Resistance Testing for the Treatment of Chronic Hepatitis C with Direct Acting Antivirals: When and for How Long?
Abstract
Conflicts of interest
References
- Sarrazin, C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; Lawitz, E.; Kwo, P.Y.; et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis C virus infection and compensated cirrhosis: An integrated analysis. Gastroenterology, pii: S0016-5085(17)30150-6. 10 Feb 2017. [Google Scholar]
- Zeuzem, S.; Mizokami, M.; Pianko, S.; et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: Prevalence and effect on treatment outcome. J. Hepatol. pii: S0168-8278(17)30011-9. Jan Jan 2017. [Google Scholar]
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015, 62, 932–954. [Google Scholar]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef] [PubMed]
- Fourati, S.; Hezode, C.; Soulier, A.; et al. HCV Resistance to daclatasvir/sofosbuvir across different genotypes in the real life [Abstract 577]. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Boston, USA, 22–25 February 2016. [Google Scholar]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.; Flamm, S.; Yang, J.C.; et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir-based regimens with ledipasvir/sofosbuvir for 24 weeks [Abstract 0005]. In Proceedings of the 50th Annual Meeting of the European Association for the Study of the Liver (EASL), Vienna, Austria, 22–26 April 2015. [Google Scholar]
- Poordad, F.; Bennett, M.; Sepe, T.E.; et al. QUARTZ-I: Retreatment of HCV genotype 1 DAA-failures with ombitasvir/paritaprevir/r, dasabuvir, and sofosbuvir [Abstract LB-20]. In Proceedings of the 66th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), San Francisco, California, USA 13–17 November 2015. [Google Scholar]
- Lawitz, E.J.; Poordad, F.; Gutiérrez, J.; et al. C-SWIFT Retreatment final results: Highly successful retreatment of gt1-infected patients with 12 weeks of elbasvir/grazoprevir plus sofosbuvir and ribavirin after failure of short-duration all-oral therapy [Abstract SAT148]. In Proceedings of the 51st Annual Meeting of the European Association for the Study of the Liver (EASL), Barcelona, Spain, 13–17 April 2016. [Google Scholar]
- Vermehren, J.; Susser, S.; Dietz, J.; et al. Retreatment of patients who failed DAA-combination therapies: Realworld experience from a large hepatitis C resistance database [Abstract PS103]. In Proceedings of the 51st Annual Meeting of the European Association for the Study of the Liver (EASL), Barcelona, Spain, 13–17 April 2016. [Google Scholar]
- Cento, V.; Barbaliscia, S.; Bonora, S.; et al. Optimal efficacy of HCV resistance-based retreatments after PI failure [Abstract 565]. In Proceedings of the Annual Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington, USA, 13–16 February 2017. [Google Scholar]
- Pérez, A.B.; Chueca, N.; García-Deltoro, M.; et al. Retreatment options after failing a first line of DAAs against hepatitis C virus [Abstract 566]. In Proceedings of the The Annual Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington, USA, 13–16 February 2017. [Google Scholar]
- Lontok, E.; Harrington, P.; Howe, A.; et al. Hepatitis C virus drug resistance-associated substitutions: State of the art summary. Hepatology 2015, 62, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Gordon, S.C.; Ramji, A.; et al. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks as a salvage regimen in NS5A inhibitor-experienced patients with genotype 1-6 infection: The phase 3 POLARIS-1 study [Abstract 194]. In Proceedings of the 67th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, Massachusetts, USA, 11–15 November 2016. [Google Scholar]
- Zeuzem, S.; Flamm, S.L.; Tong, M.J.; et al. A randomized, controlled, phase 3 trial of sofosbuvir/ velpatasvir/voxilaprevir or sofosbuvir/velpatasvir for 12 weeks in direct acting antiviral-experienced patients with genotype 1-6 HCV infection: The POLARIS-4 study [Abstract 109]. In Proceedings of the 67th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, Massachusetts, USA, 11–15 November 2016. [Google Scholar]
- Wyles, D.L.; Poordad, F.; Wang, S.; et al. SURVEYOR-II, Part 3: Efficacy and safety of ABT-493/ ABT-530 in patients with hepatitis C virus genotype 3 infection with prior treatment experience and/or cirrhosis [Abstract 113]. In Proceedings of the 67th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, Massachusetts, USA, 11–15 November 2016. [Google Scholar]
- Serfaty, L.; Pianko, S.; Ben Ari, Z.; et al. High sustained virologic response (SVR) rates in patients with chronic HCV GT1, 2 or 3 infection following 16 weeks of MK3682/grazoprevir/MK-8408 plus ribavirin after failure of 8 weeks of therapy (Part C of C-CREST-1 & 2) [Abstract 112]. In Proceedings of the 67th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, Massachusetts, USA, 11–15 November 2016. [Google Scholar]
- Wyles, D.L.; Wedemeyer, H.; Reddy, K.R.; et al. Safety and efficacy of the fixed-dose combination regimen of MK3682/grazoprevir/MK-8408 in cirrhotic or non-cirrhotic patients with chronic HCV GT1 infection who previously failed a direct-acting antiviral regimen (CSURGE) [Abstract 193]. In Proceedings of the 67th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), Boston, Massachusetts, USA, 11–15 November 2016. [Google Scholar]
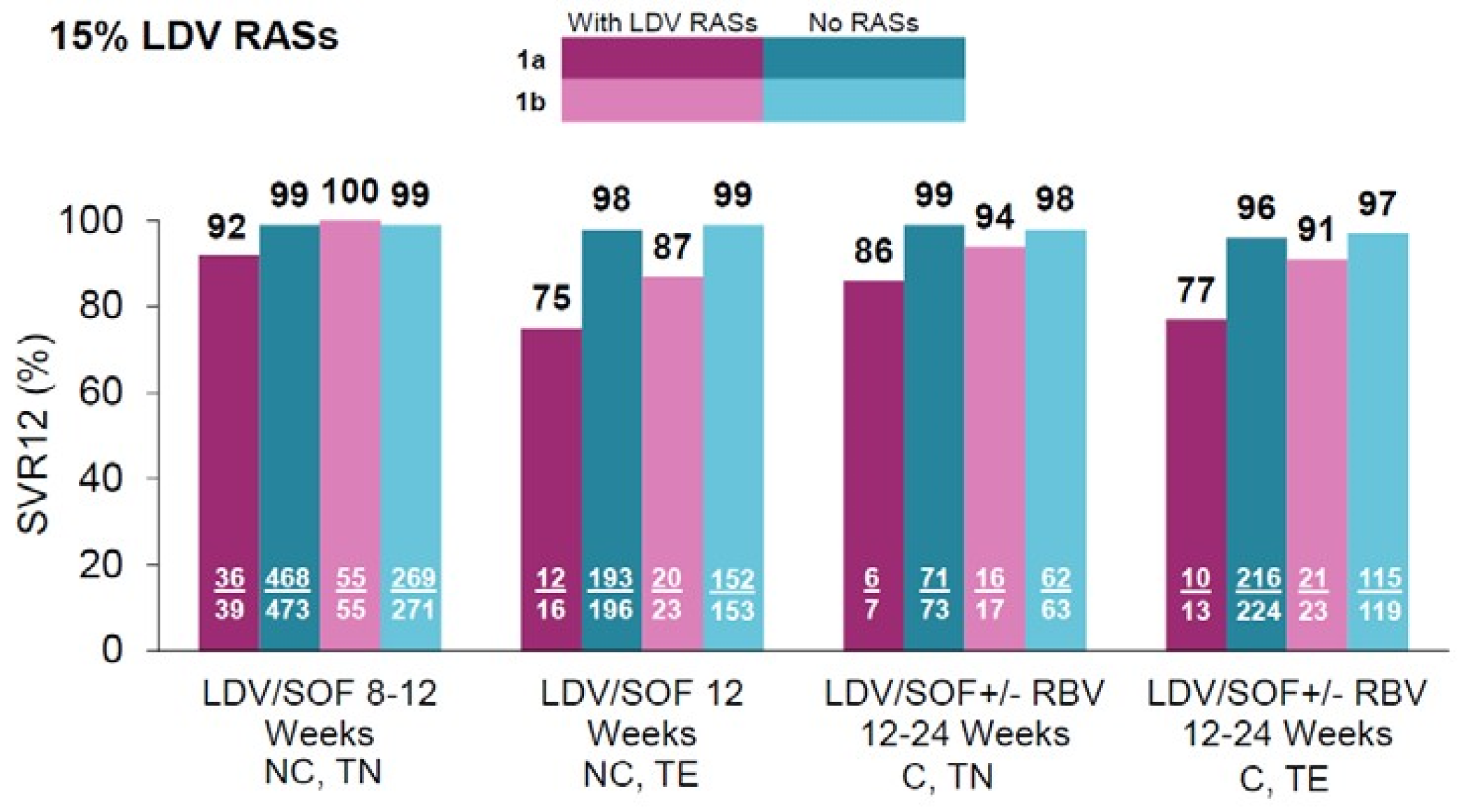
| Drugs | Study population | Study |
|---|---|---|
| SOF/VEL/VOX | 12 weeks for NS5A inhibitor-experienced GT1-6 HCV | POLARIS-1 [15] |
| SOF/VEL/VOX | 12 weeks for DAA-experienced (no NS5A inhibitors) GT1-6 HCV | POLARIS-4 [16] |
| GLE/PIB | 12 or 16 weeks for patients with GT3 HCV ± treatment experience ± cirrhosis | SURVEYOR-II/3 [17] |
| MK-3682/GZR/RZR | 16 weeks + RBV for 8-week MK-3682/GZR/(RZR or EBR) failures | C-CREST Part C [18] |
| MK-3682/GZR/RZR | 16 or 24 weeks ± RBV for GT1 HCV patients relapsing on DAAs | C-SURGE [19] |
© GERMS 2017.
Share and Cite
Pérez, A.B.; Chueca, N.; García, F. Resistance Testing for the Treatment of Chronic Hepatitis C with Direct Acting Antivirals: When and for How Long? Germs 2017, 7, 40-44. https://doi.org/10.18683/germs.2017.1107
Pérez AB, Chueca N, García F. Resistance Testing for the Treatment of Chronic Hepatitis C with Direct Acting Antivirals: When and for How Long? Germs. 2017; 7(1):40-44. https://doi.org/10.18683/germs.2017.1107
Chicago/Turabian StylePérez, Ana Belén, Natalia Chueca, and Federico García. 2017. "Resistance Testing for the Treatment of Chronic Hepatitis C with Direct Acting Antivirals: When and for How Long?" Germs 7, no. 1: 40-44. https://doi.org/10.18683/germs.2017.1107
APA StylePérez, A. B., Chueca, N., & García, F. (2017). Resistance Testing for the Treatment of Chronic Hepatitis C with Direct Acting Antivirals: When and for How Long? Germs, 7(1), 40-44. https://doi.org/10.18683/germs.2017.1107




