Identification and Typing of Strains of Wood-Rotting Basidiomycetes by Protein Profiling Using MALDI-TOF MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Protein Extraction
2.3. MALDI-TOF MS Analysis
2.4. MALDI-TOF MS Data Interpretation
3. Results
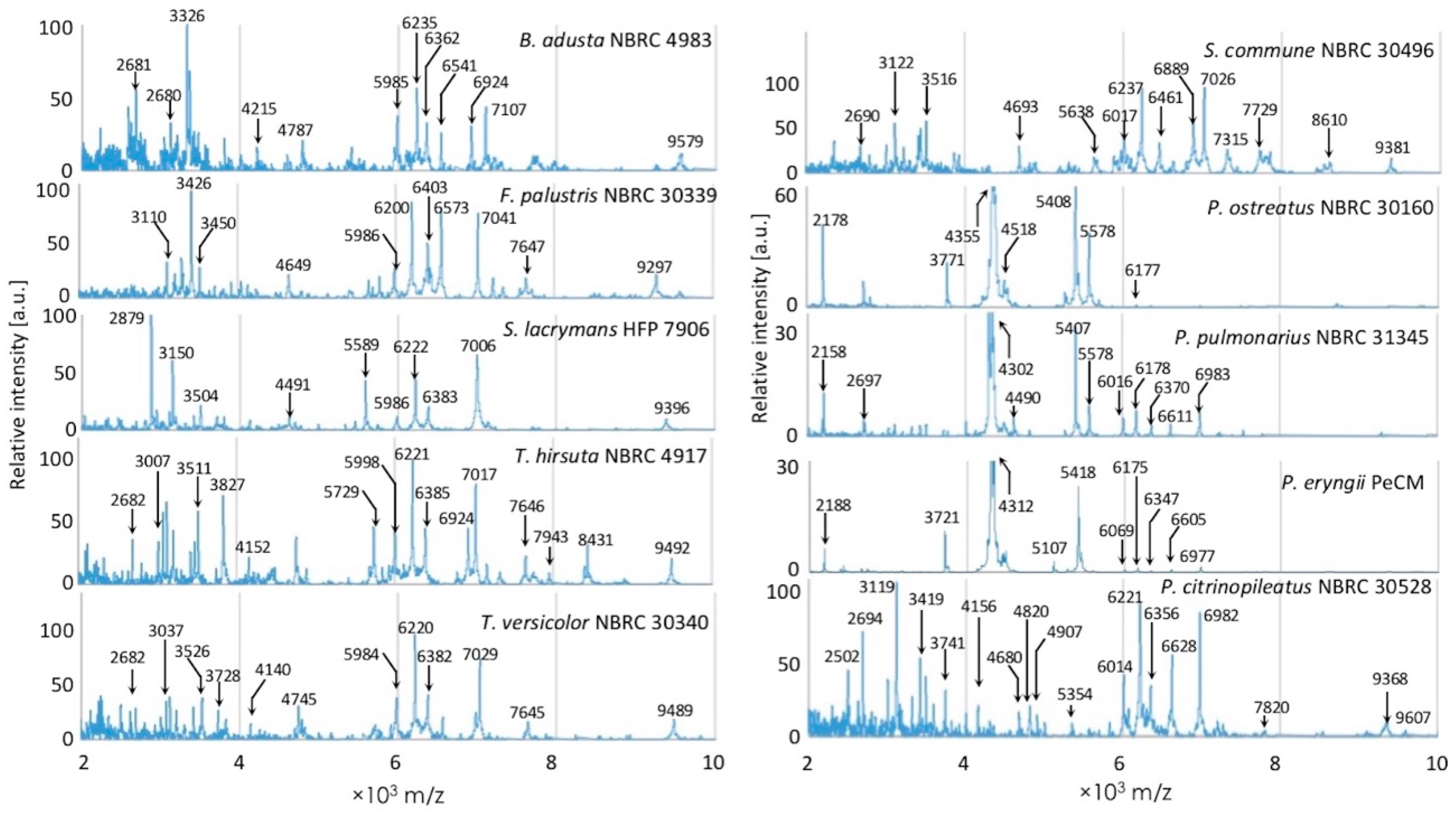
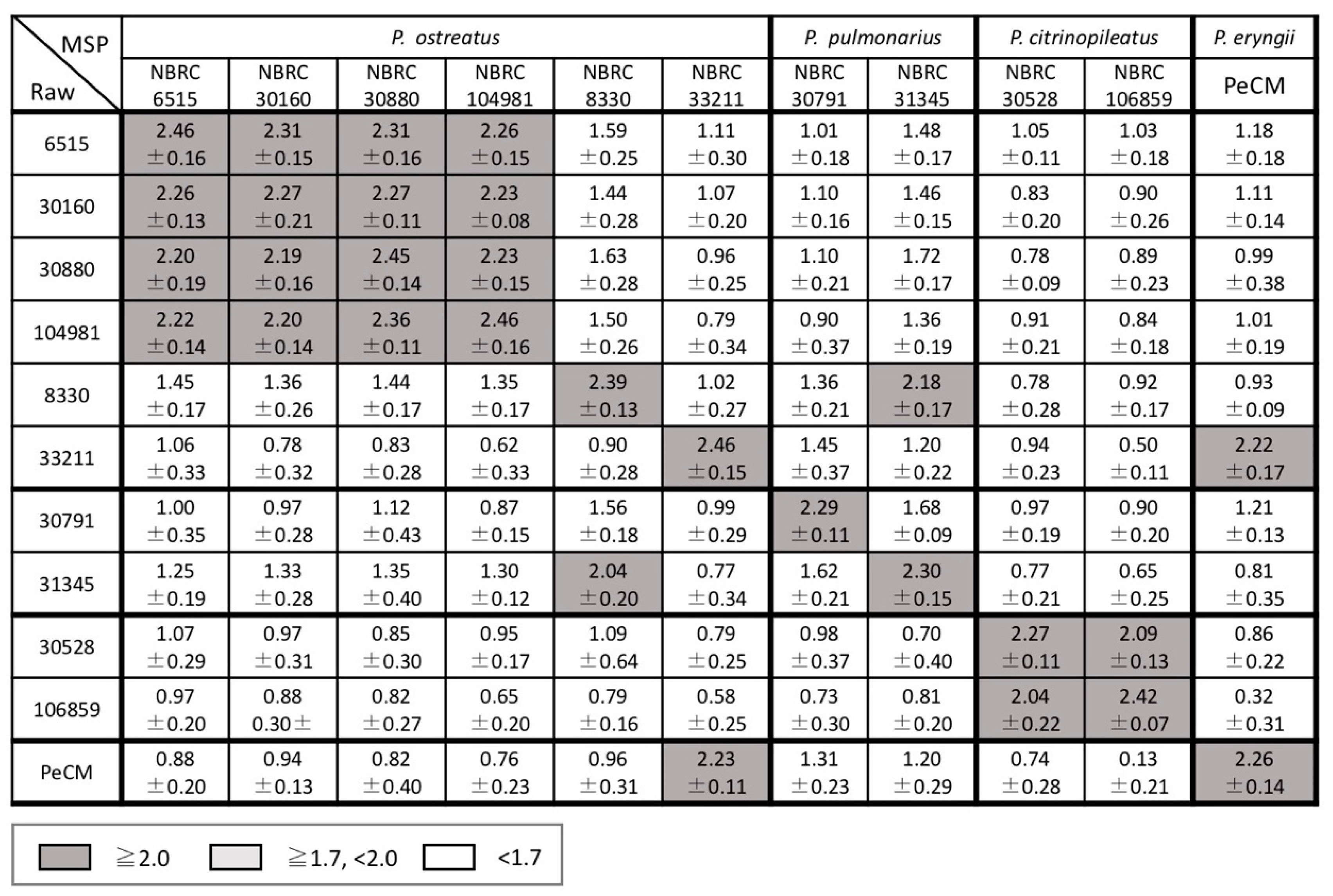
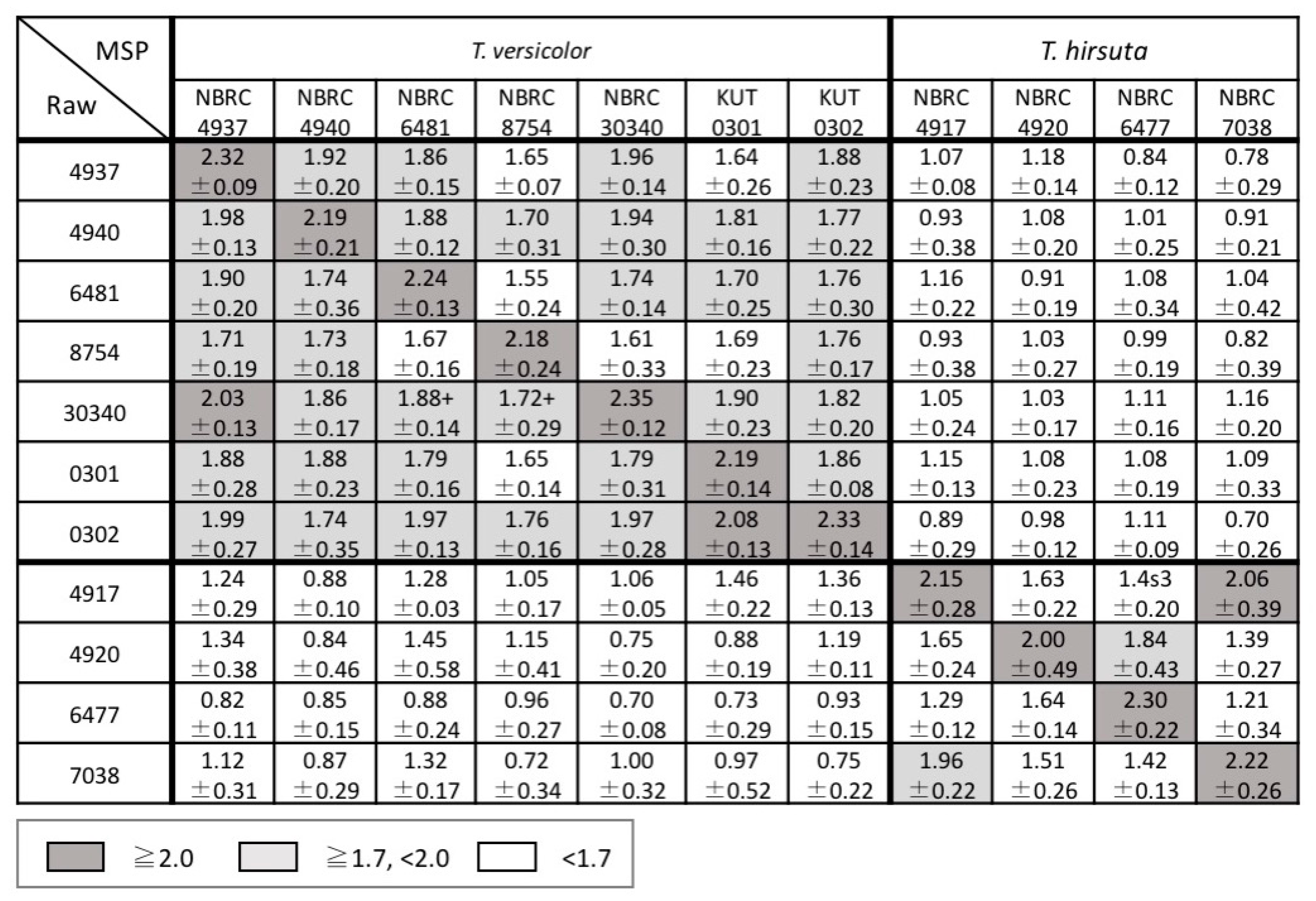
3.1. Comparison of Inter- and Intraspecific MS Patterns
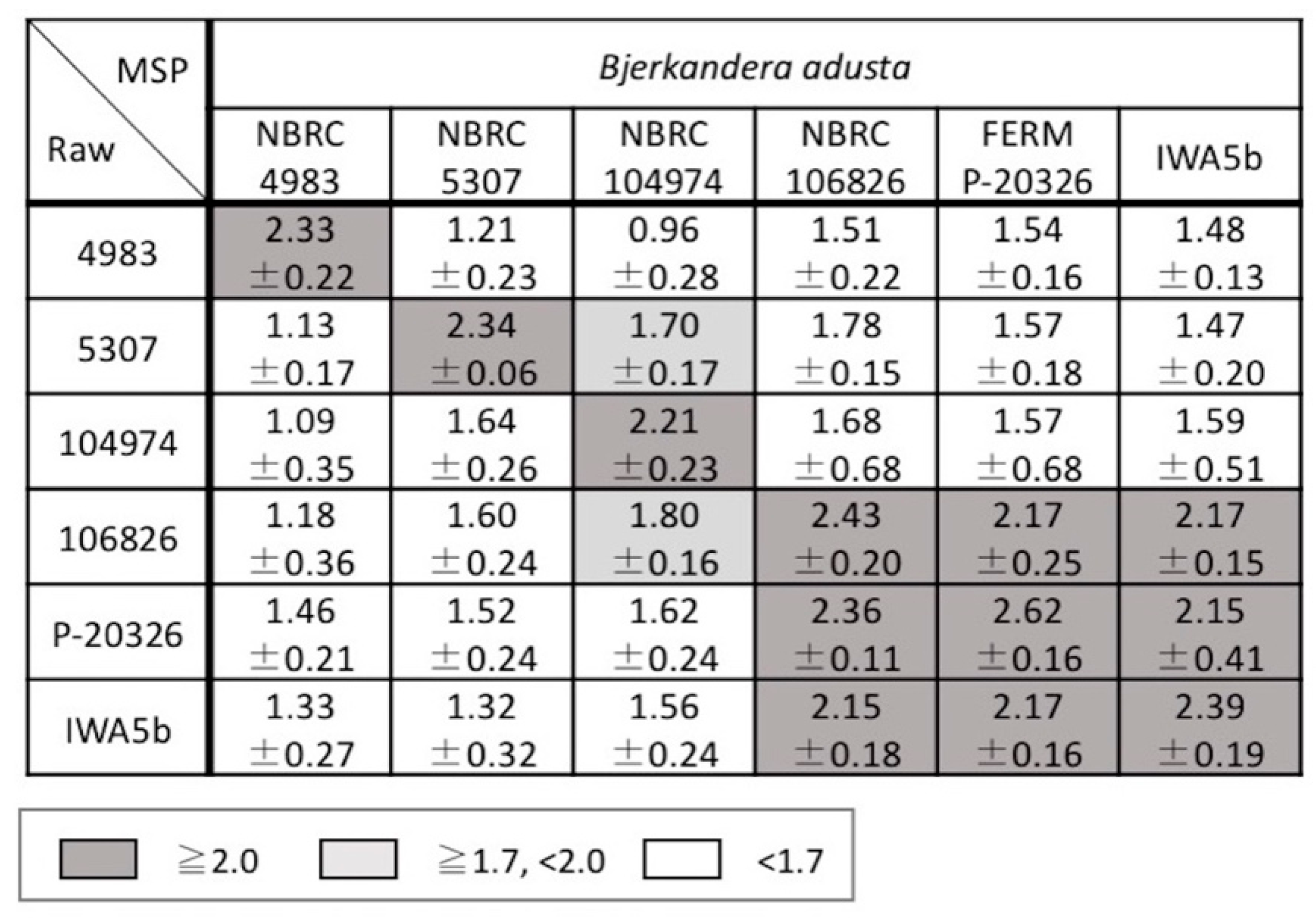
3.2. Examination of Mass Spectral Variation Using the MALDI Biotyper Score
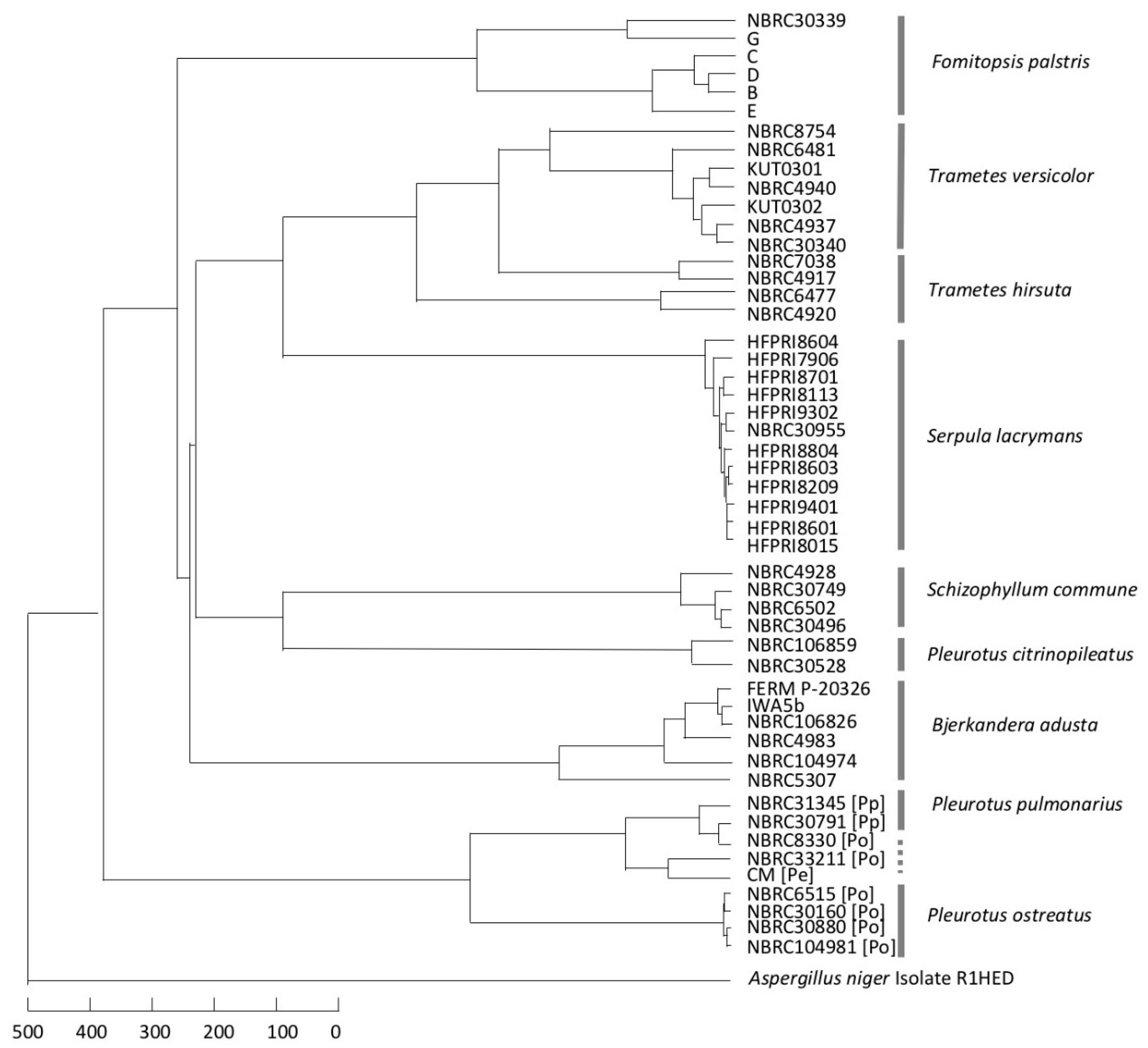
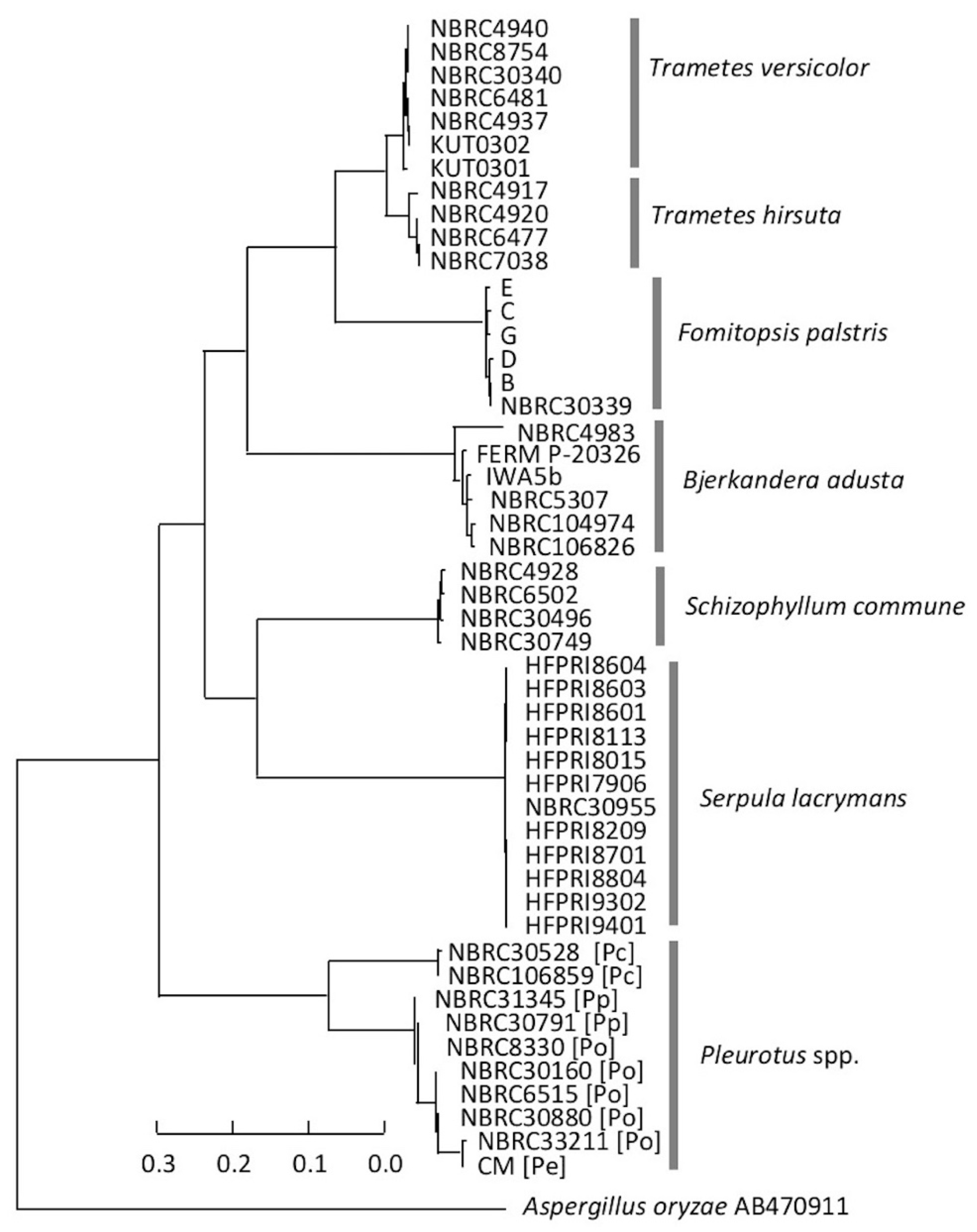
3.3. Consideration of Strain Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifert, K.A. Progress towards DNA barcoding of fungi. Mol. Ecol. Resour. 2009, 9 (Suppl. S1), 83–89. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, H.; Krüger, M.; Schüßler, A. DNA barcoding of arbuscular mycorrhizal fungi. New Phytol. 2010, 187, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Palfreyman, J.W.; Vigrow, A.; Button, D.; Herarty, B.; King, B. The use of molecular methods to identify wood decay organisms. Wood Prot. 1991, 1, 15–22. [Google Scholar]
- Palfreyman, J.W.; Vigrow, A. Molecular analysis of certain isolates of Serpula lacrymans. FEMS Microbiol. Lett. 1994, 117, 281–286. [Google Scholar] [CrossRef]
- Holland, R.D.; Wilkes, J.G.; Rafii, F.; Sutherland, J.B.; Persons, C.C.; Voorhees, K.J.; Lay, J.O., Jr. Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorp-tion/ionization with time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1227–1232. [Google Scholar] [CrossRef]
- Krishnamurthy, T.; Ross, P.L. Rapid Identification of Bacteria by Direct Matrix-assisted Laser Desorption/Ionization Mass Spectrometric Analysis of Whole Cells. Rapid Commun. Mass Spectrom. 1996, 10, 1992–1996. [Google Scholar] [CrossRef]
- Ryzhov, V.; Fenselau, C. Characterization of the Protein Subset Desorbed by MALDI from Whole Bacterial Cells. Anal. Chem. 2001, 73, 746–750. [Google Scholar] [CrossRef]
- Suarez, S.; Ferroni, A.; Lotz, A.; Jolley, K.A.; Guérin, P.; Leto, J.; Dauphin, B.; Jamet, A.; Maiden, M.C.J.; Nassif, X.; et al. Ribosomal proteins as biomarkers for bacterial identification by mass spectrometry in the clinical microbiology laboratory. J. Microbiol. Methods 2013, 94, 390–396. [Google Scholar] [CrossRef]
- Claydon, M.A.; Davey, S.N.; Edwards-Jones, V.; Gordon, D.B. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 1996, 14, 1584–1586. [Google Scholar] [CrossRef]
- Kostrzewa, M. Application of the MALDI Biotyper to clinical microbiology: Progress and potential. Expert Rev. Proteom. 2018, 15, 193–202. [Google Scholar] [CrossRef]
- Stevenson, L.G.; Drake, S.K.; Shea, Y.R.; Zelazny, A.M.; Murray, P.R. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Clinically Important Yeast Species. J. Clin. Microbiol. 2010, 48, 3482–3486. [Google Scholar] [CrossRef] [PubMed]
- Gorre, E.; Muste, C.; Owens, K.G. Introducing a Cell-Free Approach for the Identification of Brewing Yeast (Saccharomyces cerevisiae) Strains Using MALDI-TOF MS. J. Am. Soc. Mass Spectrom. 2018, 29, 2248–2259. [Google Scholar] [CrossRef] [PubMed]
- Wieme, A.D.; Spitaels, F.; Vandamme, P.; Van Landschoot, A. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry as a monitoring tool for in-house brewer’s yeast contamination: A proof of concept. J. Inst. Brew. 2014, 120, 438–443. [Google Scholar] [CrossRef]
- Delavy, M.; Dos Santos, A.R.; Heiman, C.M.; Coste, A.T. Investigating Antifungal Susceptibility in Candida Species With MALDI-TOF MS-Based Assays. Front. Cell. Infect. Microbiol. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, A.; Usbeck, J.C.; Behr, J.; Vogel, R.F. MALDI-TOF MS typing enables the classification of brewing yeasts of the genus Saccharomyces to major beer styles. PLoS ONE 2017, 12, e0181694. [Google Scholar] [CrossRef]
- Cassagne, C.; Ranque, S.; Normand, A.-C.; Fourquet, P.; Thiebault, S.; Planard, C.; Hendrickx, M.; Piarroux, R. Mould Routine Identification in the Clinical Laboratory by Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry. PLoS ONE 2011, 6, e28425. [Google Scholar] [CrossRef]
- Zvezdanova, M.E.; Escribano, P.; Ruiz, A.; Martínez-Jiménez, M.C.; Peláez, T.; Collazos, A.; Guinea, J.; Bouza, E.; Rodríguez-Sánchez, B. Increased species-assignment of filamentous fungi using MALDI-TOF MS coupled with a simplified sample processing and an in-house library. Med. Mycol. 2018, 57, 63–70. [Google Scholar] [CrossRef]
- Lau, A.F.; Walchak, R.C.; Miller, H.B.; Slechta, E.S.; Kamboj, K.; Riebe, K.; Robertson, A.E.; Gilbreath, J.J.; Mitchell, K.F.; Wallace, M.A.; et al. Multicenter Study Demonstrates Standardization Requirements for Mold Identification by MALDI-TOF MS. Front. Microbiol. 2019, 10, 2098. [Google Scholar] [CrossRef]
- Normand, A.-C.; Blaize, M.; Imbert, S.; Packeu, A.; Becker, P.; Fekkar, A.; Stubbe, D.; Piarroux, R. Identification of Molds with Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry: Performance of the Newly Developed MSI-2 Application in Comparison with the Bruker Filamentous Fungi Database and MSI-1. J. Clin. Microbiol. 2021, 59, JCM0129921. [Google Scholar] [CrossRef]
- Seyfarth, F.; Ziemer, M.; Sayer, H.G.; Burmester, A.; Erhard, M.; Welker, M.; Schliemann, S.; Straube, E.; Hipler, U.-C. The use of ITS DNA sequence analysis and MALDI-TOF mass spectrometry in diagnosing an infection with Fusarium proliferatum. Exp. Dermatol. 2008, 17, 965–971. [Google Scholar] [CrossRef]
- Fernández, A.L.; Andres, P.O.; Veciño, C.H.; Nagel, C.B.; Mujica, M.T. Subcutaneous infection by Graphium basitruncatum in a heart transplant patient. Braz. J. Infect. Dis. 2017, 21, 670–674. [Google Scholar] [CrossRef]
- Crossay, T.; Antheaume, C.; Redecker, D.; Bon, L.; Chedri, N.; Richert, C.; Guentas, L.; Cavaloc, Y.; Amir, H. New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci. Rep. 2017, 7, 14306. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood decay fungi: An analysis of worldwide research. J. Soils Sediments 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Piarroux, R.; Gabriel, F.; Grenouillet, F.; Collombon, P.; Louasse, P.; Piarroux, M.; Normand, A.-C. Using MALDI-ToF mass spectrometry to identify mushroom species: Proof of concept analysis of Amanita genus specimens. Med. Mycol. 2021, 59, 890–900. [Google Scholar] [CrossRef]
- Sugawara, R.; Yamada, S.; Tu, Z.; Sugawara, A.; Suzuki, K.; Hoshiba, T.; Eisaka, S.; Yamaguchi, A. Rapid and reliable species identification of wild mushrooms by matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS). Anal. Chim. Acta 2016, 934, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis ver-sion 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Marklein, G.; Josten, M.; Klanke, U.; Müller, E.; Horré, R.; Maier, T.; Wenzel, T.; Kostrzewa, M.; Bierbaum, G.; Hoerauf, A.; et al. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Fast and Reliable Identification of Clinical Yeast Isolates. J. Clin. Microbiol. 2009, 47, 2912–2917. [Google Scholar] [CrossRef]
- Drevinek, M.; Dresler, J.; Klimentova, J.; Pisa, L.; Hubalek, M. Evaluation of sample preparation methods for MALDI-TOF MS identification of highly dangerous bacteria. Lett. Appl. Microbiol. 2012, 55, 40–46. [Google Scholar] [CrossRef]
- Williams, T.L.; Andrzejewski, D.; Lay, J.O.; Musser, S.M. Experimental factors affecting the quality and reproducibility of MALDI TOF mass spectra obtained from whole bacteria cells. J. Am. Soc. Mass Spectrom. 2003, 14, 342–351. [Google Scholar] [CrossRef]
- Hendrickx, M. MALDI-TOF MS and Filamentous Fungal Identification: A Success Story? Curr. Fungal Infect. Rep. 2017, 11, 60–65. [Google Scholar] [CrossRef]
- Karas, M.; Gluckman, M.; Schafer, J. Ionization in matrix-assisted laser desorption/ionization: Singly charged molecular ions are the lucky survivors. J. Mass Spectrom. 2000, 35, 1–12. [Google Scholar] [CrossRef]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry: A Fundamental Shift in the Routine Practice of Clinical Microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kitagawa, K.; Nakayama, A.; Ohtani, H. On-Probe Sample Pretreatment for Direct Analysis of Lipids in Gram-Positive Bacterial Cells by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Appl. Environ. Microbiol. 2005, 71, 7539–7541. [Google Scholar] [CrossRef][Green Version]
- Mather, C.A.; Rivera, S.F.; Butler-Wu, S.M. Comparison of the Bruker Biotyper and Vitek MS Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry Systems for Identification of Mycobacteria Using Simplified Protein Extraction Protocols. J. Clin. Microbiol. 2014, 52, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.H.; Boulanger, N.; Nebbak, A.; Collin, E.; Jaulhac, B.; Almeras, L. Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus. PLoS ONE 2017, 12, e0185430. [Google Scholar] [CrossRef]
- Sakai, H.; Hattori, T.; Wada, T.; Samejima, M. Decay of wood structures by Fomitopsis palustris- Identification and physiological characteristics of the isolates. Mokuzai Hozon 2013, 39, 226–232. [Google Scholar] [CrossRef]
- Horisawa, S.; Sakuma, Y.; Takata, K.; Doi, S. Detection of intra- and interspecific variation of the dry rot fungus Serpula lacrymans by PCR-RFLP and RAPD analysis. J. Wood Sci. 2004, 50, 427–432. [Google Scholar] [CrossRef]
- Reeve, M.A.; Bachmann, D.; Caine, T.S. Identification of Penicillium species by MALDI-TOF MS analysis of spores collected by dielectrophoresis. Biol. Methods Protoc. 2019, 4, bpz018. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Z.; Wang, J.; Yang, R. Universal Sample Preparation Method for Characterization of Bacteria by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. Appl. Environ. Microbiol. 2007, 73, 1899–1907. [Google Scholar] [CrossRef]
| Scientific Name | Strain Name | Locality of Source | Acc. No. of ITS and D1D2 Sequences |
|---|---|---|---|
| Bjerkandera adusta | NBRC 4983 | - | AB733156, AB733333 |
| NBRC 5307 | - | AB592333, LC705002 | |
| NBRC 104974 | Kyoto, Japan | AB733157, AB733334 | |
| NBRC 106826 | Hokkaido, Japan | LC705000, AB733334 | |
| FERM P-20326 | Japan | LC704999, - | |
| IWA5b | Kochi, Japan | LC705001, - | |
| Fomitopsis palustris | NBRC 30339 | - | AB733120, AB733302 |
| NarF-B | Kagoshima, Japan | LC705003, - | |
| NarF-C | Kagoshima, Japan | LC705004, - | |
| NarF-D | Kagoshima, Japan | LC705005, - | |
| NarF-E | Miyagi, Japan | LC705006, - | |
| NarF-G | Gifu, Japan | LC705007, - | |
| Pleurotus citrinopileatus | NBRC 30528 | Aomori, Japan | LC713431 * |
| NBRC | Hokkaido, Japan | LC713434 * | |
| Pleurotus eryngii | PeCM | - | LC713435 * |
| Pleurotus ostreatus | NBRC 6515 | - | AB733142, AB733315 |
| NBRC 8330 | Japan | LC713430 * | |
| NBRC 30160 | Hyogo, Japan | AB733143, AB733316 | |
| NBRC 30880 | Korea | LC713432 * | |
| NBRC 33211 | Kyoto, Japan | AB733144, AB733317 | |
| NBRC 104981 | Kyoto, Japan | LC713433 * | |
| Pleurotus pulmonarius | NBRC 30791 | India | AB733145, AB733318 |
| NBRC 31345 | Tottori, Japan | LC705008, AB733319 | |
| Schizophyllum commune | NBRC 4928 | - | AB733163, AB733339 |
| NBRC 6502 | - | AB733164, AB733340 | |
| NBRC 30496 | Osaka, Japan | AB733165, AB733341 | |
| NBRC 30749 | Osaka, Japan | AB733166, AB733342 | |
| Serpula lacrymans | NBRC 30955 | Hokkaido, Japan | AB733149, AB733324 |
| HFPRI 7096 | Hokkaido, Japan | AB733420, AB733411 | |
| HFPRI 8015 | Hokkaido, Japan | AB733421, AB733412 | |
| HFPRI 8113 | Hokkaido, Japan | AB733422, AB733413 | |
| HFPRI 8209 | Hokkaido, Japan | AB733423, AB733414 | |
| HFPRI 8601 | Hokkaido, Japan | AB733424, AB733415 | |
| HFPRI 8603 | Nagano, Japan | AB733426, AB733416 | |
| HFPRI 8604 | Nagano, Japan | AB733427, AB733417 | |
| HFPRI 8701 | Toyama, Japan | AB733428, AB733418 | |
| HFPRI 8804 | Hokkaido, Japan | AB733429, AB733348 | |
| HFPRI 9302 | Hokkaido, Japan | AB733430, AB733347 | |
| HFPRI 9401 | Tokyo, Japan | AB733431, AB733419 | |
| Trametes hirsuta | NBRC 4917 | - | AB733167, AB733343 |
| NBRC 4920 | - | AB733169, AB733344 | |
| NBRC 6477 | - | AB733168, AB733345 | |
| NBRC 7038 | - | AB733170, AB733346 | |
| Trametes versicolor | NBRC 4937 | - | AB733152, AB733328 |
| NBRC 4940 | - | AB733153, AB733329 | |
| NBRC 6481 | - | LC705011, AB733330 | |
| NBRC 8754 | - | AB733154, AB733331 | |
| NBRC 30340 | - | AB733151, AB733327 | |
| KUT 0301 | Kochi, Japan | LC705009, - | |
| KUT 0302 | Kochi, Japan | LC705010, - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horisawa, S.; Iwamoto, K. Identification and Typing of Strains of Wood-Rotting Basidiomycetes by Protein Profiling Using MALDI-TOF MS. BioTech 2022, 11, 30. https://doi.org/10.3390/biotech11030030
Horisawa S, Iwamoto K. Identification and Typing of Strains of Wood-Rotting Basidiomycetes by Protein Profiling Using MALDI-TOF MS. BioTech. 2022; 11(3):30. https://doi.org/10.3390/biotech11030030
Chicago/Turabian StyleHorisawa, Sakae, and Koki Iwamoto. 2022. "Identification and Typing of Strains of Wood-Rotting Basidiomycetes by Protein Profiling Using MALDI-TOF MS" BioTech 11, no. 3: 30. https://doi.org/10.3390/biotech11030030
APA StyleHorisawa, S., & Iwamoto, K. (2022). Identification and Typing of Strains of Wood-Rotting Basidiomycetes by Protein Profiling Using MALDI-TOF MS. BioTech, 11(3), 30. https://doi.org/10.3390/biotech11030030






