Evaluation of Navify Mutation Profiler Tertiary Analysis Software Assessing for Hematologic Malignancies †
Abstract
1. Introduction
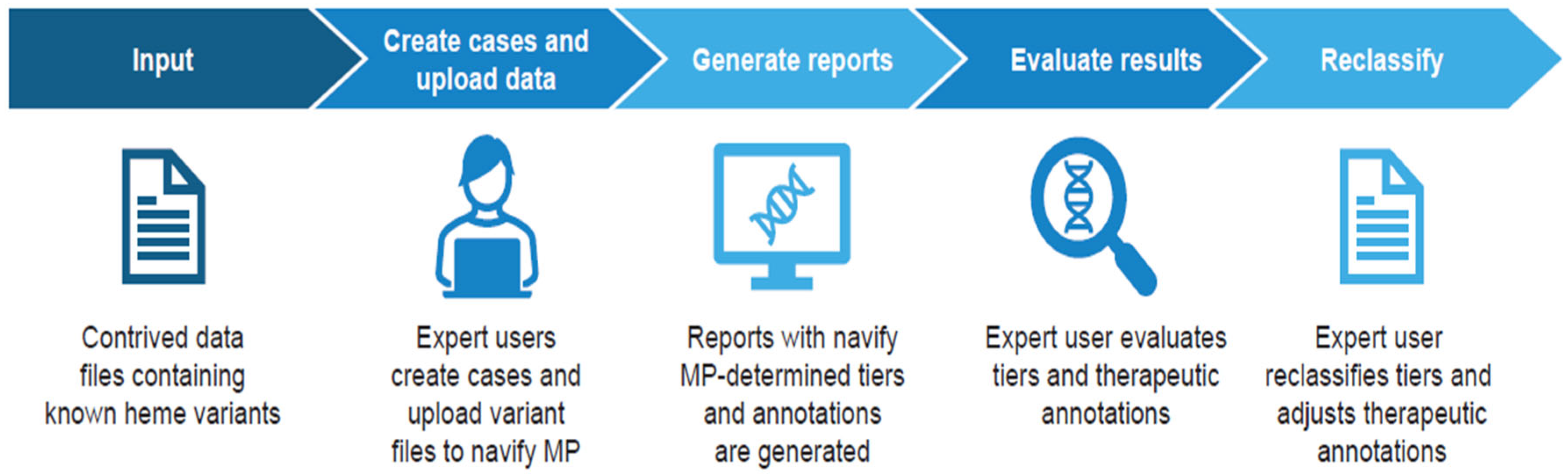
2. Materials and Methods
2.1. Study Design
2.2. Test Methods
- With the exact same filter set applied, not allowing for any deviations, the reproducibility of the software’s curation of tier classifications and treatment options of hematologic malignancies was expected to be 100%. The output from Navify MP generated by the users should match the reference result.
- Given a pre-defined filter set, the accuracy of the software’s curation of tier classifications (Tier IA vs. non-Tier IA) of individual variants from hematologic malignancies was expected to be ≥85%. This threshold of 85% was chosen due to the known concordance from the AMP’s VITAL challenge, which found an overall concordance between AMP member classifications and expected classifications of 81% [11], the subjectivity of tier IA classifications [12], and the limited total number of Tier IA variants in hematologic malignancies. As Navify MP enables expert users to reclassify variants according to their judgment, it is functionally robust to disagreement between user and software-determined classification. The goal of the acceptance criteria is to ensure the user is not overwhelmed with large numbers of disagreements, rather than to penalize the software and databases for the occasional disagreement.
- The accuracy of treatment options (Navify MP-assigned Tier IA with either “therapies approved/guidelines recommended in: matching indication” or “no approved therapies” without reclassification) for individual variants from hematologic malignancies was expected to be ≥85%. Again, 85% was chosen due to the subjectivity involved in the determination of treatment options, which are not completely prescriptive as per guidelines and ultimately rely on professional expert judgment [12].
2.3. Variant Validation and Test Articles
2.4. Replication Test of Usability Across Users
2.5. Statistical Analysis
- For replication testing: Using the exact same filter set applied without tier reclassification
- For agreement: Using pre-defined filters with tier reclassification as decided by the software users
3. Results
3.1. Hematologic Malignancies: Agreement Based on Concordance
3.1.1. Individual Variants
3.1.2. Subgroup Analysis of Biomarkers
3.1.3. Variant Combinations
3.2. Treatment
3.3. Solid Tumors: Agreement Based on Concordance
Solid Tumors: Treatment
3.4. Replication Test of Usability Across Users
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freedman, A.N.; Klabunde, C.N.; Wiant, K.; Enewold, L.; Gray, S.W.; Filipski, K.K.; Keating, N.L.; Leonard, D.G.B.; Lively, T.; McNeel, T.S.; et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United States. JCO Precis. Oncol. 2018, 2, PO.18.00169. [Google Scholar] [CrossRef] [PubMed]
- Ijzerman, M.J.; de Boer, J.; Azad, A.; Degeling, K.; Geoghegan, J.; Hewitt, C.; Hollande, F.; Lee, B.; To, Y.H.; Tothill, R.W.; et al. Towards routine implementation of liquid biopsies in cancer management: It is always too early, until suddenly it is too late. Diagnostics 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Trends in FDA-Approved Cancer Therapies. US Pharmacist. Available online: https://www.uspharmacist.com/article/trends-in-fdaapproved-cancer-therapies (accessed on 18 October 2023).
- Targeted Therapy Approved for Leukemia. NIH National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/approved-drug-list#targeted-therapy-approved-for-leukemia (accessed on 19 December 2024).
- Yang, W.; Williams, J.H.; Hogan, P.F.; Bruinooge, S.S.; Rodriguez, G.I.; Kosty, M.P.; Bajorin, D.F.; Hanley, A.; Muchow, A.; McMillan, N.; et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: An aging, better-insured population will result in shortage. J. Oncol. Pract. 2014, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Schmidt, R.L.; Aisner, D.L.; Behdad, A.; Betz, B.L.; Brown, N.; Coleman, J.F.; Corless, C.L.; Deftereos, G.; Ewalt, M.D.; et al. Multi-institutional evaluation of interrater agreement of variant classification based on the 2017 Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists standards and guidelines for the interpretation and reporting of sequence variants in cancer. J. Mol. Diagn. 2020, 22, 284–293. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.3.2024; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2024; Available online: https://www.nccn.org/ (accessed on 29 October 2024).
- NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.2.2024; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2024; Available online: https://www.nccn.org/ (accessed on 29 October 2024).
- NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myelodysplastic Syndromes V.3.2024; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2024; Available online: https://www.nccn.org/ (accessed on 29 October 2024).
- Li, M.M.; Cottrell, C.E.; Pullambhatla, M.; Roy, S.; Temple-Smolkin, R.L.; Turner, S.A.; Wang, K.; Zhou, Y.; Vnencak-Jones, C.L. Assessments of somatic variant classification using the Association for Molecular Pathology/American Society of Clinical Oncology/College of American Pathologists guidelines: A report from the Association for Molecular Pathology. J. Mol. Diagn. 2023, 25, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Khalife, R.; Love, T.M.; Sucheston-Campbell, L.; Clark, M.J.; Sorensen, H.; Krishna, S.; Magliocco, A. Comparing classifications from multiple variant annotation software solutions using real-world next generation sequencing data from oncology testing. J. Mol. Pathol. 2024, 5, 81–95. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Salifu, S.P.; Doughan, A. New clues to prognostic biomarkers of four hematological malignancies. J. Cancer 2022, 13, 2490–2503. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Johnson, A.; Shufean, A.; Kahle, M.; Yang, D.; Woodman, S.E.; Vu, T.; Moorthy, S.; Holla, V.; Meric-Bernstam, F. Operationalization of next-generation sequencing and decision support for precision oncology. JCO Clin. Cancer Inform. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nafees, A.; Khan, M.; Chow, R.; Fazelzad, R.; Hope, A.; Liu, G.; Letourneau, D.; Raman, S. Evaluation of clinical decision support systems in oncology: An updated systematic review. Crit. Rev. Oncol. Hematol. 2023, 192, 104143. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, R.; Li, Z.; Ding, J.; Xie, J.; Li, J. Challenges of providing concordant interpretation of somatic variants in non-small cell lung cancer: A multicenter study. J. Cancer 2019, 10, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Tudini, E.; Andrews, J.; Lawrence, D.M.; King-Smith, S.L.; Baker, N.; Baxter, L.; Beilby, J.; Bennetts, B.; Beshay, V.; Black, M.; et al. Shariant platform: Enabling evidence sharing across Australian clinical genetic-testing laboratories to support variant interpretation. Am. J. Hum. Genet. 2022, 109, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.L. Grappling with the data explosion in oncology. Oncol. Hematol. Rev. 2015, 11, 102. [Google Scholar] [CrossRef]

| Tier | Total Variants * | Variants in Agreement | Agreement% (95% CI) |
|---|---|---|---|
| IA | 254 | 232 | 91.34% (87.18–94.49%) |
| Non-IA | 348 | 340 | 97.70% (95.52–99.00%) |
| IB | 26 | 21 | 80.77% (60.65–93.45%) |
| IIC | 175 | 161 | 92.00% (86.94–95.56%) |
| IID | 21 | 19 | 90.48% (69.62–98.83%) |
| III | 126 | 122 | 96.83% (92.07–99.13%) |
| Overall (IA vs. non-IA) | 602 | 572 | 95.02% (92.96–96.61%) |
| For Tier IA | For Non-Tier IA | ||||
|---|---|---|---|---|---|
| Total Variants * | Agreement % (n/N; 95% CI) | Total Variants * | Agreement % (n/N; 95% CI) | ||
| Tumor Type | ALL | 52 | 86.54% (45/52; 74.21–94.41%) | 53 | 98.11% (52/53; 89.93–99.95%) |
| AML | 92 | 98.91% (91/92; 94.09–99.97%) | 55 | 100.0% (55/55; 93.51–100.0%) | |
| CML | 42 | 85.71% (36/42; 71.46–94.57%) | 35 | 100.0% (35/35; 90.00–100.0%) | |
| DLBCL | 21 | 100.0% (21/21; 83.89–100.0%) | 70 | 98.57% (69/70; 92.30–99.96%) | |
| FL | 12 | 100.0% (12/12; 73.54–100.0%) | 93 | 95.70% (89/93; 89.35–98.82%) | |
| MM | 35 | 77.14% (27/35; 59.86–89.58%) | 42 | 95.24% (40/42; 83.84–99.42%) | |
| Region | Canada | 64 | 93.75% (60/64; 84.76–98.27%) | 108 | 95.37% (103/108; 89.53–98.48%) |
| EU | 64 | 100.0% (64/64; 94.40–100.0%) | 108 | 100.0% (108/108; 96.64–100.0%) | |
| US | 126 | 85.71% (108/126; 78.37–91.31%) | 132 | 97.73% (129/132; 93.50–99.53%) | |
| Software User | User 1 | 32 | 100.0% (32/32; 89.11–100.0%) | 54 | 90.74% (49/54; 79.70–96.92%) |
| User 2 | 32 | 87.50% (28/32; 71.01–96.49%) | 54 | 100.0% (54/54; 93.40–100.0%) | |
| User 3 | 32 | 100.0% (32/32; 89.11–100.0%) | 54 | 100.0% (54/54; 93.40–100.0%) | |
| User 4 | 32 | 100.0% (32/32; 89.11–100.0%) | 54 | 100.0% (54/54; 93.40–100.0%) | |
| User 5 | 42 | 64.29% (27/42; 48.03–78.45%) | 44 | 95.45% (42/44; 84.53–99.44%) | |
| User 6 | 42 | 100.0% (42/42; 91.59–100.0%) | 44 | 100.0% (44/44; 91.96–100.0%) | |
| User 7 | 42 | 92.86% (39/42; 80.52–98.50%) | 44 | 97.73% (43/44; 87.98–99.94%) | |
| Tier | Total Variants * | Variants in Agreement | Agreement % (95% CI) |
|---|---|---|---|
| IA | 17 | 17 | 100.0% (80.49%, 100.0%) |
| Non-IA | 116 | 116 | 100.0% (96.87%, 100.0%) |
| Overall | 133 | 133 | 100.0% (97.26%, 100.0%) |
| Total Treatment Options * | User-Assigned Treatment Options | Agreement on Treatment Assignment | ||
|---|---|---|---|---|
| Agreement% (n/N) | 95% CI | |||
| Navify MP-Determined Tier IA Variant Combinations (n = 17) | 56 | 53 | 94.64% (53/56) | (85.13–98.88%) |
| Total Variants | Total Treatment Options * | Agreement on Treatment Assignment | ||
|---|---|---|---|---|
| Agreement % (n/N) | 95% CI | |||
| All Navify MP-Assigned Tier IA Individual Variants | 254 | 325 | 99.08% (322/325) | (97.33–99.81%) |
| Individual Navify MP- and User-Assigned Tier IA Variants, | 232 | 303 | 99.67% (302/303) | (98.17–99.99%) |
| Tier | Total Variants * | Variants in Agreement | Agreement % (95% CI) | |
|---|---|---|---|---|
| All Individual Variants | IA | 67 | 67 | 100.0% (94.64–100.0%) |
| Navify MP and User-Assigned Tier IA Variant Combinations | Non-IA | 94 | 92 | 97.87% (92.52–99.74%) |
| Overall | 161 | 159 | 98.76% (95.58–99.85%) | |
| TMB | IA | 5 | 5 | 100.0% (47.82–100.0%) |
| Non-IA | 9 | 9 | 100.0% (66.37–100.0%) | |
| Overall | 14 | 14 | 100.0% (76.84–100.0%) | |
| MSI | IA | 3 | 3 | 100.0% (29.24–100.0%) |
| Non-IA | 11 | 11 | 100.0% (71.51–100.0%) | |
| Overall | 14 | 14 | 100.0% (76.84–100.0%) |
| Total Variants | Total Treatment Options * | Agreement on Treatment Assignment | |||
|---|---|---|---|---|---|
| Agreement % (n/N) | 95% CI | ||||
| Navify MP-Assigned Tier IA Variants | All Individual Variants | 67 | 154 | 96.75% (149/154) | (92.59–98.94%) |
| TMB | 5 | 15 | 100.00% (15/15) | (78.20–100.00%) | |
| MSI | 3 | 3 | 100.00% (3/3) | (29.24–100.00%) | |
| Navify MP and User- Assigned Tier IA Variants | All Individual Variants | 67 | 154 | 96.75% (149/154) | (92.59–98.94%) |
| TMB | 5 | 15 | 100.00% (15/15) | (78.20–100.00%) | |
| MSI | 3 | 3 | 100.00% (3/3) | (29.24–100.00%) | |
| Tier | Total Variants * | Variants in Agreement | Replication Agreement % (95% CI) |
|---|---|---|---|
| IA | 254 | 254 | 100.0% (98.56–100.0%) |
| Non-IA | 348 | 348 | 100.0% (98.95–100.0%) |
| IB | 26 | 26 | 100.0% (86.77–100.0%) |
| IIC | 175 | 175 | 100.0% (97.91–100.0%) |
| IID | 21 | 21 | 100.0% (83.89–100.0%) |
| III | 126 | 126 | 100.0% (97.11–100.0%) |
| Overall | 602 | 602 | 100.0% (99.39–100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhrao, R.; Clark, M.J.; Chugh, S.; Capucion, L.; Krishna, S.; Yerram, R.; Niu, L.; Parham, A.; Harrell, A.; Duncan, J.; et al. Evaluation of Navify Mutation Profiler Tertiary Analysis Software Assessing for Hematologic Malignancies. J. Mol. Pathol. 2025, 6, 9. https://doi.org/10.3390/jmp6020009
Singhrao R, Clark MJ, Chugh S, Capucion L, Krishna S, Yerram R, Niu L, Parham A, Harrell A, Duncan J, et al. Evaluation of Navify Mutation Profiler Tertiary Analysis Software Assessing for Hematologic Malignancies. Journal of Molecular Pathology. 2025; 6(2):9. https://doi.org/10.3390/jmp6020009
Chicago/Turabian StyleSinghrao, Ruby, Michael J. Clark, Shikha Chugh, Lisha Capucion, Shuba Krishna, Ranga Yerram, Lili Niu, Adama Parham, Amy Harrell, John Duncan, and et al. 2025. "Evaluation of Navify Mutation Profiler Tertiary Analysis Software Assessing for Hematologic Malignancies" Journal of Molecular Pathology 6, no. 2: 9. https://doi.org/10.3390/jmp6020009
APA StyleSinghrao, R., Clark, M. J., Chugh, S., Capucion, L., Krishna, S., Yerram, R., Niu, L., Parham, A., Harrell, A., Duncan, J., Clark, K., & Javey, M. (2025). Evaluation of Navify Mutation Profiler Tertiary Analysis Software Assessing for Hematologic Malignancies. Journal of Molecular Pathology, 6(2), 9. https://doi.org/10.3390/jmp6020009





