Synergistic Effect of Bacillus subtilis B3 and β-Glucanase on Solid-State Fermentation of Sunflower Meal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid-State Fermentation of Sunflower Meal
2.3. Chemical Analysis
2.4. Physical Analysis
2.5. Microbial Analysis
2.6. In Vitro Digestibility Test
2.7. Data Analysis and Statistics
3. Results
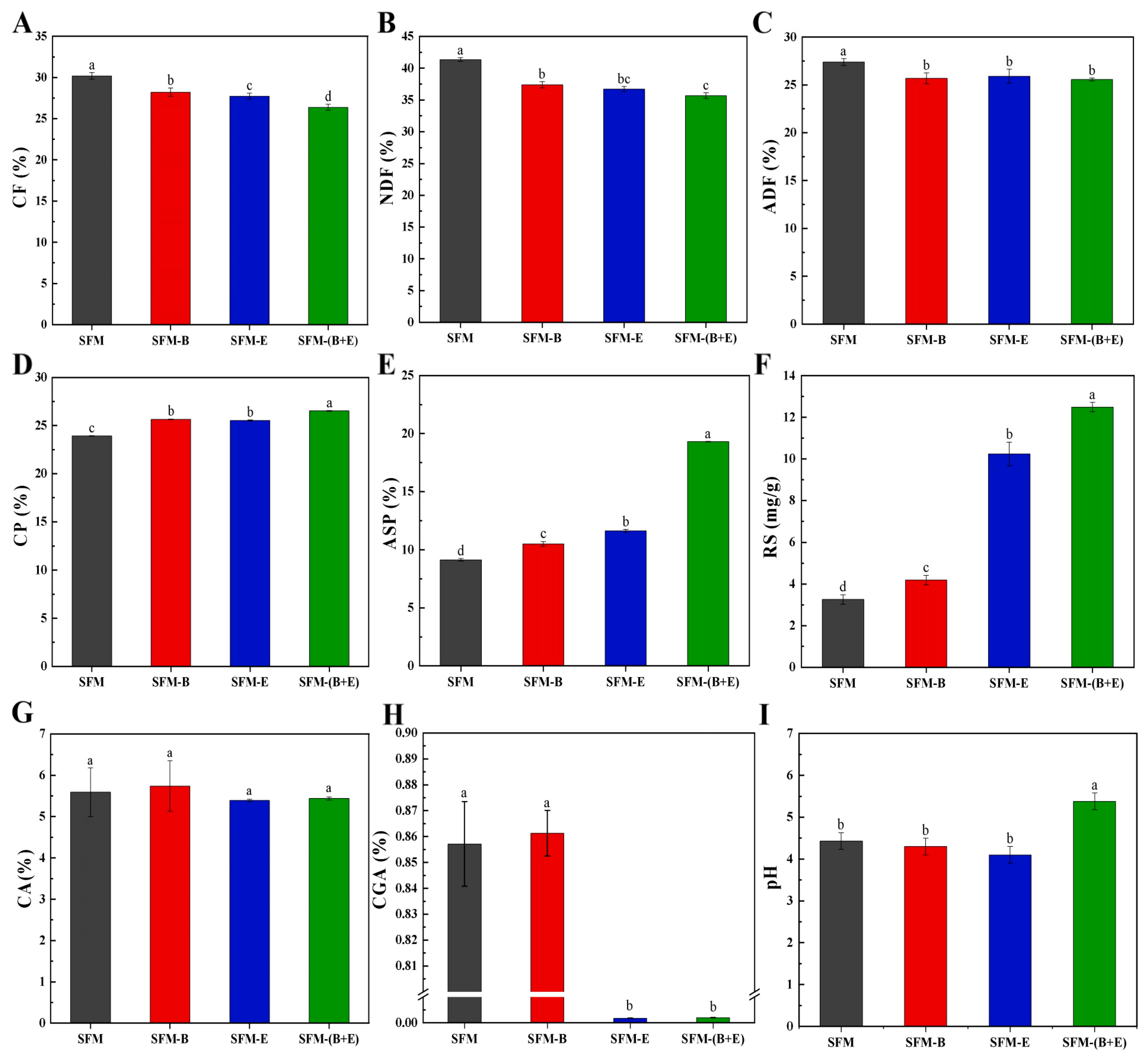
3.1. Results of Chemical Analysis
3.2. Results of Physical Analysis
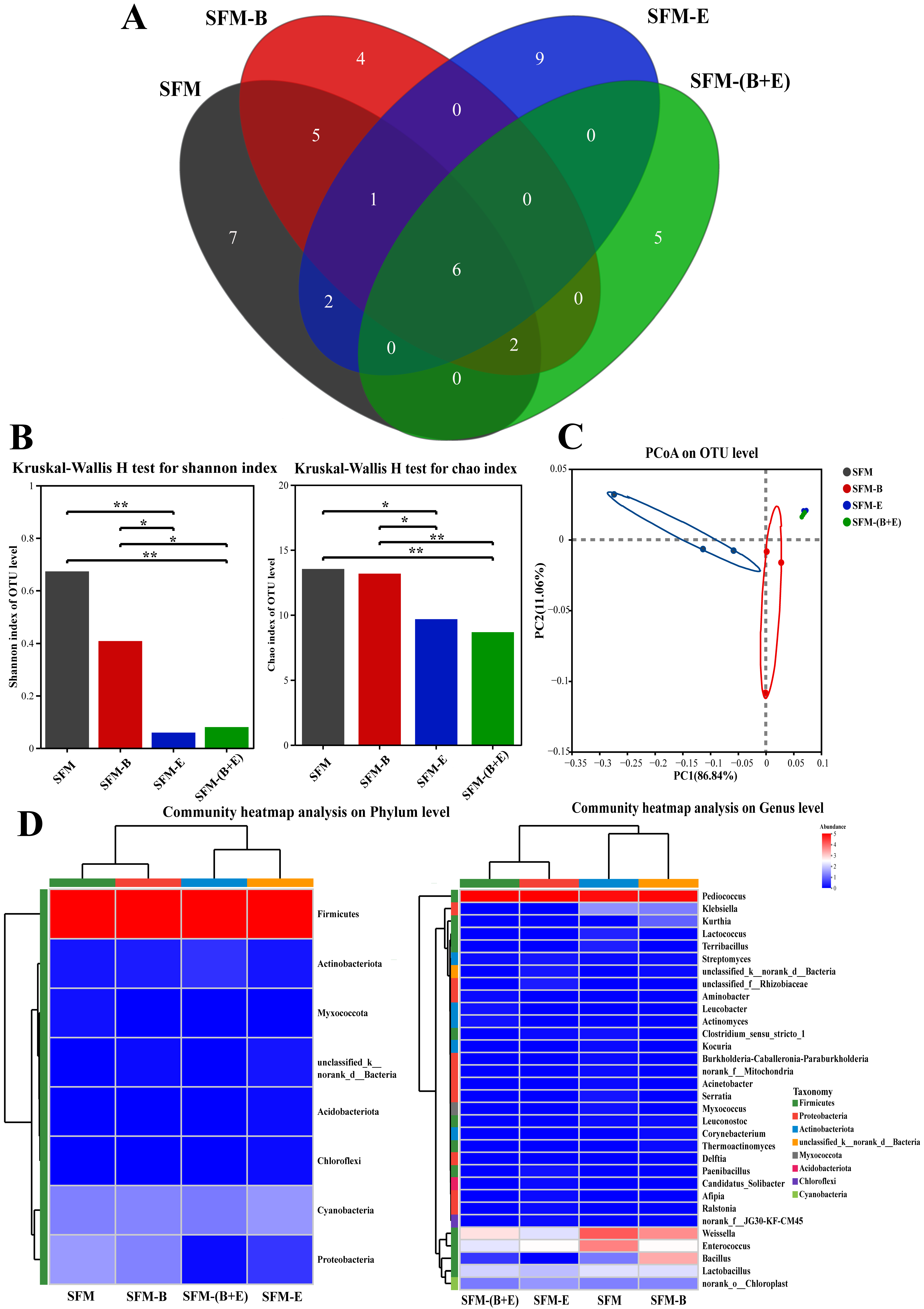
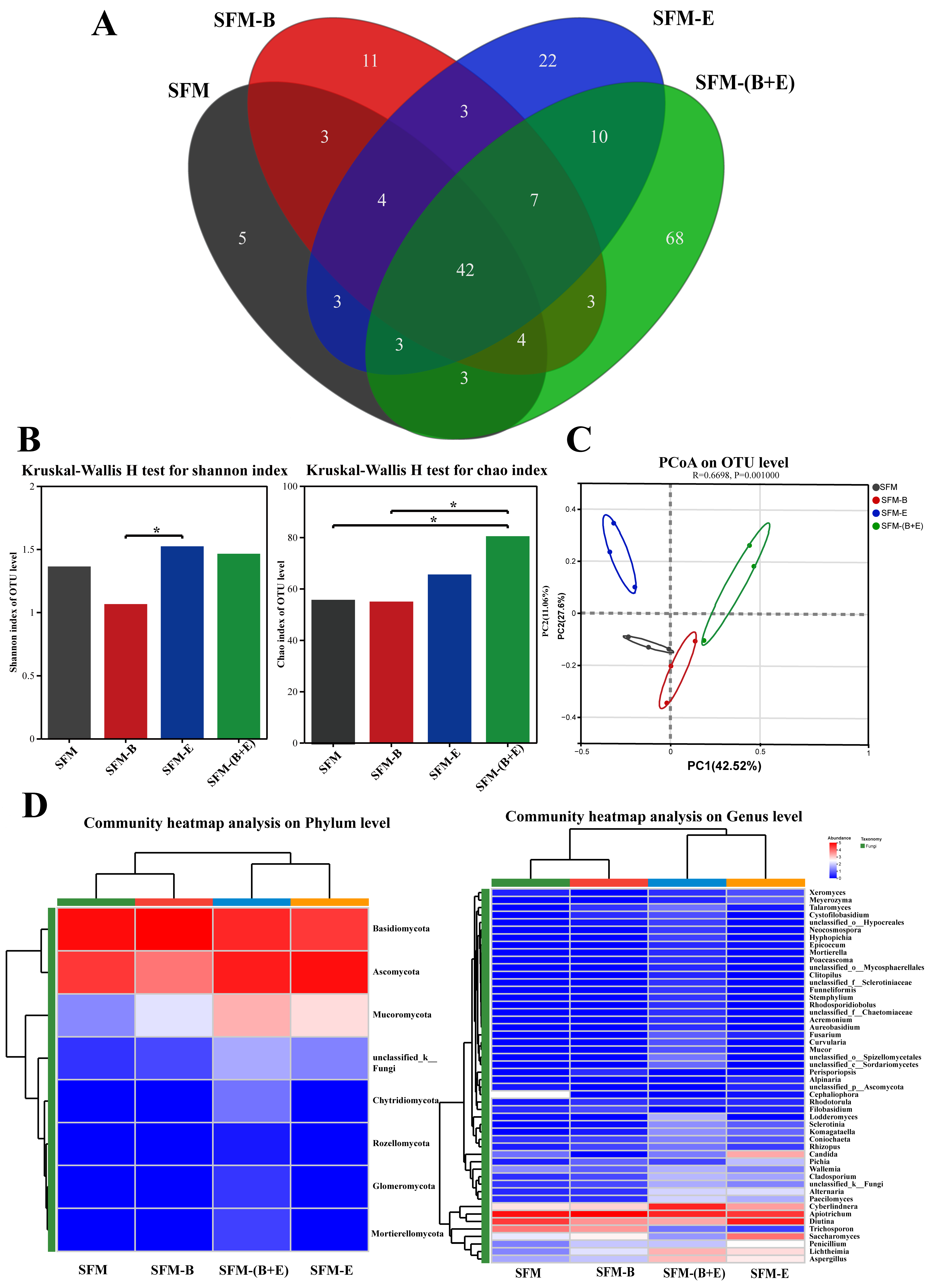
3.3. Microbial Community Analysis
3.3.1. Bacterial Community Analysis
3.3.2. Fungal Community Analysis
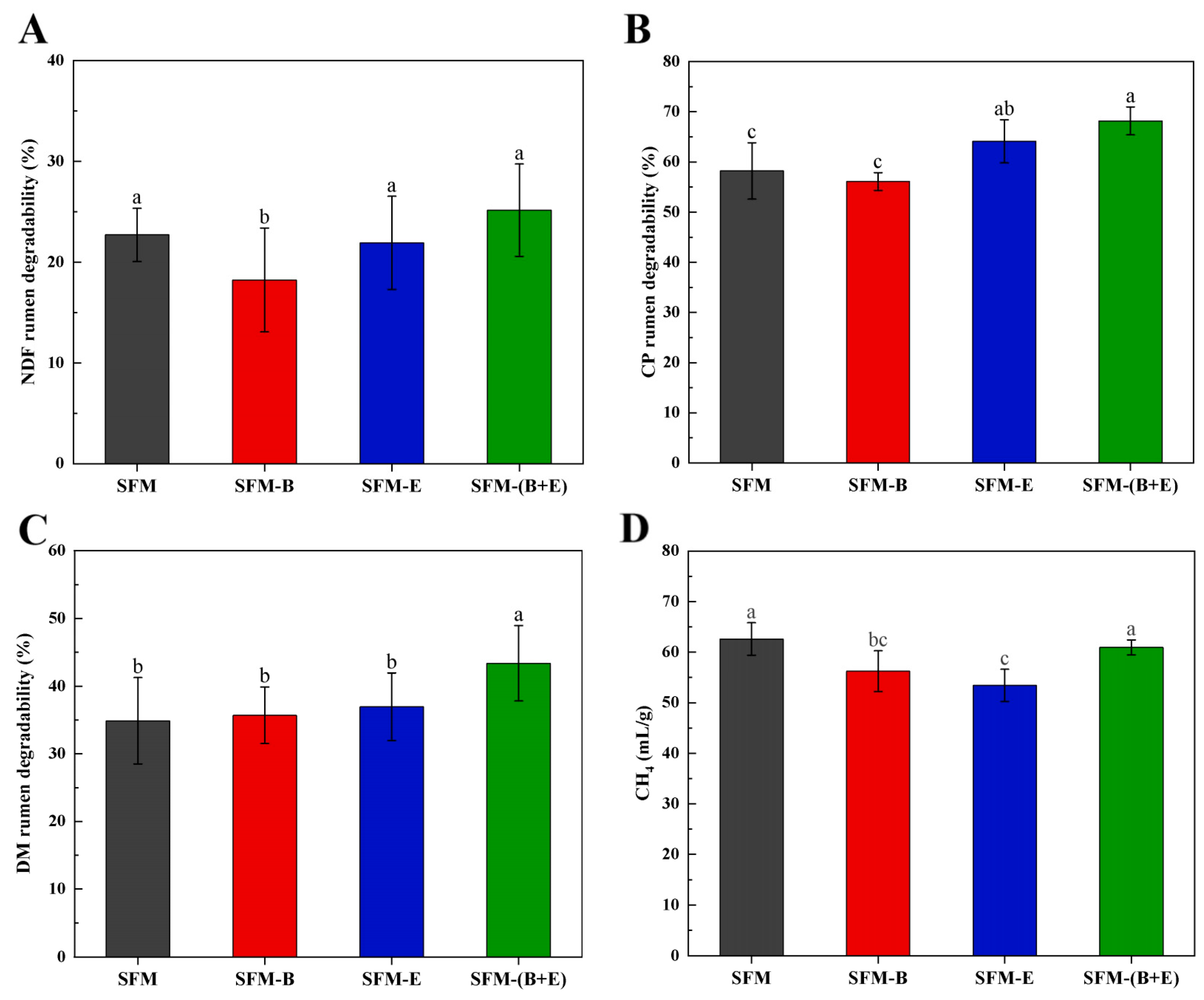
3.4. Results of In Vitro Digestibility Test
4. Discussion
4.1. Chemical Composition
4.2. Physical Structure
4.3. Microbial Community
4.4. In Vitro Digestibility and Methane Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SFM | Sunflower meal |
| SBM | Soybean meal |
| SSF | Solid-state fermentation |
| CF | Crude fiber |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
| CP | Crude protein |
| ASP | Acid-soluble protein |
| RS | Reducing sugar |
| CA | Crude ash |
| CGA | Chlorogenic acid |
| OTU | Operational taxonomic unit |
| DM | Dry matter |
| PC | Primary component |
References
- Pirgozliev, V.R.; Whiting, I.M.S.; Mansbridge, C.; Rose, S.P. Sunflower and rapeseed meal as alternative feed materials to soybean meal for sustainable egg production, using aged laying hens. Br. Poult. Sci. 2023, 64, 634–640. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; Mcclements, D.J. Sunflower meal/cake as a sustainable protein source for global food demand: Towards a zero-hunger world. Food Hydrocolloid 2024, 147 Pt A, 109329. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Mohammady, E.Y.; Elashry, M.A.; El-Haroun, E.R.; Davies, S.J. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 2018, 495, 592–601. [Google Scholar] [CrossRef]
- Mbukwane, M.J.; Nkukwana, T.T.; Plumstead, P.W.; Snyman, N. Sunflower meal inclusion rate and the effect of exogenous enzymes on growth performance of broiler chickens. Animals 2022, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Eide, L.H.; Morales-Lange, B.; Kuiper, R.V.; Dale, O.B.; Rocha, S.D.C.; Djordjevic, B.; Øverland, M. Fermented sunflower meal in diets for Atlantic salmon under commercial-like farming conditions promotes gut lactic acid bacteria and controls inflammation in the distal intestine. Aquaculture 2025, 595, 741517. [Google Scholar] [CrossRef]
- Njeri, F.M.; Patterson, R.; Gachuiri, C.K.; Kiarie, E.G. Effects of pretreating wheat middlings and sunflower meal with fiber degrading enzymes on components solubilization and utilization in broiler chickens. Transl. Anim. Sci. 2023, 7, txad108. [Google Scholar] [CrossRef] [PubMed]
- Simović, M.; Banjanac, K.; Veljković, M.; Nikolić, V.; López-Revenga, P.; Montilla, A.; Moreno, F.J.; Bezbradica, D. Sunflower meal valorization through enzyme-aided fractionation and the production of emerging prebiotics. Foods 2024, 13, 2506. [Google Scholar] [CrossRef]
- Karunaratne, N.D.; Classen, H.L.; van Kessel, A.G.; Bedford, M.R.; Ames, N.P.; Newkirk, R.W. Diet medication and beta-glucanase affect ileal digesta soluble beta-glucan molecular weight, carbohydrate fermentation, and performance of coccidiosis vaccinated broiler chickens given wheat-based diets. Anim. Nutr. 2023, 15, 288–296. [Google Scholar] [CrossRef]
- Shakarami, M.H.; Mohammadabadi, T.; Motamedi, H.; Sari, M.; Teimouri, A.Y. Isolation and identification of cellulolytic bacteria from gastrointestinal tract of Arabian horse and investigation of their effect on the nutritional value of wheat straw. J. Appl. Microbiol. 2019, 127, 344–353. [Google Scholar] [CrossRef]
- Vieira, L.; Filipe, D.; Amaral, D.; Magalhães, R.; Martins, N.; Ferreira, M.; Ozorio, R.; Salgado, J.; Belo, I.; Oliva-Teles, A.; et al. Solid-state fermentation as green technology to improve the use of plant feedstuffs as ingredients in diets for European sea bass (Dicentrarchus labrax) juveniles. Animals 2023, 13, 2692. [Google Scholar] [CrossRef]
- Soltan, M.A.; Hassaan, M.S.; Abdella, M.S.; El-Syaad, G.A.; El-Ashry, M.A. Yeast fermented sunflower meal as a replacer for fish meal in diets of the Nile tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2015, 19, 65–72. [Google Scholar] [CrossRef]
- Dharmakar, P.; Aanand, S.; Sampath Kumar, J.S.; Ande, M.P.; Padmavathy, P.; Pereira, J.J. Solid-state fermentation of sunflower meal using commercial yeast for use as an improved nutrient source in aquafeed. Int. J. Appl. Res. 2022, 8, 375–378. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhang, Z.; Ding, L.; Hang, S. Combination of fiber-degrading enzymatic hydrolysis and lactobacilli fermentation enhances utilization of fiber and protein in rapeseed meal as revealed in simulated pig digestion and fermentation in vitro. Anim. Feed Sci. Technol. 2021, 278, 115001. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, X.; Bao, X.; Li, M.; Gao, Y.; Qin, S.; Yang, H.; Pu, L.; Hong, L.; Zhang, J. Effects of bacterial enzyme cooperative fermentation diet on growth performance, blood biochemical indices, and fecal microflora of growing—Finishing pigs. Fermentation 2024, 10, 610. [Google Scholar] [CrossRef]
- GB/T 22492-2008; Soybean Protein Flour. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 6438-2025; Determination of Crude Ash in Feeds. Standardization Administration of the People’s Republic of China: Beijing, China, 2025.
- González-Pérez, S.; Merck, K.B.; Vereijken, J.M.; van Koningsveld, G.A.; Gruppen, H.; Voragen, A.G.J. Isolation and characterization of undenatured chlorogenic acid free sunflower (Helianthus annuus) proteins. J. Agric. Food Chem. 2002, 50, 1713–1719. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Phesatcha, B.; Phesatcha, K.; Viennaxay, B.; Matra, M.; Totakul, P.; Wanapat, M. Cricket meal (Gryllus bimaculatus) as a protein supplement on in vitro fermentation characteristics and methane mitigation. Insects 2022, 13, 129. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Li, J.; Zhu, B.; Gao, T. Anaerobic solid-state fermentation of soybean meal with Bacillus sp. to improve nutritional quality. Front. Nutr. 2021, 8, 706977. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, B.; Liu, G.; Shi, H.; Wang, J. Effect of Bacillus subtilis and Lactobacillus plantarum on solid-state fermentation of soybean meal. J. Sci. Food Agric. 2023, 103, 6070–6079. [Google Scholar] [CrossRef]
- Lu, D.L.; Zhang, M.S.; Wang, F.B.; Dai, Z.J.; Li, Z.W.; Ni, J.T.; Feng, W.J.; Zhang, F.G.; Dai, J.; Wang, H.N.; et al. Nutritional value improvement of soybean meal through solid-state fermentation by proteases-enhanced Streptomyces sp. SCUT-3. Int. J. Biol. Macromol. 2025, 298, 140035. [Google Scholar] [CrossRef]
- Du, Z.; Yamasaki, S.; Oya, T.; Cai, Y. Cellulase-lactic acid bacteria synergy action regulates silage fermentation of woody plant. Biotechnol. Biofuels Bioprod. 2023, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Xu, L.; Tian, Y.; Lv, M.; Fang, P.; Dong, K.; Lin, Q.; Cao, Z. Effects of synergistic fermentation of tea bee pollen with bacteria and enzymes on growth and intestinal health of Apis cerana cerana. Curr. Res. Microb. Sci. 2025, 8, 100343. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 155, 494–507. [Google Scholar] [CrossRef]
- GB 10376-1989; Sunflower Meal (Solvent) for Feedstuffs. Standardization Administration of the People’s Republic of China: Beijing, China, 1989.
- Announcement No. 217; Feedadditive-Chlorogenicacid. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- Bongartz, V.; Böttger, C.; Wilhelmy, N.; Schulze-Kaysers, N.; Südekum, K.-H.; Schieber, A. Protection of protein from ruminal degradation by alkali-induced oxidation of chlorogenic acid in sunflower meal. J. Anim. Physiol. Anim. Nutr. 2017, 102, e209–e215. [Google Scholar] [CrossRef]
- Verde, C.L.; Pacioles, C.T.; Paterson, N.; Chin, J.; Owens, C.P.; Senger, L.W. Hydrolysis of chlorogenic acid in sunflower flour increases consumer acceptability of sunflower flour cookies by improving cookie color. J. Food Sci. 2023, 88, 3538–3550. [Google Scholar] [CrossRef]
- Lin, B.; Yan, J.; Zhong, Z.; Zheng, X. A Study on the preparation of microbial and nonstarch polysaccharide enzyme synergistic fermented maize cob feed and its feeding efficiency in finishing pigs. BioMed Res. Int. 2020, 2020, 8839148. [Google Scholar] [CrossRef]
- Ma, L.L.; Lu, Y.Y.; Yan, H.; Wang, X.; Yi, Y.L.; Shan, Y.Y.; Liu, B.F.; Zhou, Y.; Lü, X. Screening of cellulolytic vacteria from rotten wood of Qinling (China) for biomass degradation and cloning of cellulases from Bacillus methylotrophicus. BMC Biotechnol. 2020, 20, 2. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohydr. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef]
- Sreechithra, T.V.; Sakhare, S.D. Impact of processing techniques on the nutritional quality, antinutrients, and in vitro protein digestibility of milled soybean fractions. Food Chem. 2025, 485, 144565. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, X.; Chen, C.; Ma, H.; Tan, Z.; Zhang, M.; Duan, Y.; Qin, G.; Wang, Y.; Jiao, Z.; et al. Combined effects of lactic acid bacteria and protease on the fermentation quality and microbial community during 50 Kg soybean meal fermentation simulating actual production scale. Microorganisms 2024, 12, 1339. [Google Scholar] [CrossRef]
- Kidane, A.; Gregersen Vhile, S.; Ferneborg, S.; Skeie, S.; Olsen, M.A.; Torunn Mydland, L.; Øverland, M.; Prestl Kken, E. Cyberlindnera jadinii yeast as a protein source in early- to mid-lactation dairy cow diets: Effects on feed intake, ruminal fermentation, and milk production. J. Dairy Sci. 2022, 105, 2343–2353. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, H.; Liu, K.; Wu, Y.; Zhang, B.; Ma, C.; Liu, H. Effect of Cyberlindnera jadinii supplementation on growth performance, serum immunity, antioxidant status, and intestinal health in winter fur growing raccoon dogs (Nyctereutes procyonoides). Front. Vet. Sci. 2023, 10, 1154808. [Google Scholar] [CrossRef] [PubMed]
- Itani, K.; Marcussen, C.; Rocha, S.D.C.; Kathiresan, P.; Mydland, L.T.; Press, C.M.; Xie, Z.; Tauson, A.H.; Øverland, M. Effect of Cyberlindnera jadinii yeast on growth performance, nutrient digestibility, and gut health of broiler chickens from 1 to 34 d of age. Poultry Sci. 2023, 102, 103127. [Google Scholar] [CrossRef] [PubMed]
- Jannathulla, R.; Dayal, J.S.; Ambasankar, K.; Muralidhar, M. Effect of Aspergillus niger fermented soybean meal and sunflower oil cake on growth, carcass composition and haemolymph indices in Penaeus vannamei Boone, 1931. Aquaculture 2018, 486, 1–8. [Google Scholar] [CrossRef]
- Scheftgen, A.J.; Skarlupka, J.H.; Jewell, K.A.; Suen, G. Correlating feed efficiency with ruminal bacterial, fungal, and archaeal community composition in dairy cows over two lactations. Dairy 2025, 6, 8. [Google Scholar] [CrossRef]
- Morsy, T.A.; Gouda, G.A.; Kholif, A.E. In vitro fermentation and production of methane and carbon dioxide from rations containing Moringa oleifera leave silage as a replacement of soybean meal: In vitro assessment. Environ. Sci. Pollut. Res. 2022, 29, 69743–69752. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Shi, H.; Zhao, P.; Ma, Z.; Li, X.; Wang, X.; Xue, F. Synergistic Effect of Bacillus subtilis B3 and β-Glucanase on Solid-State Fermentation of Sunflower Meal. BioTech 2025, 14, 92. https://doi.org/10.3390/biotech14040092
Chen S, Shi H, Zhao P, Ma Z, Li X, Wang X, Xue F. Synergistic Effect of Bacillus subtilis B3 and β-Glucanase on Solid-State Fermentation of Sunflower Meal. BioTech. 2025; 14(4):92. https://doi.org/10.3390/biotech14040092
Chicago/Turabian StyleChen, Shuqi, Haoran Shi, Peng Zhao, Zengqiang Ma, Xiaolong Li, Xiangyu Wang, and Feiyan Xue. 2025. "Synergistic Effect of Bacillus subtilis B3 and β-Glucanase on Solid-State Fermentation of Sunflower Meal" BioTech 14, no. 4: 92. https://doi.org/10.3390/biotech14040092
APA StyleChen, S., Shi, H., Zhao, P., Ma, Z., Li, X., Wang, X., & Xue, F. (2025). Synergistic Effect of Bacillus subtilis B3 and β-Glucanase on Solid-State Fermentation of Sunflower Meal. BioTech, 14(4), 92. https://doi.org/10.3390/biotech14040092




