1. Introduction
Immunomodulators regulate the immune system’s response, either enhancing or suppressing it to combat diseases like cancer [
1,
2,
3]. In cancer therapy, immunostimulation activates immune cells, such as T cells, enabling them to target and destroy cancer cells more effectively [
1,
2,
3]. Agents that enhance pathways, such as interferon production, have become vital, especially with the advent of immune checkpoint inhibitors (ICIs) like PD-1 and CTLA-4 blockers [
4,
5,
6,
7,
8,
9]. Imiquimod, a well-known small-molecule immunomodulator, exemplifies this potential by activating the immune system through binding to toll-like receptor 7 (TLR7) [
10,
11]. This binding stimulates cytokine production, particularly interferons, which are crucial for initiating immune responses [
10,
11]. Imiquimod’s ability to promote immunostimulation highlights how small molecules can enhance the immune system’s capability to target and eliminate abnormal or cancerous cells.
Despite the success of agents like Imiquimod, the range of available small-molecule immunostimulatory agents remains limited. Expanding this range is crucial for advancing cancer treatments, as these molecules can directly modulate immune signaling pathways, potentially reversing immune exhaustion and reactivating suppressed immune cells within the tumor microenvironment. This broadening of immunomodulator types could enhance cancer immunotherapy, particularly for patients who do not respond to existing treatments or develop resistance.
The One-Bead-Two-Compound (OB2C) library method is an advanced platform for high-throughput small-molecule discovery [
12,
13]. Each bead in the OB2C library displays a known cell adhesion or cell-capturing ligand alongside a random library compound. The barcode or chemical tag for identification is embedded within the bead to avoid interference during screening. When live cells interact with the OB2C library, the capture ligand brings the library compound in close proximity to the target cells, facilitating a direct interaction between the compound and the cells [
12,
13]. This allows for efficient screening, where beads that elicit a desired biochemical or cellular response can be quickly identified using a reporter system. The OB2C method has been particularly successful in identifying novel therapeutic candidates, such as Galectin-1 inhibitors, which have shown significant promise in preclinical xenograft models by inhibiting tumor growth [
13]. Its efficiency in screening large libraries in a single experiment makes OB2C a powerful and effective tool for discovering new cancer therapies.
In this study, we utilized the OB2C combinatorial library method to identify novel small-molecule immunomodulators for cancer therapy. In this approach, each OB2C bead was functionalized to present two distinct compounds, one to facilitate an immune cell interaction and another from a library of candidate molecules. Upon incubation with immune cells, beads associated with activated cells were identified by detecting intracellular IFN-γ production using immunocytochemistry (ICC). From an OB2C library comprising 1764 compounds, Kib-IM-4 emerged as a potent immunomodulator. Kib-IM-4 significantly enhanced IFN-γ production in peripheral blood mononuclear cells (PBMCs) and promoted immune cell activation. In addition, Kib-IM-4 boosted the cytolytic activity of PBMCs against prostate cancer cells, resulting in a substantial reduction in tumor cell viability. These findings demonstrate the utility of the OB2C platform in discovering functional immunomodulators and highlight Kib-IM-4’s potential as a promising candidate for further development in cancer immunotherapy.
2. Methods
2.1. Construction of OB2C Library
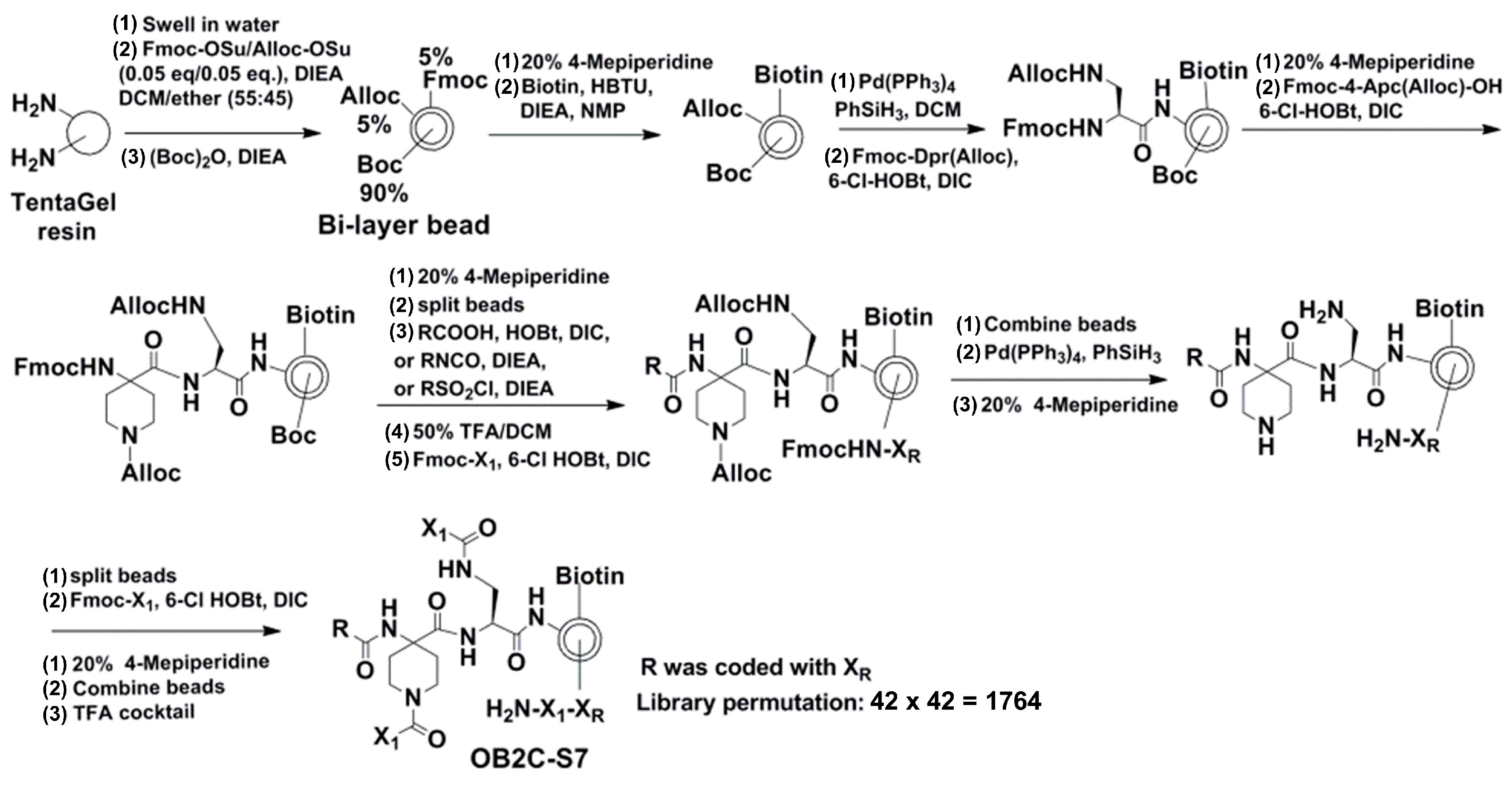
The OB2C library was constructed using a bi-layer bead system based on TentaGel resin [
13]. Each bead displayed biotin and a benzimidazole-based compound, with a tripeptide coding tag embedded inside to prevent interference with the screening process. The synthesis started by swelling TentaGel resin, followed by coupling Alloc-protected Fmoc amino acids and biotin using HBTU-mediated reactions. The beads were split and functionalized with 42 different carboxylic acids, isocyanates, or acyl sulfonyl chlorides to generate the first chemical diversity (R group). A second round of splitting and coupling introduced 42
l-/
d-amino acids (X1 group) to add further diversity. The resulting OB2C library contained 1764 unique compounds displayed on the bead surface for high-throughput screening.
2.2. Immunomodulator Screening Using Jurkat Cells
First, neutravidin (5 nmol) was reacted with LLP2A-biotin at a 1:2 molar ratio for 20 min. The OB2C beads were then adhered to a 12-well culture plate using 80% dimethylformamide. The mixture of neutravidin and LLP2A-biotin was added to the wells containing the beads. After 20 min of incubation, Jurkat cells (5 × 105) were seeded into the wells and incubated at 37 °C for 20 min. Positive controls involved LLP2A beads incubated with Jurkat cells stimulated using a cell stimulation cocktail (eBioscience, San Diego, CA, USA) containing 50 ng/mL PMA and 1.35 µM ionomycin) in the presence of 5 μg/mL Brefeldin A (eBioscience), while negative controls involved LLP2A beads incubated with Jurkat cells without stimulation. The activation cocktail stimulated protein kinase C (PKC) and increased intracellular calcium, promoting IFN-γ production. Brefeldin A was included during the incubation to block protein transport and prevent cytokine secretion, enhancing intracellular detection. Unbound cells were gently washed off using PBS, and the cell-bound beads were incubated at 37 °C for an additional 24 h. Following the incubation, the cells were fixed with 4% paraformaldehyde for 20 min. For the ICC assay, nonspecific protein binding was blocked by adding 5% BSA, and the cell membrane was permeabilized using 0.5% Triton X-100. Rabbit anti-human IFN-γ (Cell Signaling Technology, Danvers, MA, USA) was used as the primary antibody at a 1:100 dilution in PBS, and the beads were incubated with the primary antibody overnight at 4 °C. After washing with PBS, the beads were incubated with the secondary antibody, a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, for 1 h at room temperature. HRP activity was detected using diaminobenzidine tetrahydrochloride as a substrate for 3 min, according to the manufacturer’s instructions (BioGenex, Fremont, CA, USA). In addition, the significant difference in cell numbers between the positive and negative controls can be attributed to PMA-induced α4β1 integrin expression, which enhances Jurkat cell adhesion and retention during washing in the positive control. In contrast, the absence of PMA in the negative control results in weaker adhesion and greater cell loss during washing, leading to lower cell numbers and reduced IFN-γ detection.
2.3. Decoding of Positive Beads
Positive beads were isolated and treated with 6 mol/L guanidine HCl (pH 1.0) to remove any bound cells, proteins, or biomolecules produced by the cells. After thorough washing with water, the beads were chemically decoded using standard Edman microsequencing on an ABI Procise 494 system.
2.4. Cytokine Production Assays with PBMCs
To evaluate the immunostimulatory potential of the resynthesized compounds, soluble forms of Kib-IM-4 were added to freshly isolated PBMCs at concentrations of 10 µM, 20 µM, and 40 µM. Cytokine secretion, including IFN-γ, IL-1β, IL-2, IL-6, and TNF-α, was measured using Luminex xMAP technology. The treated PBMCs were incubated for 24, 48, and 72 h, and the cytokine levels in the culture supernatants were analyzed to determine the time- and dose-dependent effects of Kib-IM-4 on immune activation.
2.5. PBMC Clustering and Activation Assay
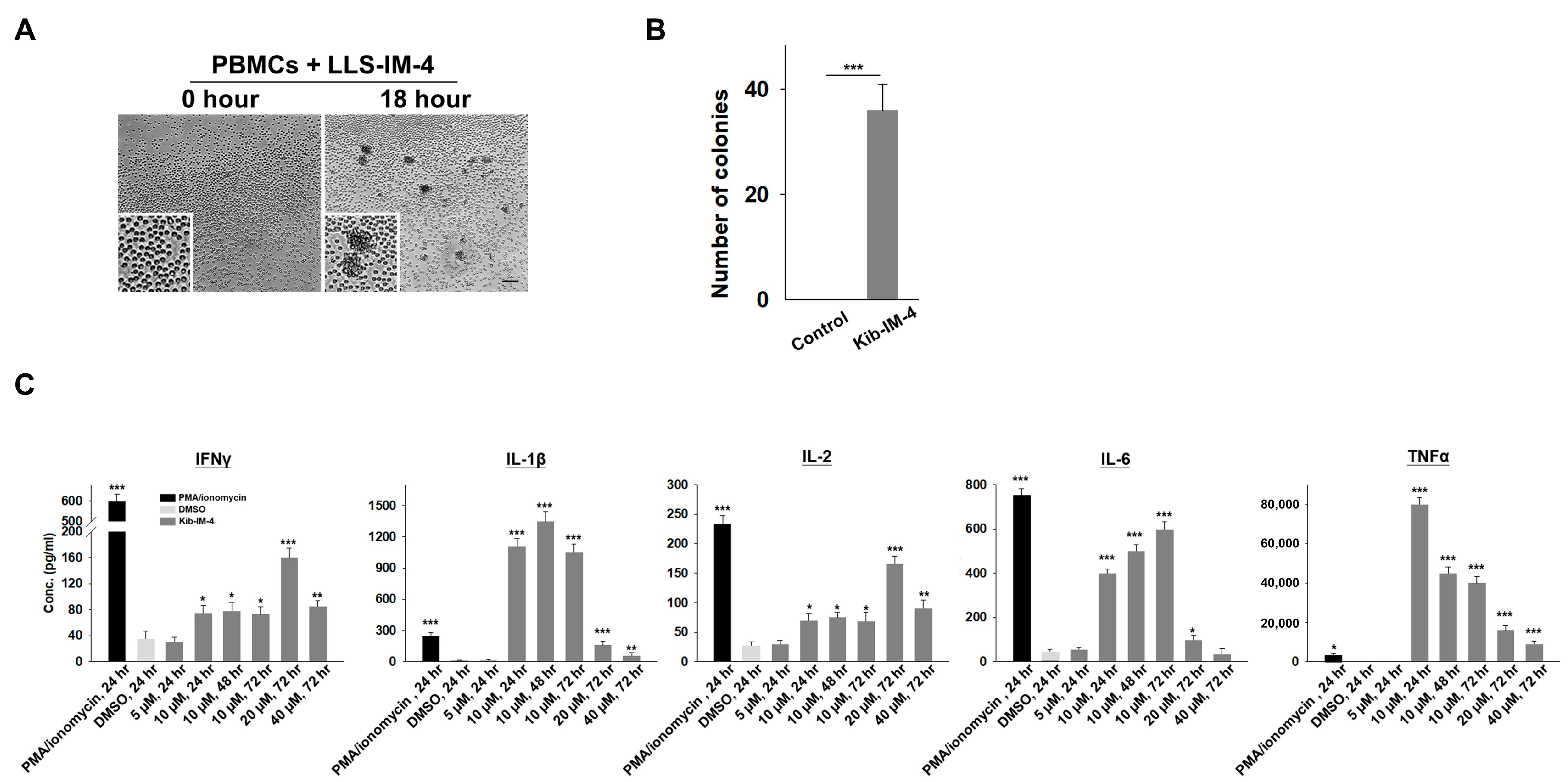
Microscopy (Olympus, Center Valley, PA, USA) was used to assess PBMC clustering as an indicator of immune activation after treatment with Kib-IM-4. PBMCs were treated with soluble Kib-IM-4 at 10 µM and incubated for 72 h. The formation of cell clusters was visualized and documented as evidence of immune cell activation. Three days after treatment, colonies were imaged for size measurement, and those with diameters exceeding 40 µm were counted from three separate wells.
2.6. Cytolytic Activity of PBMCs Co-Cultured with Prostate Cancer Cells
To assess the cytolytic potential of Kib-IM-4-stimulated PBMCs, a co-culture system was set up with prostate cancer cell lines (PC3, DU145, LNCaP, and 22RV1). PBMCs were treated with 10 µM Kib-IM-4 and co-cultured with cancer cells at effector-to-target ratios of 2:1 and 5:1. Microscopy was used to visualize interactions between the PBMCs and prostate cancer cells. After 72 h, cell viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA) to determine the cytotoxic effects of Kib-IM-4-stimulated PBMCs on prostate cancer cells.
2.7. Statistical Analysis
All in vitro experimental studies were performed in triplicate, with each experiment independently repeated twice. Statistical comparisons between variables were performed using either a one-way ANOVA or Student’s
t-test, depending on the data type. The results are expressed as mean ± standard deviation (SD), with a significance threshold set at
p < 0.05. Z-factor, a statistical measure of assay quality, was used to assess the robustness and reliability of the OB2C screening for immunomodulator discovery [
14]. A Z-factor between 0.5 and 1.0 indicates a highly reliable screening system capable of effectively distinguishing between active and inactive compounds.
3. Results
3.1. Construction of the OB2C Library for High-Throughput Screening
The OB2C library utilized a bi-layer bead system built on a TentaGel resin base, with each bead displaying biotin and a benzimidazole-based compound. A tripeptide coding tag was placed inside the bead to avoid interference with the screening process. The synthesis started by swelling the TentaGel resin and coupling Alloc-protected Fmoc amino acids and biotin through HBTU-mediated reactions. The beads were then split and functionalized with 42 different carboxylic acids, isocyanates, or acyl sulfonyl chlorides to create the first chemical diversity (R group). Another round of splitting and coupling was performed using 42 L-/D-amino acids (X1 group) to introduce the second diversity. The final OB2C-S7 library contained 1764 unique compounds (42 × 42) displayed on the bead surface for high-throughput screening (
Figure 1).
3.2. Development of OB2C Screening for Immunomodulator Discovery
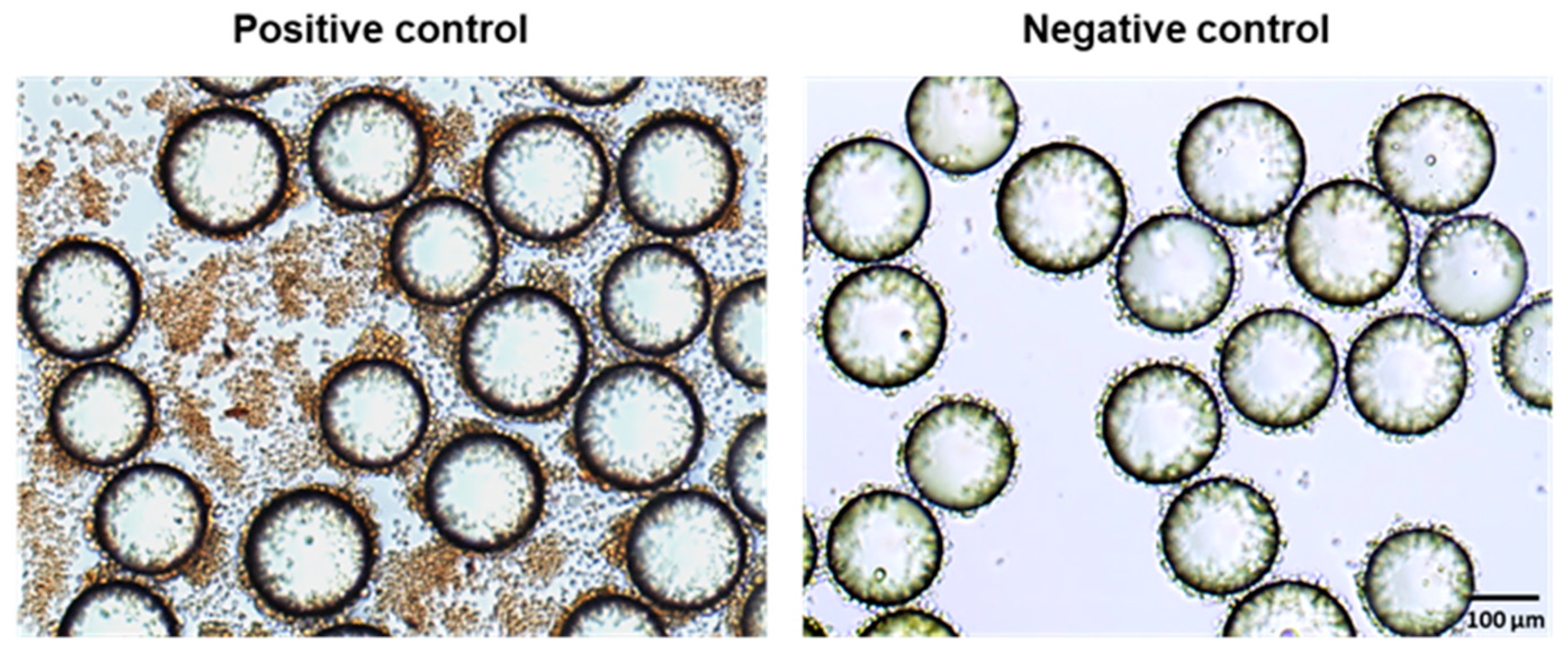
Jurkat cells, a lymphoid T cell line that produces minimal IFN-γ under normal conditions, can be activated through the stimulation of PKC by PMA/ionomycin to induce IFN-γ production. Thus, we utilized Jurkat cells in the experiments to develop the OB2C screening methodology. The detection of IFN-γ was achieved using an HRP-conjugated anti-IFN-γ antibody as the probe to identify immunomodulatory beads. Human IgG-HRP was used as a background control in ICC on beads. The positive control involved LLP2A beads incubated with PMA/ionomycin-stimulated Jurkat cells and Brefeldin A, and the negative control was LLP2A beads incubated without PMA/ionomycin (
Figure 2). In the positive control (
Figure 2), IFN-γ production in non-bead-associated cells is likely due to the unintended binding of the LLP2A-biotin-neutravidin mixture to the wells, which captured some Jurkat cells. PMA/ionomycin stimulation elevated the IFN-γ signal to 180% relative to the background, while cells without PMA/ionomycin showed only a 3% increase. This resulted in an average Z-factor of 0.89, which is an excellent indicator for a reliable screening system. This value confirmed that the assay can distinguish active from inactive compounds effectively.
3.3. Identification of Immunomodulatory Compounds from the OB2C Library
LLP2A was incorporated into the OB2C libraries as a cell-capturing ligand for Jurkat cells, due to its high affinity for α4β1 integrin, which is commonly activated in malignant lymphoid cells [
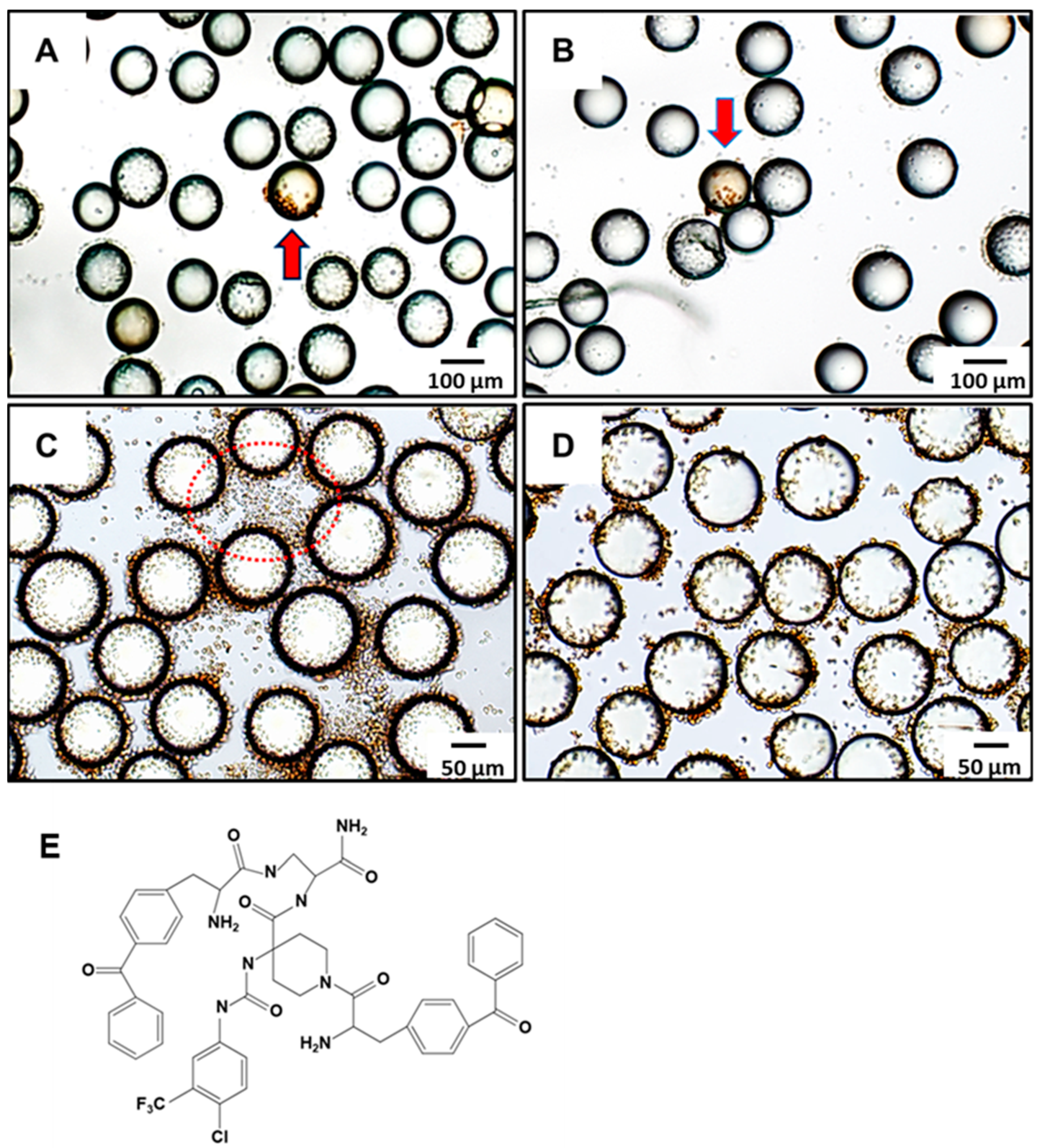
15]. LLP2A facilitated the attachment of suspension cells to the bead surface, enabling an interaction between the displayed small molecules on the beads and the cell surface receptors. This setup was designed to test the hypothesis that random compounds might activate immune responses by interacting with receptors like Toll-like receptors. Brefeldin A was used to block protein transport during activation, enhancing intracellular cytokine staining and aiding in the detection of immune responses. Beads displaying compounds that induced IFN-γ production were identified by brown-stained cells, indicating positive immunomodulatory activity. In
Figure 3A,B, red arrows indicate two positive beads that triggered IFN-γ expression, as demonstrated by the brown staining of the attached Jurkat cells. These beads correspond to the Kib-IM-1 and Kib-IM-4 compounds, which were identified from a 1764-compound OB2C library. Beads were resynthesized with Kib-IM-1 and Kib-IM-4 for validation, as shown in
Figure 3C,D. The brown-stained cells indicate successful IFN-γ production, confirming the immunomodulatory effect of these compounds. In
Figure 3C, non-bead-associated cells do not produce IFN-γ, as they are not exposed to the small molecules synthesized on the beads, highlighting the essential role of bead-associated molecules in IFN-γ production.This comparison underscores the selective immunomodulatory activity of Kib-IM-1 and Kib-IM-4, aligning with the initial screening results. The structure of Kib-IM-4 is shown in
Figure 3E.
3.4. Impact of Kib-IMs on PBMC Clustering and IFN-γ Production
To evaluate the immunostimulatory potential of Kib-IM compounds on immune cells, we resynthesized the compounds in a soluble form and treated PBMCs at various concentrations. Cytokine secretion from these stimulated cells was analyzed using Luminex xMAP technology. When PBMCs were treated with the soluble form of Kib-IM-4, microscopy images revealed a key aspect of its immunostimulatory activity: the formation of PBMC clusters (
Figure 4A,B). These pronounced clumping and aggregation patterns are hallmarks of immune cell activation, indicating a stimulated immune environment where PBMCs interact more intensely, potentially enhancing immune surveillance and tumor-targeting capabilities. We measured IFN-γ secretion levels following treatment with 5 µM, 10 µM, 20 µM, and 40 µM of Kib-IM-4 at 24, 48, and 72 h (
Figure 4C). Under basal conditions, unstimulated PBMCs did not produce cytokines or upregulate activation markers. After treatment with Kib-IM-4, significant IFN-γ production was observed (
Figure 4B). In addition, the production of other key immune mediators, such as IL-1β, IL-2, IL-6, and TNF-α, was significantly increased, further demonstrating the robust immunostimulatory effects of Kib-IM-4 (
Figure 4B). Unlike Kib-IM-4, Kib-IM-1 did not induce PBMC clustering or significantly enhance IFN-γ production. This observed clustering, along with the significant increase in cytokine secretion, particularly IFN-γ, suggested that Kib-IM-4 may have the ability to enhance the immune cell function and activate key pathways involved in immune responses.
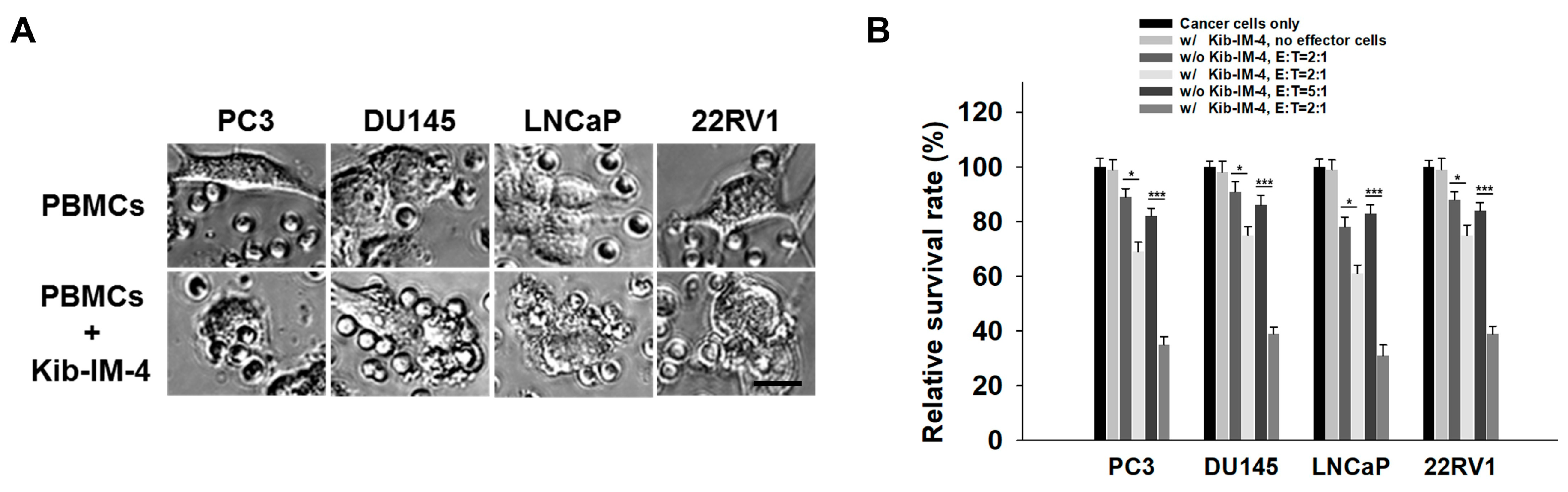
3.5. Enhanced Cytolytic Activity of Kib-IM-4-Stimulated PBMCs Against Prostate Cancer Cells
To assess the cytolytic potential of Kib-IM-4-stimulated PBMCs, we co-cultured them with prostate cancer cells including PC3, DU145, LNCaP, and 22RV1. Microscopy images clearly show PBMCs actively engaging with and attacking prostate cancer cells following Kib-IM-4 exposure, resulting in significant immune cell interactions and the disruption of cancer cells (
Figure 5A). In contrast, untreated PBMCs displayed minimal to no interaction with the cancer cells (
Figure 5A). The quantification reveals a significant reduction in the survival rates of prostate cancer cell lines when co-cultured with Kib-IM-4-treated PBMCs. This effect was particularly pronounced at a higher PBMC-to-cancer cell ratio (5:1), while the effect was still substantial, but less pronounced at a lower ratio (2:1), demonstrating the dose-dependent nature of the immune response (
Figure 5B). These findings underscore the therapeutic potential of Kib-IM-4 in enhancing immune-mediated tumor destruction.
4. Discussion
The findings in this study highlight the efficiency and potential of the OB2C library method in discovering small-molecule immunomodulators that activate immune responses against cancer cells. Specifically, we identified Kib-IM-4, which significantly increased IFN-γ production in PBMCs and promoted immune cell clustering. Furthermore, Kib-IM-4 enhanced the cytolytic activity of PBMCs against prostate cancer cells, resulting in a marked reduction in tumor cell viability. These results emphasize the potential of Kib-IM-4 in advancing immune-mediated cancer therapies.
As noted, the concentration of Kib-IM-4 required to achieve T cell activation falls within the 10–40 µM range, which is relatively high for therapeutic applicability. However, it is important to note that this study is in the discovery stage and serves as a proof of concept. We utilized the OB2C technology to identify novel immunomodulators for cancer therapy. The identified compound, Kib-IM-4, represents an initial hit from the screening process. Further optimization, including structure–activity relationship (SAR) studies and lead compound refinement, will be necessary to enhance its potency and reduce the effective concentration required for the activity. This initial proof-of-concept underscores the potential of the OB2C platform to identify functional small molecules, paving the way for subsequent development and therapeutic optimization. Target protein identification is a crucial next step in understanding the molecular mechanisms behind Kib-IM-4’s immunostimulatory effects. Employing techniques such as affinity-based proteomics or RNA sequencing could help uncover the specific signaling pathways and receptor interactions involved. Furthermore, in vivo studies using prostate cancer models are warranted to evaluate the therapeutic potential of Kib-IM-4. Prostate cancer serves as a suitable model given its immunosuppressive tumor microenvironment and the need for novel immune-modulating therapies. These experiments will provide crucial insights into whether Kib-IM-4 can effectively reduce tumor growth, further supporting its potential as a hit compound for future development in cancer immunotherapy.
Small-molecule immunomodulators like those discovered in this study have a clear path toward clinical development. Unlike protein-based therapeutics, which often face hurdles such as poor stability and delivery challenges, small molecules can be more easily optimized for pharmacokinetics and scaled for manufacturing [
16,
17,
18]. Their ability to penetrate tissues more efficiently also enhances their potential to act within the tumor microenvironment [
18,
19]. The significant enhancement in PBMC cytolytic activity against prostate cancer cells upon treatment with Kib-IM-4, as shown in our co-culture experiments, points to the therapeutic relevance of these compounds in activating immune responses to target tumors. However, we acknowledge the potential risks of non-specific T cell activation, which could lead to substantial side effects, including excessive immune activation and off-target tissue damage. To mitigate these risks, future efforts will focus on optimizing the specificity of these compounds by targeting pathways uniquely active within tumor microenvironments.
Although current immunotherapies, such as immune checkpoint inhibitors, have significantly advanced cancer treatment, their efficacy is often limited by immune resistance or insufficient immune activation in some patients [
16,
20]. The small molecules identified in this study offer a new strategy to complement or enhance existing treatments, providing a multi-pronged approach to cancer immunotherapy. By directly modulating immune cell signaling pathways, these compounds may help to overcome the limitations associated with protein-based immunotherapies, offering new hope for patients who do not respond to current treatments.
In conclusion, the results presented here demonstrate that the OB2C platform is a highly effective tool for identifying novel small-molecule immunomodulators. The immunostimulatory effects of Kib-IM compounds on PBMCs and their ability to trigger anti-cancer immune responses provide a solid foundation for further preclinical and clinical development. These small molecules hold promise for improving the efficacy of cancer immunotherapy, addressing critical unmet needs in the field.
Author Contributions
H.-C.W. and T.-C.S. performed the experimental studies, conducted the analysis, and carried out the computational studies. H.-C.W. and T.-C.S. wrote the original draft. T.-C.S. supervised the work. H.-C.W. and T.-C.S. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the United States National Institutes of Health [R43CA271932]; and the United States Department of Defense [W81XWH-22-1-0941, HT9425-23-1-0203] to T.-C.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We gratefully acknowledge the support and resources provided by Johnson & Johnson Innovation—JLABS at the Texas Medical Center (JLABS@TMC)—including access to laboratory space, instruments, and personnel assistance, which were essential for the completion of this project.
Conflicts of Interest
Dr. Tsung-Chieh Shih is a founder of Kibio Inc. and is affiliated with its Department of Research and Development. The remaining authors declare no competing interests.
References
- Petroni, G.; Buqué, A.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 2021, 39, 310–345. [Google Scholar] [CrossRef]
- Dhanak, D.; Edwards, J.P.; Nguyen, A.; Tummino, P.J. Small-Molecule Targets in Immuno-Oncology. Cell Chem. Biol. 2017, 24, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Cheng, K.; Bi, H.; Chen, J. Small molecule-based immunomodulators for cancer therapy. Acta Pharm. Sin. B 2022, 12, 4287–4308. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Thompson, C.B. At the Bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef]
- Callahan, M.K.; Wolchok, J.D. At the Bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.-W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.L.; Gerster, J.F.; Imbertson, L.M.; Reiter, M.J.; Miller, R.L.; Gibson, S.J.; Wagner, T.L.; Tomai, M.A. Cytokine induction by the immunomodulators imiquimod and S-27609. J. Leukoc. Biol. 1995, 58, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shih, T.-C.; Deng, X.; Anwar, L.; Ahadi, S.; Kumaresan, P.; Lam, K.S. Design, synthesis, and application of OB2C combinatorial peptide and peptidomimetic libraries. Methods Mol. Biol. 2015, 1248, 3–22. [Google Scholar] [PubMed]
- Shih, T.C.; Liu, R.; Fung, G.; Bhardwaj, G.; Ghosh, P.M.; Lam, K.S. A Novel Galectin-1 Inhibitor Discovered through One-Bead Two-Compound Library Potentiates the Antitumor Effects of Paclitaxel in vivo. Mol. Cancer Ther. 2017, 16, 1212–1223. [Google Scholar] [CrossRef]
- Zhuo, M. Z factor: A new index for measuring academic research output. Mol. Pain 2008, 4, 53. [Google Scholar] [CrossRef]
- Peng, L.; Liu, R.; Marik, J.; Wang, X.; Takada, Y.; Lam, K.S. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat. Chem. Biol. 2006, 2, 381–389. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, P.; Shrimali, R.; Adurthi, S.; Ramachandra, R.; Satyam, L.; Dhudashiya, A.; Samiulla, D.; Sunilkumar, K.B.; Ramachandra, M. A novel peptide therapeutic targeting PD1 immune checkpoint with equipotent antagonism of both ligands and a potential for better management of immune-related adverse events. J. Immunother. Cancer 2013, 1, O24. [Google Scholar] [CrossRef]
- Beck, H.; Härter, M.; Haß, B.; Schmeck, C.; Baerfacker, L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov. Today 2022, 27, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.; Saung, M.T.; Zheng, L.; Murphy, A.G. Small molecule immunomodulation: The tumor microenvironment and overcoming immune escape. J. Immunother. Cancer 2019, 7, 224. [Google Scholar] [CrossRef]
- Karasarides, M.; Cogdill, A.P.; Robbins, P.B.; Bowden, M.; Burton, E.M.; Butterfield, L.H.; Cesano, A.; Hammer, C.; Haymaker, C.L.; Horak, C.E.; et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol. Res. 2022, 10, 372–383. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).