Peptide Inhibitor Assay for Allocating Functionally Important Accessible Sites Throughout a Protein Chain: Restriction Endonuclease EcoRI as a Model Protein System
Abstract
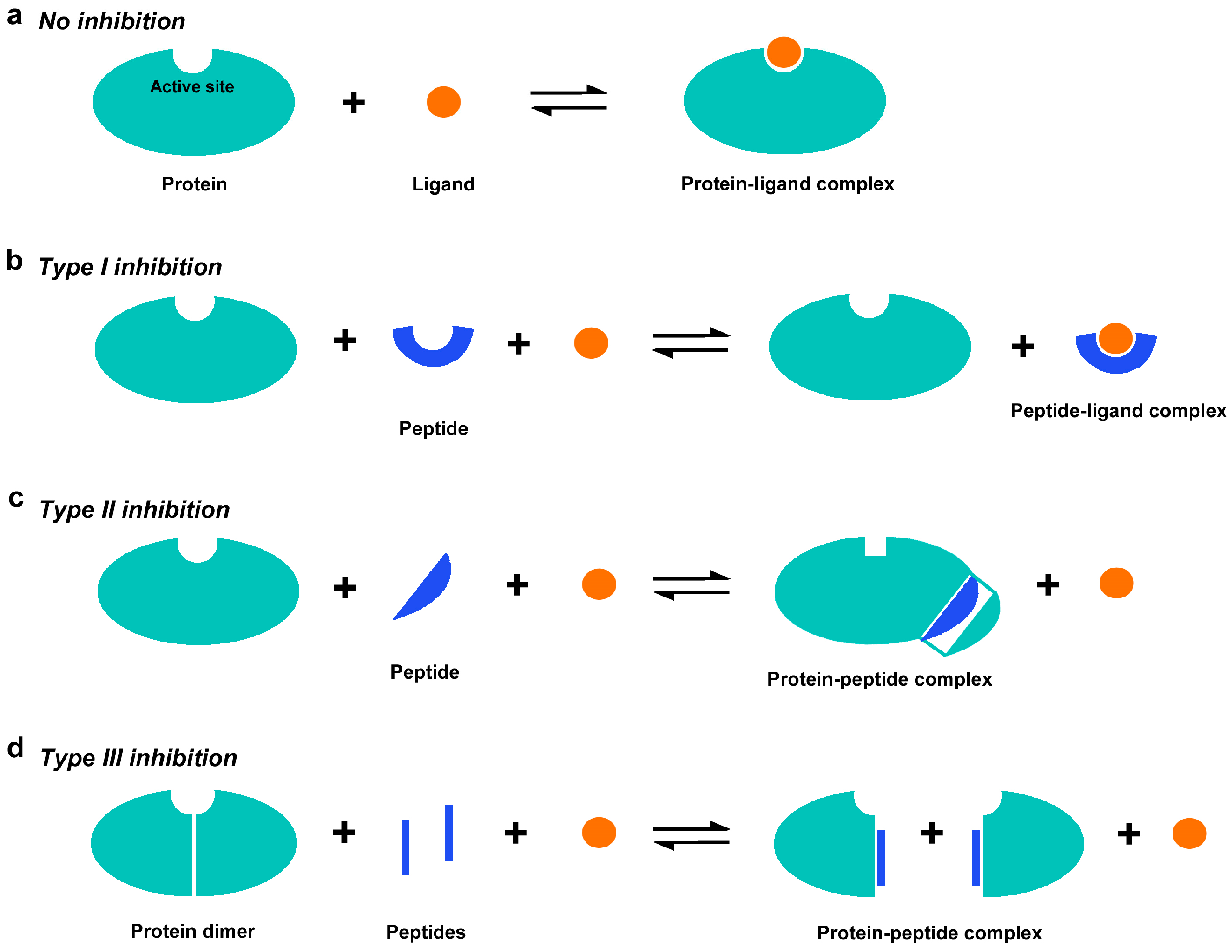
1. Introduction
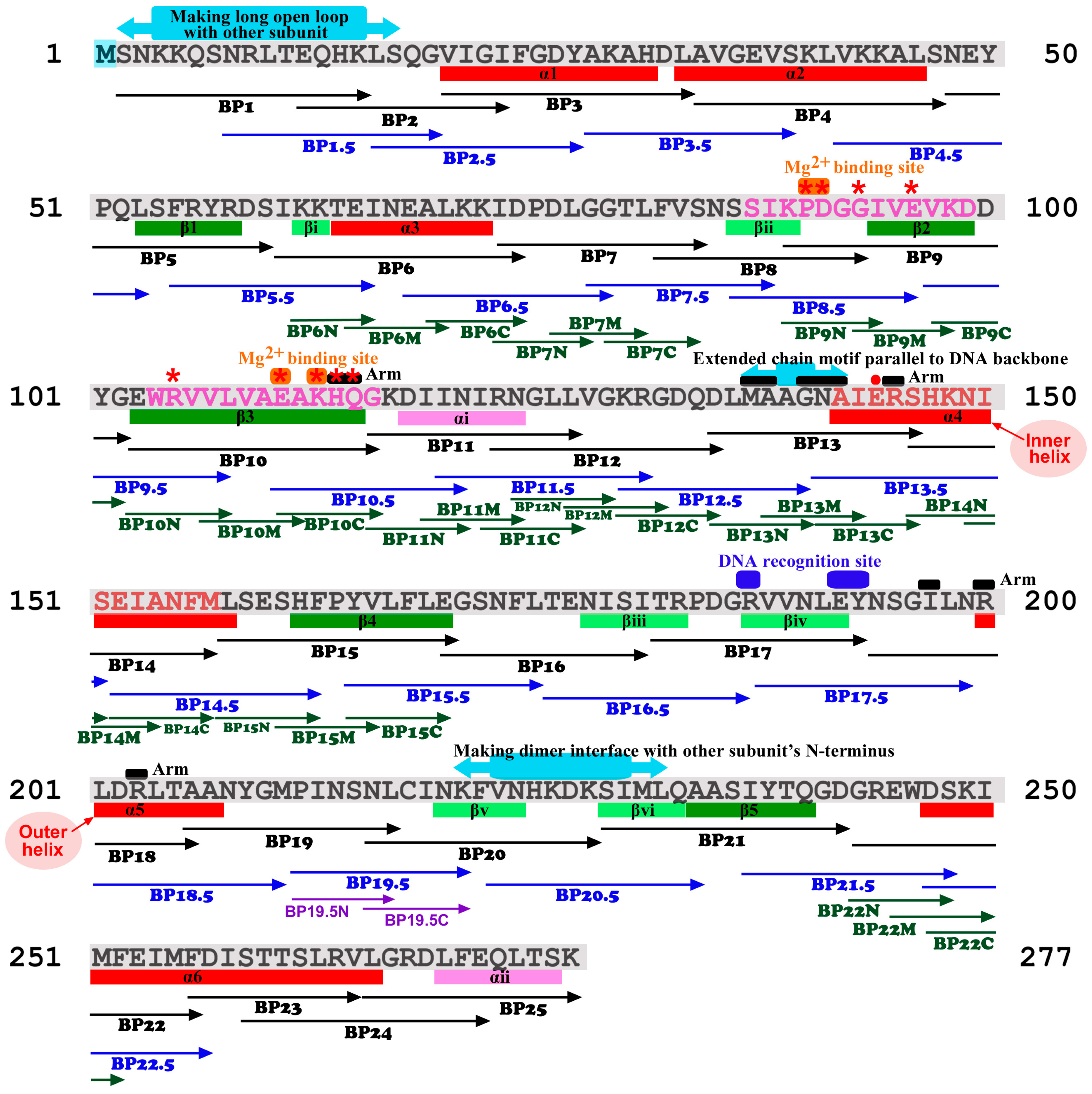
2. Materials and Methods
2.1. Plasmid
2.2. EcoRI Sequence and Synthetic Peptides
2.3. Enzymatic Digestion with EcoRI
2.4. Quantification of EcoRI Activity
2.5. Statistics
3. Results
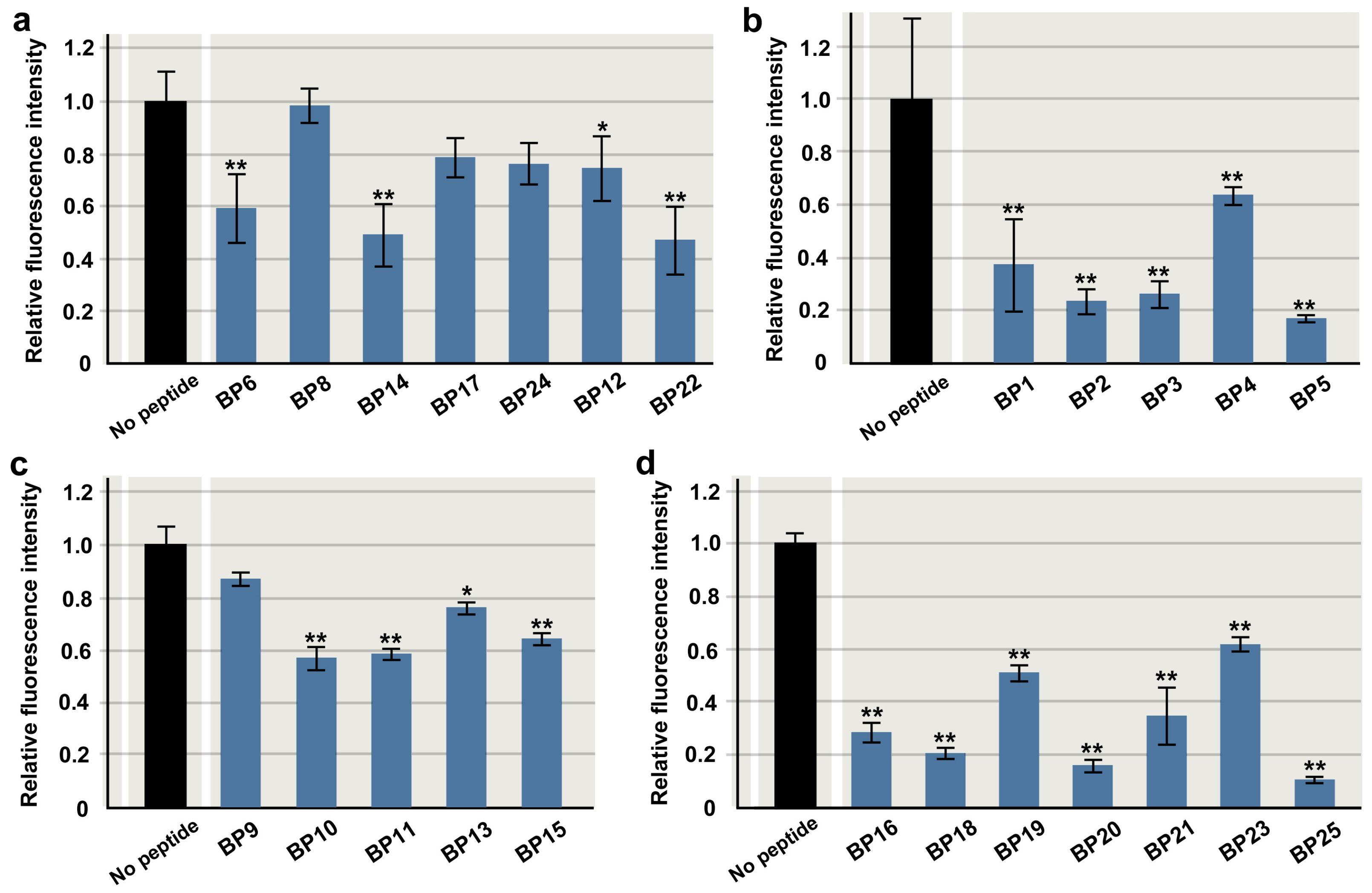
3.1. Initially Chosen Seven Peptides
3.2. Amino-Terminus Five Peptides
3.3. Other 13 Peptides to Cover the Entire Protein Chain
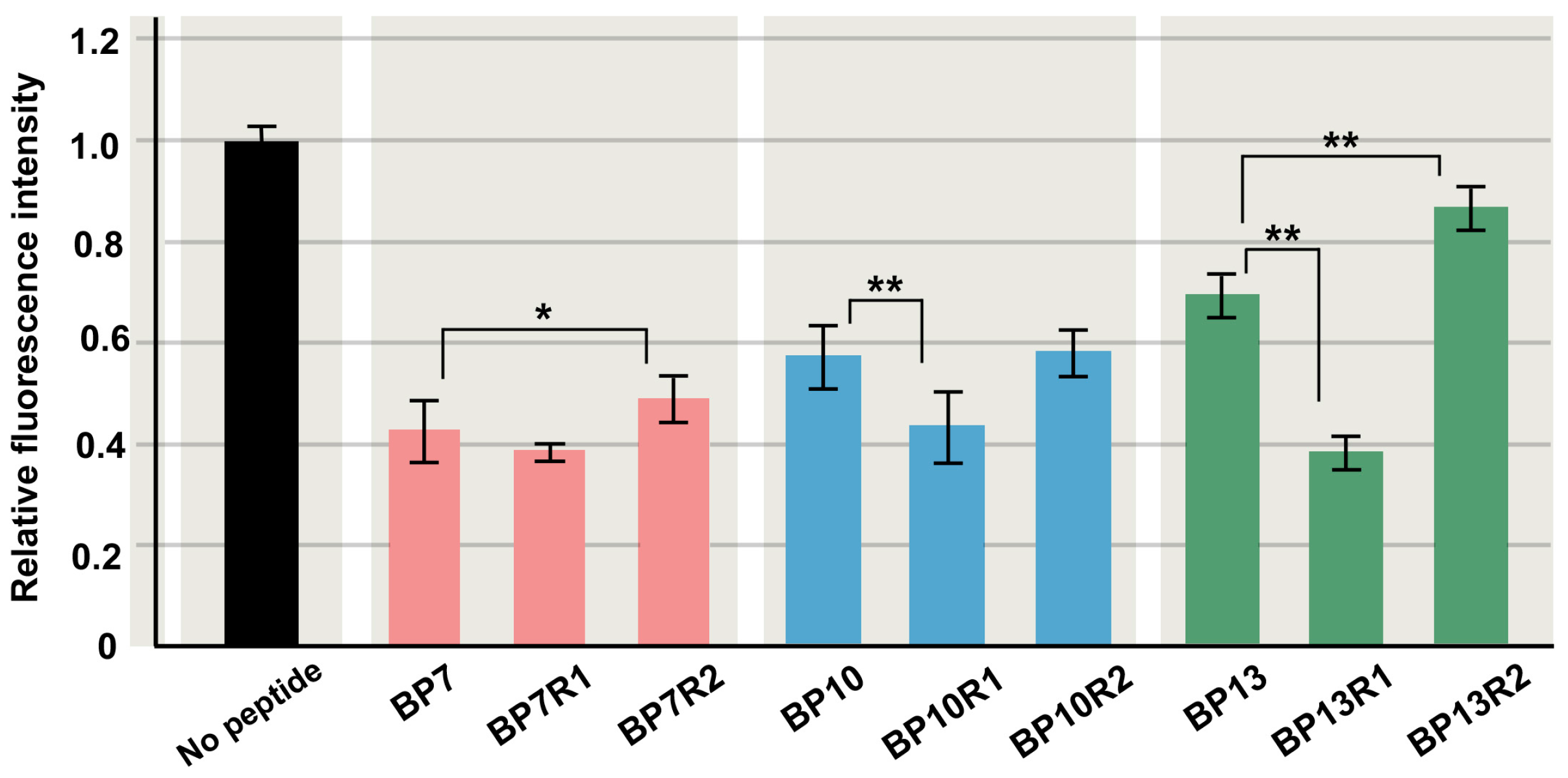
3.4. Randomized Peptides with the Same Amino Acid Contents
3.5. Additional 22 Staggered Peptides
3.6. Nonspecific Nuclease Activity with BP19.5
3.7. Short Peptides
3.8. Overview of the Inhibitory Effects
3.9. Potential Correlations Among Physicochemical Factors
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Liddington, R.C. Structural basis of protein-protein interactions. Methods Mol. Biol. 2004, 261, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kieber-Emmons, T.; Murali, R.; Greene, M.I. Therapeutic peptides and peptidomimetics. Curr. Opin. Biotechnol. 1997, 8, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ripka, A.S.; Rich, D.H. Peptidomimetic design. Curr. Opin. Chem. Biol. 1998, 2, 441–452. [Google Scholar] [CrossRef]
- Ko, E.; Liu, J.; Burgess, K. Minimalist and universal peptidomimetics. Chem. Soc. Rev. 2011, 40, 4411–4421. [Google Scholar] [CrossRef]
- Perez, J.J. Designing peptidomimetics. Curr. Top. Med. Chem. 2018, 18, 566–590. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. Mechanisms inspired targeting peptides. Adv. Exp. Med. Biol. 2020, 1248, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef]
- Reese, H.R.; Shanahan, C.C.; Proulx, C.; Menegatti, S. Peptide science: A “rule model” for new generations of peptidomimetics. Acta Biomater. 2020, 102, 35–74. [Google Scholar] [CrossRef]
- Eichler, J. Synthetic peptide arrays and peptide combinatorial libraries for the exploration of protein-ligand interactions and the design of protein inhibitors. Comb. Chem. High Throughput Screen. 2005, 8, 135–143. [Google Scholar] [CrossRef]
- Boschetti, E.; Righetti, P.G. The art of observing rare protein species in proteomes with peptide ligand libraries. Proteomics 2009, 9, 1492–1510. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.W.; Muttenthaler, M.; Freissmuth, M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr. Pharm. Des. 2010, 16, 3071–3088. [Google Scholar] [CrossRef]
- Peczuh, M.W.; Hamilton, A.D. Peptide and protein recognition by designed molecules. Chem. Rev. 2000, 100, 2479–2494. [Google Scholar] [CrossRef]
- Veselovsky, A.V.; Ivanov, Y.D.; Ivanov, A.S.; Archakov, A.I.; Lewi, P.; Janssen, P. Protein-protein interactions: Mechanisms and modification by drugs. J. Mol. Recognit. 2002, 15, 405–422. [Google Scholar] [CrossRef]
- Sillerud, L.O.; Larson, R.S. Design and structure of peptide and peptidomimetic antagonists of protein-protein interaction. Curr. Protein Pept. Sci. 2005, 6, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Salo-Ahen, O.M.; Ferrari, S.; Ponterini, G.; Cruciani, G.; Carosati, E.; Tochowicz, A.M.; Mangani, S.; Wade, R.C.; Costi, M.P. Homodimeric enzymes as drug targets. Curr. Med. Chem. 2010, 17, 826–846. [Google Scholar] [CrossRef] [PubMed]
- Groner, B.; Weber, A.; Mack, L. Increasing the range of drug targets: Interacting peptides provide leads for the development of oncoprotein inhibitors. Bioengineered 2012, 3, 320–325. [Google Scholar] [CrossRef]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of α-helix-mediated protein-protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef]
- Bowman, M.J.; Chmielewski, J. Novel strategies for targeting the dimerization interface of HIV protease with cross-linked interfacial peptides. Biopolymers 2002, 66, 126–133. [Google Scholar] [CrossRef]
- Lowman, H.B. Bacteriophage display and discovery of peptide leads for drug development. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 401–424. [Google Scholar] [CrossRef]
- Andrieu, J.; Re, F.; Russo, L.; Nicotra, F. Phage-displayed peptides targeting specific tissues and organs. J. Drug Target. 2019, 27, 555–565. [Google Scholar] [CrossRef]
- Vanhee, P.; van der Sloot, A.M.; Verschueren, E.; Serrano, L.; Rousseau, F.; Schymkowitz, J. Computational design of peptide ligands. Trends Biotechnol. 2011, 29, 231–239. [Google Scholar] [CrossRef]
- London, N.; Raveh, B.; Schueler-Furman, O. Peptide docking and structure-based characterization of peptide binding: From knowledge to know-how. Curr. Opin. Struct. Biol. 2013, 23, 894–902. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-based design of inhibitors of protein-protein interactions: Mimicking peptide binding epitopes. Angew Chem. Int. Ed. Engl. 2015, 54, 8896–8927. [Google Scholar] [CrossRef]
- Kilburg, D.; Gallicchio, E. Recent advances in computational models for the study of protein-peptide interactions. Adv. Protein Chem. Struct. Biol. 2016, 105, 27–57. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.A.; Deber, C.M. Therapeutic design of peptide modulators of protein-protein interactions in membranes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 577–585. [Google Scholar] [CrossRef]
- Wichapong, K.; Poelman, H.; Ercig, B.; Hrdinova, J.; Liu, X.; Lutgens, E.; Nicolaes, G.A. Rational modulator design by exploitation of protein-protein complex structures. Future Med. Chem. 2019, 11, 1015–1033. [Google Scholar] [CrossRef] [PubMed]
- Kanakaveti, V.; Shanmugam, A.; Ramakrishnan, C.; Anoosha, P.; Sakthivel, R.; Rayala, S.K.; Gromiha, M.M. Computational approaches for identifying potential inhibitors on targeting protein interactions in drug discovery. Adv. Protein Chem. Struct. Biol. 2020, 121, 25–47. [Google Scholar] [CrossRef]
- Singh, S.S.; Jois, S.D. Homo- and heterodimerization of proteins in cell signaling: Inhibition and drug design. Adv. Protein Chem. Struct. Biol. 2018, 111, 1–59. [Google Scholar] [CrossRef]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.; Meyer, E.F., Jr.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M. The Protein Data Bank: A computer-based archival file for macromolecular structures. J. Mol. Biol. 1977, 112, 535–542. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Bryant, P.; Pozzati, G.; Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gillam, S.; Smith, M. Site-specific mutagenesis using synthetic oligodeoxyribonucleotide primers: I. Optimum conditions and minimum ologodeoxyribonucleotide length. Gene 1979, 8, 81–97. [Google Scholar] [CrossRef]
- Gillam, S.; Smith, M. Site-specific mutagenesis using synthetic oligodeoxyribonucleotide primers: II. In vitro selection of mutant DNA. Gene 1979, 8, 99–106. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Bondos, S.E.; Dunker, A.K.; Uversky, V.N. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Commun. Signal. 2022, 20, 20. [Google Scholar] [CrossRef]
- Kulkarni, P.; Bhattacharya, S.; Achuthan, S.; Behal, A.; Jolly, M.K.; Kotnala, S.; Mohanty, A.; Rangarajan, G.; Salgia, R.; Uversky, V. Intrinsically disordered proteins: Critical components of the wetware. Chem. Rev. 2022, 122, 6614–6633. [Google Scholar] [CrossRef]
- Trivedi, R.; Nagarajaram, H.A. Intrinsically disordered proteins: An overview. Int. J. Mol. Sci. 2022, 23, 14050. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Prediction of protein confirmation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Garnier, J.; Osguthorpe, D.J.; Robson, B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J. Mol. Biol. 1978, 120, 97–120. [Google Scholar] [CrossRef]
- de Brevern, A.G.; Etchebest, C.; Hazout, S. Bayesian probabilistic approach for predicting backbone structures in terms of protein blocks. Proteins 2000, 41, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.C. Prediction of protein cellular attributes using pseudo amino acid composition. Proteins 2001, 43, 246–255. [Google Scholar] [CrossRef]
- de Brevern, A.G.; Valadié, H.; Hazout, S.; Etchebest, C. Extension of a local backbone description using a structural alphabet: A new approach to the sequence-structure relationship. Protein Sci. 2002, 11, 2871–2886. [Google Scholar] [CrossRef] [PubMed]
- Figureau, A.; Soto, M.A.; Tohá, J. A pentapeptide-based method for protein secondary structure prediction. Protein Eng. 2003, 16, 103–107. [Google Scholar] [CrossRef]
- Otaki, J.M.; Ienaka, S.; Gotoh, T.; Yamamoto, H. Availability of short amino acid sequences in proteins. Protein Sci. 2005, 14, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Vries, J.K.; Liu, X.; Bahar, I. The relationship between n-gram patterns and protein secondary structure. Proteins 2007, 68, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Tsutsumi, M.; Gotoh, T.; Yamamoto, H. Secondary structure characterization based on amino acid composition and availability in proteins. J. Chem. Inf. Model. 2010, 50, 690–700. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Otaki, J.M. Parallel and antiparallel β-strands differ in amino acid composition and availability of short constituent sequences. J. Chem. Inf. Model. 2011, 51, 1457–1464. [Google Scholar] [CrossRef]
- Kanduc, D. Pentapeptides as minimal functional units in cell biology and immunology. Curr. Protein Pept. Sci. 2013, 14, 111–120. [Google Scholar] [CrossRef]
- Motomura, K.; Nakamura, M.; Otaki, J.M. A frequency-based linguistic approach to protein decoding and design: Simple concepts, diverse applications, and the SCS Package. Comput. Struct. Biotechnol. J. 2013, 5, e201302010. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tanwar, D.K.; Penha, E.D.S.; Wold, Y.I.; Koonin, E.V.; Basu, M.K. Grammar of protein domain architectures. Proc. Natl. Acad. Sci. USA 2019, 116, 3636–3645. [Google Scholar] [CrossRef]
- Simons, K.T.; Kooperberg, C.; Huang, E.; Baker, D. Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. J. Mol. Biol. 1997, 268, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.T.; Bonneau, R.; Ruczinski, I.; Baker, D. Ab initio protein structure prediction of CASP III targets using ROSETTA. Proteins 1999, 37 (Suppl. S3), 171–176. [Google Scholar] [CrossRef]
- Loenen, W.A.; Dryden, D.T.; Raleigh, E.A.; Wilson, G.G.; Murray, N.E. Highlights of the DNA cutters: A short history of the restriction enzymes. Nucleic Acids Res. 2014, 42, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Pingoud, A.; Jeltsch, A. Recognition and cleavage of DNA by type-II restriction endonucleases. Eur. J. Biochem. 1997, 246, 1–22. [Google Scholar] [CrossRef]
- Wright, D.J.; Jack, W.E.; Modrich, P. The kinetic mechanism of EcoRI endonuclease. J. Biol. Chem. 1999, 274, 31896–31902. [Google Scholar] [CrossRef] [PubMed]
- Piatt, S.C.; Loparo, J.J.; Price, A.C. The role of noncognate sites in the 1D search mechanism of EcoRI. Biophys. J. 2019, 116, 2367–2377. [Google Scholar] [CrossRef]
- D’Acunto, M. Protein-DNA target search relies on quantum walk. BioSystems 2021, 201, 104340. [Google Scholar] [CrossRef] [PubMed]
- McClarin, J.A.; Frederick, C.A.; Wang, B.C.; Greene, P.; Boyer, H.W.; Grable, J.; Rosenberg, J.M. Structure of the DNA-Eco RI endonuclease recognition complex at 3 Å resolution. Science 1986, 234, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Grable, J.C.; Love, R.; Greene, P.J.; Rosenberg, J.M. Refinement of Eco RI endonuclease crystal structure: A revised protein chain tracing. Science 1990, 249, 1307–1309. [Google Scholar] [CrossRef]
- Heitman, J. How the EcoRI endonuclease recognizes and cleaves DNA. BioEssays 1992, 14, 445–454. [Google Scholar] [CrossRef]
- Wolfes, H.; Alves, J.; Fliess, A.; Geiger, R.; Pingoud, A. Site directed mutagenesis experiments suggest that Glu 111, Glu 144 and Arg 145 are essential for endonucleolytic activity of EcoRI. Nucleic Acids Res. 1986, 14, 9063–9080. [Google Scholar] [CrossRef]
- Geiger, R.; Rüter, T.; Alves, J.; Fliess, A.; Wolfes, H.; Pingoud, V.; Urbanke, C.; Maass, G.; Pingoud, A.; Düsterhöft, A.; et al. Genetic engineering of EcoRI mutants with altered amino acid residues in the DNA binding site: Physicochemical investigations give evidence for an altered monomer/dimer equilibrium for the Gln144Lys145 and Gln144Lys145Lys200 mutants. Biochemistry 1989, 28, 2667–2677. [Google Scholar] [CrossRef]
- Alves, J.; Rüter, T.; Geiger, R.; Fliess, A.; Maass, G.; Pingoud, A. Changing the hydrogen-bonding potential in the DNA binding site of EcoRI by site-directed mutagenesis drastically reduces the enzymatic activity, not, however, the preference of this restriction endonuclease for cleavage within the site-GAATTC-. Biochemistry 1989, 28, 2678–2684. [Google Scholar] [CrossRef]
- Oelgeschläger, T.; Geiger, R.; Rüter, T.; Alves, J.; Fliess, A.; Pingoud, A. Probing the function of individual amino acid residues in the DNA binding site of the EcoRI restriction endonuclease by analysing the toxicity of genetically engineered mutants. Gene 1990, 89, 19–27. [Google Scholar] [CrossRef]
- Grabowski, G.; Maass, G.; Alves, J. Asp-59 is not important for the catalytic activity of the restriction endonuclease EcoRI. FEBS Lett. 1996, 381, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, T.; Heitman, J.; Kiss, A. Mutational analysis of the function of Met137 and Ile197, two amino acids implicated in sequence-specific DNA recognition by the EcoRI endonuclease. Biol. Chem. 1998, 379, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A.; Alves, J.; Oelgeschläger, T.; Wolfes, H.; Maass, G.; Pingoud, A. Mutational analysis of the function of Gln115 in the EcoRI restriction endonuclease, a critical amino acid for recognition of the inner thymidine residue in the sequence -GAATTC- and for coupling specific DNA binding to catalysis. J. Mol. Biol. 1993, 229, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Jeltsch, A.; Wolfes, H.; Maass, G.; Alves, J. Site-directed mutagenesis in the catalytic center of the restriction endonuclease EcoRI. Gene 1995, 157, 113–118. [Google Scholar] [CrossRef]
- Muir, R.S.; Flores, H.; Zinder, N.D.; Model, P.; Soberon, X.; Heitman, J. Temperature-sensitive mutants of the EcoRI endonuclease. J. Mol. Biol. 1997, 274, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, P.J.; Rosenberg, J.M.; Jen-Jacobson, L. Structural and thermodynamic basis for enhanced DNA binding by a promiscuous mutant EcoRI endonuclease. Structure 2007, 15, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Sapienza, P.J.; Niu, T.; Kurpiewski, M.R.; Grigorescu, A.; Jen-Jacobson, L. Thermodynamic and structural basis for relaxation of specificity in protein-DNA recognition. J. Mol. Biol. 2014, 426, 84–104. [Google Scholar] [CrossRef]
- Flores, H.; Osuna, J.; Heitman, J.; Soberón, X. Saturation mutagenesis of His114 of EcoRI reveals relaxed-specificity mutants. Gene 1995, 157, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Küster, W.; Alves, J. Asn141 is essential for DNA recognition by EcoRI restriction endonuclease. FEBS Lett. 1998, 438, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Watrob, H.; Liu, W.; Chen, Y.; Bartlett, S.G.; Jen-Jacobson, L.; Barkley, M.D. Solution conformation of EcoRI restriction endonuclease changes upon binding of cognate DNA and Mg2+ cofactor. Biochemistry 2001, 40, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Y.; Watrob, H.; Bartlett, S.G.; Jen-Jacobson, L.; Barkley, M.D. N-termini of EcoRI restriction endonuclease dimer are in close proximity on the protein surface. Biochemistry 1998, 37, 15457–15465. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A.; Alves, J.; Urbanke, C.; Maass, G.; Eckstein, H.; Lianshan, Z.; Bayer, E.; Pingoud, A. A dodecapeptide comprising the extended chain-α4 region of the restriction endonuclease EcoRI specifically binds to the EcoRI recognition site. J. Biol. Chem. 1995, 270, 5122–5129. [Google Scholar] [CrossRef]
- Brickner, M.; Chmielewski, J. Inhibiting the dimeric restriction endonuclease EcoRI using interfacial helical peptides. Chem. Biol. 1998, 5, 339–343. [Google Scholar] [CrossRef]
- Asai, H.; Kasai, H.; Matsuda, Y.; Yamazaki, N.; Nagawa, F.; Sakano, H.; Tsuboi, A. Genomic structure and transcription of a murine odorant receptor gene: Differential initiation of transcription in the olfactory and testicular cells. Biochem. Biophys. Res. Commun. 1996, 221, 240–247. [Google Scholar] [CrossRef]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoon, Y.C.; Lee, A.S.; Kang, N.; Koo, J.; Rhyu, M.R.; Park, J.H. Expression of human olfactory receptor 10J5 in heart aorta, coronary artery, and endothelial cells and its functional role in angiogenesis. Biochem. Biophys. Res. Commun. 2015, 460, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the Expasy server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Harpaz, Y.; Gerstein, M.; Chothia, C. Volume changes on protein folding. Structure 1994, 2, 641–649. [Google Scholar] [CrossRef]
- Joshi, P.; Vendruscolo, M. Druggability of intrinsically disordered proteins. Adv. Exp. Med. Biol. 2015, 870, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Ambadipudi, S.; Zweckstetter, M. Targeting intrinsically disordered proteins in rational drug discovery. Expert Opin. Drug Discov. 2016, 11, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Nakasone, W.; Nakamura, M. Self and nonself short constituent sequences of amino acids in the SARS-CoV-2 proteome for vaccine development. COVID 2021, 1, 555–574. [Google Scholar] [CrossRef]
- Otaki, J.M.; Nakasone, W.; Nakamura, M. Nonself mutations in the spike protein suggest an increase in the antigenicity and a decrease in the virulence of the Omicron variant of SARS-CoV-2. COVID 2022, 2, 407–418. [Google Scholar] [CrossRef]
- Mizuno, Y.; Nakasone, W.; Nakamura, M.; Otaki, J.M. In silico and in vitro evaluation of the molecular mimicry of the SARS-CoV-2 spike protein by common short constituent sequences (cSCSs) in the human proteome: Toward safer epitope design for vaccine development. Vaccines 2024, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Dahal, A.; Sonju, J.J.; Kousoulas, K.G.; Jois, S.D. Peptides and peptidomimetics as therapeutic agents for COVID-19. Pept. Sci. 2022, 114, e24245. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, J.; Wang, D.; Su, Y.; Xing, Z.; Sun, F.; Chen, F. Therapeutic polypeptides and peptidomimetics: Powerful tools for COVID-19 treatment. Clin. Drug Investig. 2023, 43, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, J.; Singh, H.; Kaur, S.; Prakash, A.; Medhi, B. Peptides and peptidomimetics as a therapeutic candidate for the treatment of COVID-19: A brief review. Indian J. Pharmacol. 2023, 55, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, Y.; Otaki, J.M. Protein delivery to insect epithelial cells in vivo: Potential application to functional molecular analysis of proteins in butterfly wing development. BioTech 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, Y.; Otaki, J.M. Antibody-mediated protein knockdown reveals Distal-less functions for eyespots and parafocal elements in butterfly wing color pattern development. Cells 2024, 13, 1476. [Google Scholar] [CrossRef] [PubMed]







| Name | Amino Acid Number | Amino Acid Sequence | Length (aa) | Molecular Weight | Isoelectric pI | Mean Hydrophobicity |
|---|---|---|---|---|---|---|
| BP1 | 2–15 | SNKKQSNRLTEQHK | 14 aa | 1697.87 | 10.29 | −2.529 |
| BP2 | 12–23 | EQHKLSQGVIGI | 12 aa | 1308.50 | 6.85 | −0.183 |
| BP3 | 20–33 | VIGIFGDYAKAHDL | 14 aa | 1518.73 | 5.21 | 0.514 |
| BP4 | 34–47 | AVGEVSKLVKKALS | 14 aa | 1428.74 | 9.7 | 0.471 |
| BP5 | 48–60 | NEYPQLAFRYRDS | 13 aa | 1658.79 | 6.07 | −1.508 |
| BP6 * | 61–74 | IKKTEINEALKKID | 14 aa | 1642.96 | 8.43 | −0.800 |
| BP7 | 73–84 | IDPDLGGTLFVS | 12 aa | 1233.38 | 3.56 | 0.683 |
| BP8 * | 82–93 | FVSNSSIKPDGG | 12 aa | 1207.31 | 5.84 | −0.350 |
| BP9 | 89–102 | KPDGGIVEVKDDYG | 14 aa | 1491.62 | 4.23 | −0.929 |
| BP10 | 103–116 | EWRVVLVAEAKHQG | 14 aa | 1621.86 | 6.86 | −0.243 |
| BP11 | 116–127 | GKDIINIRNGLL | 12 aa | 1325.57 | 8.75 | 0.117 |
| BP12 * | 123–135 | RNGLLVGKRGDQD | 13 aa | 1427.58 | 8.75 | −1.254 |
| BP13 | 135–146 | DLMAAGNAIERS | 12 aa | 1247.39 | 4.37 | −0.050 |
| BP14 * | 146–157 | SHKNISEIANFM | 12 aa | 1390.58 | 6.47 | −0.308 |
| BP15 | 158–170 | LSESHFPYVLFLE | 13 aa | 1580.80 | 4.51 | 0.500 |
| BP16 | 170–181 | EGSNFLTENISI | 12 aa | 1323.42 | 3.80 | −0.092 |
| BP17 * | 182–193 | TRPDGRVVNLEY | 12 aa | 1418.57 | 5.74 | −0.942 |
| BP18 | 194–206 | NSGILNRLDRLTA | 13 aa | 1442.64 | 9.60 | −0.285 |
| BP19 | 206–217 | AANYGMPINSNL | 12 aa | 1264.42 | 5.57 | −0.067 |
| BP20 | 216–228 | NLCINKFVNHKDK | 13 aa | 1572.85 | 9.20 | −0.854 |
| BP21 | 229–242 | SIMLQAASIYTQGD | 14 aa | 1497.68 | 3.80 | 0.271 |
| BP22 * | 243–252 | GREWDSKIMFEIMF | 14 aa | 1789.10 | 4.68 | −0.186 |
| BP23 | 252–265 | FEIMFDISTTSLRV | 14 aa | 1658.93 | 4.37 | 0.714 |
| BP24 * | 259–272 | STTSLRVLGRDLFE | 14 aa | 1593.80 | 5.79 | −0.071 |
| BP25 | 266–277 | LGRDLFEQLTSK | 12 aa | 1406.60 | 6.07 | −0.550 |
| Name | Amino Acid Number | Amino Acid Sequence | Length (aa) | Molecular Weight | Isoelectric pI | Mean Hydrophobicity |
|---|---|---|---|---|---|---|
| BP7R1 | n.a. | VGLILSTGDFDP | 12 aa | 1233.38 | 3.56 | 0.683 |
| BP7R2 | n.a. | DGLTPFSDVLGI | 12 aa | 1233.38 | 3.56 | 0.683 |
| BP10R1 | n.a. | VEGQELAVKAHRWV | 14 aa | 1621.86 | 6.73 | −0.243 |
| BP10R2 | n.a. | AGRKQEVHAVWVEL | 14 aa | 1621.86 | 6.80 | −0.243 |
| BP13R1 | n.a. | EALDIAMGRNSA | 12 aa | 1247.39 | 4.37 | −0.050 |
| BP13R2 | n.a. | LAMRGASIADEN | 12 aa | 1247.39 | 4.37 | −0.050 |
| Name | Amino Acid Number | Amino Acid Sequence | Length (aa) | Molecular Weight | Isoelectric pI | Mean Hydrophobicity |
|---|---|---|---|---|---|---|
| BP1.5 | 8–19 | NRLTEQHKLSQG | 12 aa | 1410.55 | 8.75 | −1.658 |
| BP2.5 | 16–27 | LSQGVIGIFGDY | 12 aa | 1268.43 | 3.80 | 0.792 |
| BP3.5 | 28–39 | AKAHDLAVGEVS | 12 aa | 1196.33 | 5.32 | 0.192 |
| BP4.5 | 42–52 | VKKALSNEYPQL | 12 aa | 1389.61 | 8.47 | −0.700 |
| BP5.5 | 55–66 | FRYRDSIKKTEI | 12 aa | 1555.80 | 9.70 | −1.233 |
| BP6.5 | 65–79 | EALKKIDPDLGG | 12 aa | 1255.43 | 4.56 | −0.567 |
| BP7.5 | 78–88 | GGTLFVSNSSI | 11 aa | 1081.19 | 5.52 | 0.718 |
| BP8.5 | 86–96 | SSIKPDGGIVE | 11 aa | 1101.22 | 4.37 | −0.155 |
| BP9.5 | 97–109 | VKDDYGEWRVVL | 12 aa | 1478.67 | 4.56 | −0.425 |
| BP10.5 | 111–121 | EAKHQGKDIIN | 11 aa | 1252.39 | 6.85 | −1.327 |
| BP11.5 | 120–131 | INIRNGLLVGKR | 12 aa | 1352.65 | 12.01 | 0.008 |
| BP12.5 | 130–140 | KRGDQDLMAAG | 11 aa | 1161.30 | 5.96 | −0.945 |
| BP13.5 | 141–151 | NAIERSHKNIS | 11 aa | 1268.40 | 8.75 | −1.173 |
| BP14.5 | 152–163 | EIANFMLSESHF | 12 aa | 1424.59 | 4.51 | 0.192 |
| BP15.5 | 165–175 | YVLFLEGSNFL | 11 aa | 1301.50 | 4.00 | 1.064 |
| BP16.5 | 176–187 | TENISITRPDGR | 12 aa | 1358.47 | 5.74 | −1.225 |
| BP17.5 | 188–199 | VVNLEYNSGILN | 12 aa | 1334.49 | 4.00 | 0.333 |
| BP18.5 | 201–211 | LDRLTAANYGM | 11 aa | 1224.40 | 5.84 | −0.073 |
| BP19.5 | 223–234 | PINSNLCINK | 10 aa | 1383.63 | 8.57 | −0.508 |
| BP20.5 | 237–252 | VNHKDKSIMLQA | 12 aa | 1426.46 | 4.03 | −1.542 |
| BP21.5 | 246–257 | IYTQGDGREWDS | 12 aa | 1375.62 | 4.03 | 0.291 |
| BP22.5 | 212–221 | DSKIMFEIMFD | 11 aa | 1115.31 | 8.64 | −0.150 |
| Name | Amino Acid Number | Amino Acid Sequence | Length (aa) | Molecular Weight | Isoelectric pI | Mean Hydrophobicity |
|---|---|---|---|---|---|---|
| BP19.5N | 212–217 | PINSNL | 6 aa | 656.74 | 5.96 | −0.183 |
| BP19.5C | 216–221 | NLCINK | 6 aa | 703.85 | 8.22 | −0.017 |
| Name | Amino Acid Number | Amino Acid Sequence | Length (aa) | Molecular Weight | Isoelectric pI | Mean Hydrophobicity |
|---|---|---|---|---|---|---|
| BP6N | 61–66 | IKKTEI | 6 aa | 730.90 | 8.59 | −0.500 |
| BP6M | 65–70 | EINEAL | 6 aa | 687.75 | 3.80 | −0.067 |
| BP6C | 69–74 | ALKKID | 6 aa | 686.85 | 8.64 | −0.200 |
| BP7N | 73–78 | IDPDLG | 6 aa | 628.68 | 3.56 | −0.117 |
| BP7M | 76–81 | DLGGTL | 6 aa | 574.63 | 3.80 | 0.433 |
| BP7C | 79–84 | GTLFVS | 6 aa | 622.72 | 5.52 | 1.483 |
| BP9N | 89–94 | KPDGGI | 6 aa | 585.66 | 5.84 | −0.883 |
| BP9M | 93–98 | GIVEVK | 6 aa | 643.78 | 6.00 | 0.850 |
| BP9C | 97–102 | VKDDYG | 6 aa | 695.73 | 4.21 | −1.400 |
| BP10N | 103–108 | EWRVVL | 6 aa | 800.96 | 6.10 | 0.550 |
| BP10M | 107–112 | VLVAEA | 6 aa | 600.71 | 4.00 | 2.050 |
| BP10C | 111–116 | EAKHQG | 6 aa | 668.71 | 6.85 | −2.117 |
| BP11N | 116–121 | GKDIIN | 6 aa | 658.75 | 5.84 | −0.383 |
| BP11M | 119–124 | IINIRN | 6 aa | 741.89 | 9.75 | 0.333 |
| BP11C | 121–127 | IRNGLL | 6 aa | 684.84 | 9.75 | 0.617 |
| BP12N | 124–129 | NGLLVG | 6 aa | 571.67 | 5.52 | 1.250 |
| BP12M | 126–132 | LVGKRG | 6 aa | 628.77 | 11.00 | −0.200 |
| BP12C | 130–135 | KRGDQD | 6 aa | 717.74 | 5.96 | −3.217 |
| BP13N | 135–140 | DLMAAG | 6 aa | 576.67 | 3.80 | 0.900 |
| BP13M | 138–143 | AAGNAI | 6 aa | 515.57 | 5.57 | 1.000 |
| BP13C | 141–146 | NAIERS | 6 aa | 688.74 | 6.00 | −1.000 |
| BP14N | 146–151 | SHKNIS | 6 aa | 684.75 | 8.49 | −1.283 |
| BP14M | 149–154 | NISEIA | 6 aa | 645.71 | 4.00 | 0.500 |
| BP14C | 152–157 | EIANFM | 6 aa | 723.84 | 4.00 | 0.667 |
| BP15N | 158–163 | LSESHF | 6 aa | 718.76 | 5.24 | −0.283 |
| BP15M | 161–166 | SHFPYV | 6 aa | 748.84 | 6.46 | 0.017 |
| BP15C | 165–170 | YVLFLE | 6 aa | 782.93 | 4.00 | 1.633 |
| BP22N | 243–248 | GREWDS | 6 aa | 748.75 | 4.37 | −2.267 |
| BP22M | 245–250 | EWDSKI | 6 aa | 776.84 | 4.37 | −1.350 |
| BP22C | 247–252 | DSKIMF | 6 aa | 739.88 | 5.80 | 0.167 |
| Name | Aliphatic Index | Hydrophilic Residues (%) | Number of Aromatic Residues | Instability Index | Net Charge at pH7 |
|---|---|---|---|---|---|
| BP1 | 27.86 | 79 | 1 | 118.93 | 3.09 |
| BP2 | 121.67 | 42 | 1 | 46.85 | 0.09 |
| BP3 | 118.57 | 21 | 3 | 14.07 | −0.91 |
| BP4 | 132.14 | 43 | 0 | −10.29 | 2.00 |
| BP5 | 37.69 | 54 | 3 | 29.55 | 0.00 |
| BP6 | 118.57 | 57 | 0 | 18.61 | 1.00 |
| BP7 | 121.67 | 25 | 1 | 6.09 | −2.00 |
| BP8 | 56.67 | 50 | 1 | 15.86 | 0.00 |
| BP9 | 69.29 | 43 | 1 | −12.92 | −2.00 |
| BP10 | 104.29 | 36 | 2 | −1.45 | 0.09 |
| BP11 | 162.50 | 42 | 0 | 36.47 | 1.00 |
| BP12 | 82.31 | 54 | 0 | 27.46 | 1.00 |
| BP13 | 90.00 | 42 | 0 | 85.61 | −1.00 |
| BP14 | 73.33 | 50 | 2 | 85.09 | 0.09 |
| BP15 | 112.31 | 31 | 4 | 45.70 | −1.91 |
| BP16 | 97.50 | 50 | 1 | 49.31 | −2.00 |
| BP17 | 80.83 | 42 | 1 | 18.93 | 0.00 |
| BP18 | 127.69 | 46 | 0 | 11.72 | 1.00 |
| BP19 | 81.67 | 33 | 1 | 38.71 | 0.00 |
| BP20 | 82.31 | 54 | 2 | 39.13 | 2.04 |
| BP21 | 97.86 | 36 | 1 | 21.12 | −1.00 |
| BP22 | 55.71 | 43 | 3 | 20.00 | −1.00 |
| BP23 | 104.29 | 36 | 2 | 45.61 | −1.00 |
| BP24 | 104.29 | 43 | 0 | 23.04 | 0.00 |
| BP25 | 97.50 | 50 | 1 | 25.22 | 0.00 |
| BP7R1 | 121.67 | 25 | 1 | 15.07 | −2.00 |
| BP7R2 | 121.67 | 25 | 1 | 18.14 | −2.00 |
| BP10R1 | 104.29 | 36 | 2 | 49.77 | 0.09 |
| BP10R2 | 104.29 | 36 | 2 | 33.89 | 0.09 |
| BP13R1 | 90.00 | 42 | 0 | 19.45 | −1.00 |
| BP13R2 | 90.00 | 42 | 0 | −18.34 | −1.00 |
| BP1.5 | 65.00 | 58 | 1 | 69.98 | 1.09 |
| BP2.5 | 121.67 | 25 | 2 | 18.14 | -1.00 |
| BP3.5 | 105.83 | 33 | 1 | −11.27 | −0.91 |
| BP4.5 | 97.50 | 50 | 1 | 33.10 | 1.00 |
| BP5.5 | 65.00 | 58 | 2 | 29.87 | 2.00 |
| BP6.5 | 105.83 | 42 | 0 | −0.98 | −1.00 |
| BP7.5 | 97.27 | 36 | 1 | 30.10 | 0.00 |
| BP8.5 | 97.27 | 45 | 0 | 0.95 | −1.00 |
| BP9.5 | 105.00 | 42 | 2 | −19.12 | −1.00 |
| BP10.5 | 80.00 | 55 | 1 | 1.37 | 0.09 |
| BP11.5 | 154.17 | 42 | 0 | 56.56 | 3.00 |
| BP12.5 | 53.64 | 45 | 0 | 59.74 | 0.00 |
| BP13.5 | 80.00 | 64 | 1 | 150.45 | 1.09 |
| BP14.5 | 73.33 | 42 | 2 | 36.15 | −1.91 |
| BP15.5 | 132.73 | 27 | 3 | −4.57 | −1.00 |
| BP16.5 | 65.00 | 50 | 0 | 71.60 | 0.00 |
| BP17.5 | 145.83 | 42 | 1 | 18.14 | −1.00 |
| BP18.5 | 89.09 | 27 | 1 | −5.48 | 0.00 |
| BP19.5 | 117.00 | 50 | 0 | 32.68 | 0.95 |
| BP20.5 | 97.50 | 50 | 1 | 48.99 | 1.09 |
| BP21.5 | 32.50 | 50 | 2 | −0.67 | −2.00 |
| BP22.5 | 70.91 | 45 | 2 | 47.61 | −2.00 |
| BP19.5N | 130.00 | 50 | 0 | 8.33 | 0.00 |
| BP19.5C | 130.00 | 50 | 0 | 47.80 | 0.95 |
| BP6N | 130.00 | 50 | 0 | 58.38 | 1.00 |
| BP6M | 146.67 | 50 | 0 | 40.43 | −2.00 |
| BP6C | 146.67 | 50 | 0 | −19.97 | 1.00 |
| BP7N | 130.00 | 33 | 0 | -4.23 | −2.00 |
| BP7M | 130.00 | 17 | 0 | 14.75 | −1.00 |
| BP7C | 113.33 | 17 | 1 | −5.82 | 0.00 |
| BP9N | 65.00 | 33 | 0 | −10.38 | 0.00 |
| BP9M | 161.67 | 33 | 0 | −24.77 | 0.00 |
| BP9C | 48.33 | 50 | 1 | −10.62 | −1.00 |
| BP10N | 161.67 | 33 | 1 | −16.72 | 0.00 |
| BP10M | 195.00 | 17 | 0 | 8.33 | −1.00 |
| BP10C | 16.67 | 50 | 1 | 8.33 | 0.09 |
| BP11N | 130.00 | 50 | 0 | −5.82 | 0.00 |
| BP11M | 195.00 | 50 | 0 | 102.13 | 1.00 |
| BP11C | 195.00 | 33 | 0 | 3.85 | 1.00 |
| BP12N | 178.33 | 17 | 0 | −30.87 | 0.00 |
| BP12M | 113.33 | 33 | 0 | 20.22 | 2.00 |
| BP12C | 0.00 | 83 | 0 | 80.62 | 0.00 |
| BP13N | 98.33 | 17 | 0 | 28.90 | −1.00 |
| BP13M | 115.00 | 17 | 0 | −5.82 | 0.00 |
| BP13C | 81.67 | 67 | 0 | 154.80 | 0.00 |
| BP14N | 65.00 | 67 | 1 | 121.03 | 1.09 |
| BP14M | 146.67 | 50 | 0 | 145.77 | −1.00 |
| BP14C | 81.67 | 33 | 1 | 15.38 | −1.00 |
| BP15N | 65.00 | 50 | 1 | 55.25 | −0.91 |
| BP15M | 48.33 | 17 | 2 | 23.15 | 0.09 |
| BP15C | 178.33 | 17 | 1 | 8.33 | −1.00 |
| BP22N | 0.00 | 67 | 1 | 15.38 | −1.00 |
| BP22M | 65.00 | 67 | 1 | 1.23 | −1.00 |
| BP22C | 65.00 | 50 | 1 | 26.28 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaki, J.M. Peptide Inhibitor Assay for Allocating Functionally Important Accessible Sites Throughout a Protein Chain: Restriction Endonuclease EcoRI as a Model Protein System. BioTech 2025, 14, 1. https://doi.org/10.3390/biotech14010001
Otaki JM. Peptide Inhibitor Assay for Allocating Functionally Important Accessible Sites Throughout a Protein Chain: Restriction Endonuclease EcoRI as a Model Protein System. BioTech. 2025; 14(1):1. https://doi.org/10.3390/biotech14010001
Chicago/Turabian StyleOtaki, Joji M. 2025. "Peptide Inhibitor Assay for Allocating Functionally Important Accessible Sites Throughout a Protein Chain: Restriction Endonuclease EcoRI as a Model Protein System" BioTech 14, no. 1: 1. https://doi.org/10.3390/biotech14010001
APA StyleOtaki, J. M. (2025). Peptide Inhibitor Assay for Allocating Functionally Important Accessible Sites Throughout a Protein Chain: Restriction Endonuclease EcoRI as a Model Protein System. BioTech, 14(1), 1. https://doi.org/10.3390/biotech14010001





