent advances in targeting COX-2 for cancer therapy: A review. RSC Med. Chem. 2025, 16, 2974–3002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tighe, S.; Zhu, Y.-T. COX-2 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1277, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.R.; Pelly, V.S.; Moeini, A.; Chiang, S.-C.; Flanagan, E.; Bromley, C.P.; Clark, C.; Earnshaw, C.H.; Koufaki, M.A.; Bonavita, E.; et al. Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations. Nat. Commun. 2022, 13, 2063. [Google Scholar] [CrossRef]
- Tretiakova, M.; Zynger, D.L.; Luan, C.; Andeen, N.K.; Finn, L.S.; Kocherginsky, M.; Teh, B.T.; Yang, X.J. Glypican 3 overexpression in primary and metastatic Wilms tumors. Virchows Arch. 2014, 466, 67–76. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Roberts, S.S.; Bender, J.G.; Shukla, N.; Wexler, L.H. Immunotherapeutic targeting of GPC3 in pediatric solid em-bryonal tumors. Front. Oncol. 2019, 9, 108. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Tanaka, S.; Souzaki, R.; Miyoshi, K.; Kohashi, K.; Oda, Y.; Nakatsura, T.; Taguchi, T. Glypican-3 expression in pediatric malignant solid tumors. Eur. J. Pediatr. Surg. 2015, 25, 138–144. [Google Scholar] [CrossRef]
- Hont, A.B.; Cruz, C.R.; Ulrey, R.; Gerken, C.; Leung, A.K.; Sabzevari, H.; Lee, T.; Wu, P.E.; Kapur, R.; Lucas, K.; et al. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor antigen-specific cytotoxic T lymphocytes: A phase I study. J. Clin. Oncol. 2019, 37, 2349–2359. [Google Scholar] [CrossRef]
- Basta-Jovanovic, G.; Radojevic-Skodric, S.; Brasanac, D.; Bogdanovic, J.; Vukotic, D.; Jovanovic, O. Prognostic value of survivin expression in Wilms tumor. J. BUON 2012, 17, 168–173. [Google Scholar]
- Nian, Q.; Lin, Y.; Zeng, J.; Zhang, Y.; Liu, R. Multifaceted functions of the Wilms tumor 1 protein: From its expression in various malignancies to targeted therapy. Transl. Oncol. 2025, 52, 102237. [Google Scholar] [CrossRef]
- Bolitho, A.; Liu, H. Epigenetic Regulation in Wilms Tumor. Biomedicines 2025, 13, 1678. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, L.; Chang, X.; Chen, K.; He, T.; Shi, J.; Lv, F.; Pan, L.; Wu, Y.; Cheng, Q.; et al. Integrative proteogenomic characterization of Wilms tumor. Nat. Commun. 2025, 16, 771. [Google Scholar] [CrossRef]
- Giannikopoulos, P.; Parham, D.M. Pediatric sarcomas: The next generation of molecular studies. Cancers 2022, 14, 2515. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Hayashi, M.; Verneris, M.R.; Lee-Sherick, A.B. Targeting tumor-associated macrophages in the pediatric sarcoma tumor microenvironment. Front. Oncol. 2020, 10, 581107. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Roth, M.E.; Gill, J.; Piperdi, S.; Chinai, J.M.; Geller, D.S.; Hoang, B.H.; Park, A.; Fremed, M.A.; Zang, X.; et al. Immune in-filtration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci. Rep. 2016, 6, 30093. [Google Scholar] [CrossRef]
- Rodrigues, J.; Sarmento, B.; Pereira, C.L. Osteosarcoma tumor microenvironment: The key for the successful development of bio-logically relevant 3D in vitro models. Vitr. Model. 2022, 1, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Nirala, B.K.; Yamamichi, T.; Petrescu, D.I.; Shafin, T.N.; Yustein, J.T. Decoding the impact of tumor microenvironment in osteosarcoma progression and metastasis. Cancers 2023, 15, 5108. [Google Scholar] [CrossRef]
- Bender, J.L.G.; Adamson, P.C.; Reid, J.M.; Xu, L.; Baruchel, S.; Shaked, Y.; Kerbel, R.S.; Cooney-Qualter, E.M.; Stempak, D.; Chen, H.X.; et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children’s Oncology Group study. J. Clin. Oncol. 2008, 26, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ Macrophages in Inflammatory and Malignant Diseases. Int. J. Mol. Sci. 2020, 21, 5497. [Google Scholar] [CrossRef]
- Zając, A.E.; Czarnecka, A.M.; Rutkowski, P. The Role of Macrophages in Sarcoma Tumor Microenvironment and Treatment. Cancers 2023, 15, 5294. [Google Scholar] [CrossRef]
- Schürch, C.M.; Förster, S.; Brühl, F.; Yang, S.H.; Felley-Bosco, E.; Hewer, E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology 2017, 7, e1373235. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.H.; Lee, Y.; Park, J.S.; Song, J.S.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Weissman, I.L.; et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, Á.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. CCL2 Shapes Macrophage Polarization by GM-CSF and M-CSF. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W.; Okabe, Y.; et al. CCL2-induced chemokine cascade promotes breast cancer metastasis. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.G.; Marina, N.; Glade-Bender, J. Angiogenesis and vascular targeting in Ewing sarcoma. Cancer 2010, 116, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Maas, M.L.; Gustafson, M.P.; Dickman, P.; Adams, R.H.; Watanabe, M.; Johnson, S.; McCormick, J.; DeRidder, A.; Yamada, T.; et al. Increased CTLA-4+ T cells in aggressive pediatric sarcoma. J. Immunother. Cancer 2015, 3, 35. [Google Scholar] [CrossRef]
- Tian, H.; Cao, J.; Li, B.; Nice, E.C.; Mao, H.; Zhang, Y.; Huang, C. Managing the immune microenvironment of osteosarcoma: The outlook for osteosarcoma treatment. Bone Res. 2023, 11, 11. [Google Scholar] [CrossRef]
- Arndt, C.A.; Koshkina, N.V.; Inwards, C.Y.; Hawkins, D.S.; Krailo, M.D.; Villaluna, D.; Anderson, P.; Zhu, L.; Pendergrass, T.; Speights, R.; et al. Inhaled GM-CSF for recurrent osteosarcoma. Clin. Cancer Res. 2010, 16, 4024–4030. [Google Scholar] [CrossRef]
- Lagmay, J.P.; Krailo, M.D.; Dang, H.; Kim, A.; Hawkins, D.S.; Beaty, O., 3rd; Womer, R.B.; Speights, R.; Pappo, A.S.; Meyers, P.A.; et al. Out-come of recurrent osteosarcoma in phase II trials. J. Clin. Oncol. 2016, 34, 3031–3038. [Google Scholar] [CrossRef]
- Iribarren, K.; Buque, A.; Mondragon, L.; Xie, W.; Lévesque, S.; Pol, J.; Zitvogel, L.; Kepp, O.; Kroemer, G. Anticancer effects of anti-CD47 immu-notherapy in vivo. OncoImmunology 2019, 8, 1550619. [Google Scholar] [CrossRef]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of osteosarcoma lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Shulman, D.S.; Turpin, B.; Shusterman, S.; Gore, L.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and lim-itations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, E.K.; Magnan, H.D.; Meyers, P.A.; Chou, A.J.; Ambati, S.R.; Wexler, L.H. Off-label use of bevacizumab in relapsed and refractory pediatric sarcoma patients: The Memorial Sloan Kettering Cancer Center experience. J. Clin. Oncol. 2016, 34, 10569. [Google Scholar] [CrossRef]
- Navid, F.; Baker, S.D.; McCarville, M.B.; Stewart, C.F.; Billups, C.A.; Wu, J.; Davidoff, A.M.; Spunt, S.L.; Furman, W.L.; McGregor, L.M.; et al. Phase I and clinical pharmacology study of bevaci-zumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin. Cancer Res. 2013, 19, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, R.; Sharma, N.; Reebel, L.; Rodal, M.B.; Peck, A.; West, B.L.; Marimuthu, A.; Severson, P.; Karlin, D.A.; Dowlati, A.; et al. Phase Ib study of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther. Adv. Med. Oncol. 2019, 11, 1758835919854238. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Pawlik, T.M.; Radwan, F.; Abdel-Hakeem, M.; Abdel-Ghany, S.; Wadan, A.-H.S.; Elzawahri, M.; El-Hashash, A.; Arneth, B. Precision nanomedicine: Navigating the tumor microenvironment for enhanced cancer immunotherapy and targeted drug delivery. Mol. Cancer 2025, 24, 160. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, W.; Che, W.; Xu, Y.; Jin, C.; Dong, L.; Xia, Q. Nanomedicines targeting metabolic pathways in the tumor microenvi-ronment: Future perspectives and the role of AI. Metabolites 2025, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Huang, A.; Yan, H.; Ju, X.; Xiang, L.; Yuan, J. Artificial intelligence: Illuminating the depths of the tumor microenvironment. J. Transl. Med. 2024, 22, 799. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Zhang, Y.; Li, J.; Zhang, K. Advancements in tumor-targeted nanoparticles: Design strategies and multifunctional therapeutic approaches. Nanomaterials 2025, 15, 1262. [Google Scholar] [CrossRef]
- Maksymova, L.; Pilger, Y.A.; Nuhn, L.; Van Ginderachter, J.A. Nanobodies targeting the tumor microenvironment and their for-mulation as nanomedicines. Mol. Cancer 2025, 24, 65. [Google Scholar] [CrossRef]
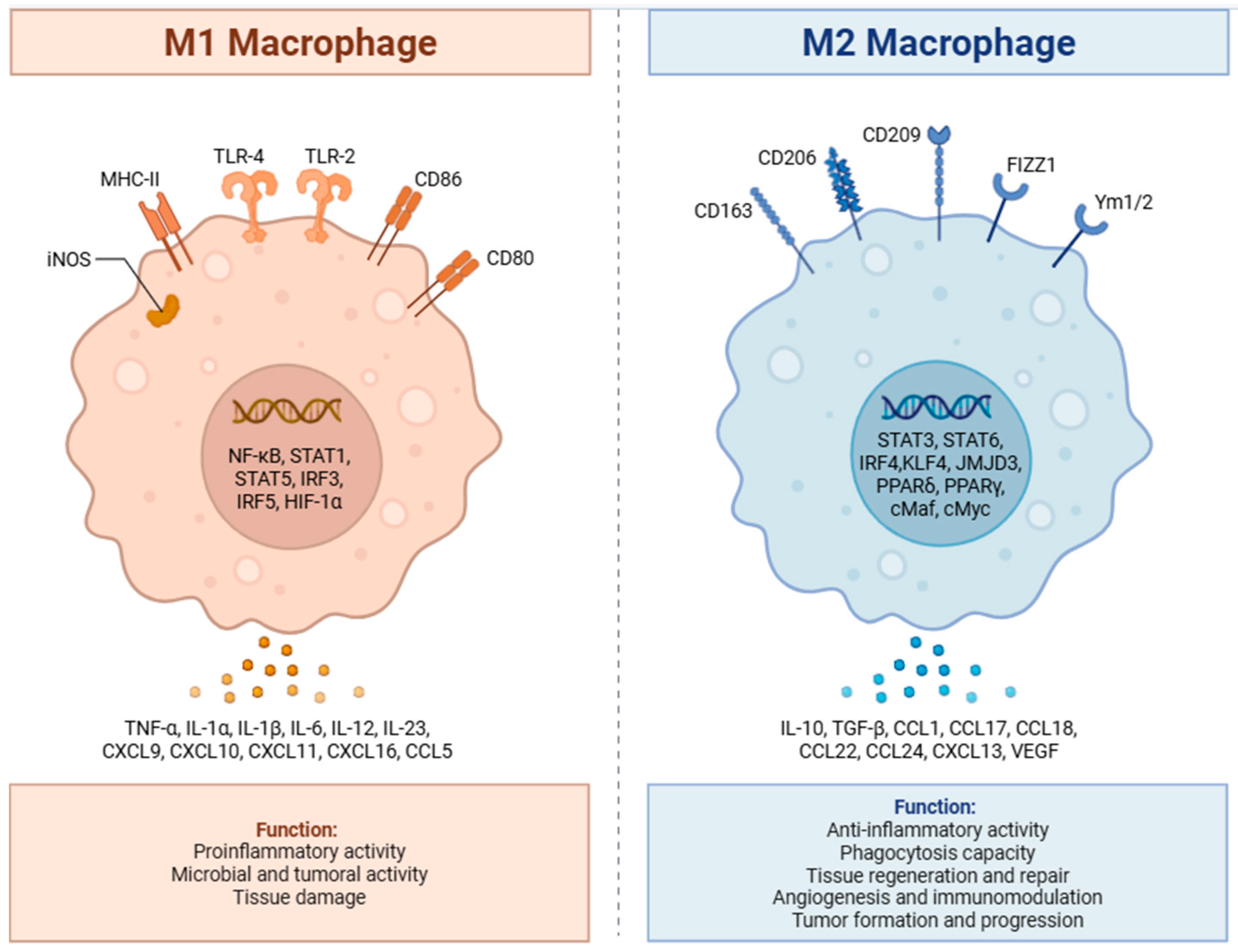
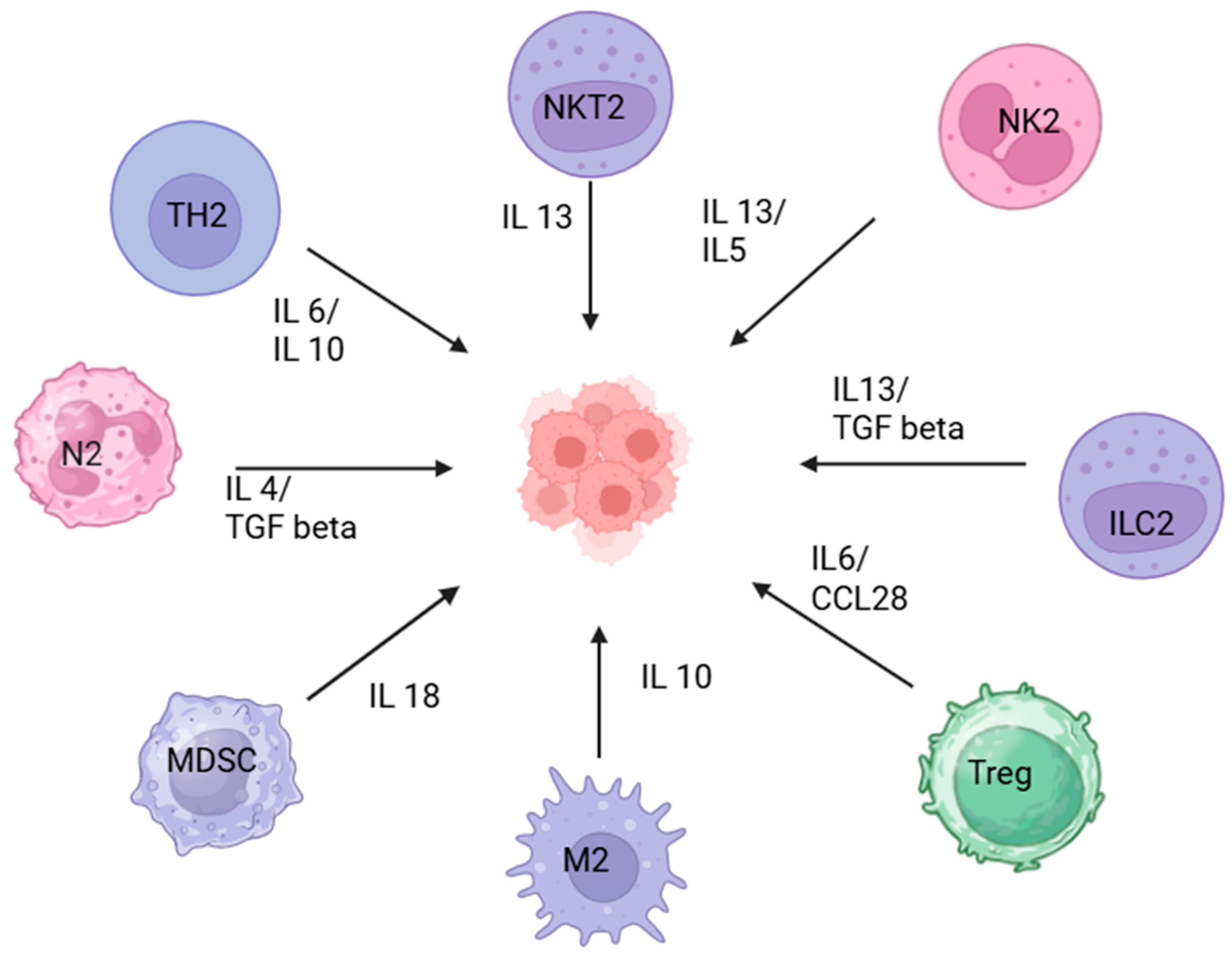
| Tumor Type | Major TME Molecules | Key Pathways/Mechanisms | Possible Therapeutic Targets |
|---|---|---|---|
| Acute lymphoblastic leukemia (ALL) | IL-7, IL-8, IL-15, CXCR4–CXCL12, NOTCH1/2, VEGF, HIF-1α, VLA-4/VCAM-1, integrin–laminin | Cytokine-mediated proliferation; PI3K/AKT, STAT, JAK pathways; CAM-DR; hypoxia-induced angiogenesis | CXCR4 inhibitors; NOTCH blockers; anti-VEGF (Bevacizumab); HIF-1α inhibitors (Echinomycin); proteasome inhibitors (Bortezomib, Carfilzomib) |
| Acute myeloid leukemia (AML) | PD-1/PD-L1, CTLA-4, TIM-3/Galectin-9, IDO, TGF-β, PGE2, IL-10, IL-7, IL-2, HIF-1α | Immune evasion via Tregs, MDSCs, and inhibitory checkpoints; hypoxia-driven angiogenesis | ICIs (TIM-3 inhibitors), CD123 ADC, CLL-1 ADC, CAR-T/NK (CD33, CD123), IL-15 super agonists |
| B-cell lymphomas (BL, DLBCL) | TAMs (CD163+, CD206+), MDSCs, TANs, CAFs, PD-L1, CXCR2, TLR9, HIF-2α | M2 macrophage polarization; stromal remodeling; T cell exhaustion | CSF-1/CSF-1R blockade, CCR2 inhibitors, CD47–SIRPα blockade, miR-155 (M1 repolarization), CAR-T/NK (CD19, CD22) |
| Tumor Type | Major TME Molecules | Key Pathways/Mechanisms | Possible Therapeutic Targets |
|---|---|---|---|
| Hodgkin lymphoma (cHL) | EBV (LMP1, LMP2A, EBNA1), PD-1/PD-L1, CTLA-4, LAG3, TIM3, TARC (CCL17), IL-6, IL-10 | T cell exhaustion; cytokine-mediated immune suppression; EBV-driven NF-κB activation | ICIs (Nivolumab, Pembrolizumab, Ipilimumab), LAG3 inhibitors (Relatlimab), TIM3 inhibitors |
| Neuroblastoma (NB) | GD2, CSF1R, CXCL12, CSF1, CCL2, PD-1, LAG3, CTLA-4, TIGIT, CD47, VEGF | T cell exhaustion; M2 macrophage infiltration; ADCC via NK cells | Anti-GD2 (Dinutuximab, Naxitamab), IL-2/GM-CSF, PD-1 + CTLA-4 blockade, anti-CD47 (Magrolimab), anti-CSF1R, Bevacizumab |
| Renal Tumors (WT, MRK, CCSK, RCC) | PD-L1, COX-2, B7-H3 (CD276), VEGF, HIF-1α, TAMs (CD68+, CD163+), IL-6/pSTAT3 | TAM-mediated angiogenesis and immunosuppression; checkpoint activation | ICIs (Nivolumab, Pembrolizumab, Ipilimumab), anti–B7-H3 (MGA271, CAR-T), COX-2 inhibitors (Celecoxib), anti-VEGF |
| Sarcomas (RMS, NRSTS, EWS, OGS) | TAMs (M2: CD206, CD163), PD-L1/PD-L2, CTLA-4, CD47, ARG1, IDO1/2, VEGF, CSF1R | Immunosuppression, angiogenesis, “don’t eat-me” signaling, macrophage-driven metastasis | L-MTP-PE (TLR4 agonist), anti-CD47, CSF1R inhibitors (Pexidartinib), PD-1/CTLA-4 inhibitors, Bevacizumab, ARG1/IDO inhibitors |
| Target | Therapy Type | Tumor Types | Clinical Insights | Reference |
|---|---|---|---|---|
| PD-1/PD-L1 | Checkpoint inhibitors (nivolumab, pembrolizumab, atezolizumab) | WT, MRTK, TFE-RCC | PD-L1 expressed in 14–35% of WT; higher in anaplastic/metastatic WT; anecdotal responses in MRTK | [169,170,171] |
| CTLA-4 | ICI (ipilimumab) | INI-deficient tumors | Used in combination trials | [172] |
| B7-H3 (CD276) | Monoclonal antibodies, CAR-T | WT, RCC | High expression in WT and RCC; linked to metastasis and poor prognosis; active trials | [173,174,175] |
| COX-2 | Celecoxib (COX-2 inhibitor) | WT, RCC | Expressed in WT including anaplastic/favorable histology; linked to TAM recruitment and angiogenesis | [176,177,178] |
| Glypican-3 (GPC3) | Peptide vaccine, CAR-T, monoclonal antibodies | WT, MRTK | Expressed in 30–77% of WT and 43–65% of MRTK; activates Hedgehog pathway | [179,180,181,182] |
| Tumor-Associated Antigens (WT1, PRAME, survivin) | TAA-specific T cells | WT, solid tumors | 73% disease stabilization in early trials | [183,184,185,186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Batra, S.; Prabhakar, P.; Mohapatra, D.; George, N.G.; Goel, N.; Rishi, B.; Misra, A.; Singh, A. Tumor Microenvironment: Current Understanding and Therapeutic Implications in Adult and Pediatric Cancers. Onco 2026, 6, 2. https://doi.org/10.3390/onco6010002
Batra S, Prabhakar P, Mohapatra D, George NG, Goel N, Rishi B, Misra A, Singh A. Tumor Microenvironment: Current Understanding and Therapeutic Implications in Adult and Pediatric Cancers. Onco. 2026; 6(1):2. https://doi.org/10.3390/onco6010002
Chicago/Turabian StyleBatra, Satyendra, Prashant Prabhakar, Debabrata Mohapatra, Noreen Grace George, Neha Goel, Bhavika Rishi, Aroonima Misra, and Amitabh Singh. 2026. "Tumor Microenvironment: Current Understanding and Therapeutic Implications in Adult and Pediatric Cancers" Onco 6, no. 1: 2. https://doi.org/10.3390/onco6010002
APA StyleBatra, S., Prabhakar, P., Mohapatra, D., George, N. G., Goel, N., Rishi, B., Misra, A., & Singh, A. (2026). Tumor Microenvironment: Current Understanding and Therapeutic Implications in Adult and Pediatric Cancers. Onco, 6(1), 2. https://doi.org/10.3390/onco6010002






