Abstract
Background: Mortality data, including the risk factors for mortality in HIV-infected children with pulmonary TB (PTB) being treated for PTB and who are on antiretroviral therapy (ART), are scarce in Nigeria. We determined the mortality rate and risk factors for mortality among such children, at the pediatric HIV clinic of the Jos University Teaching Hospital (JUTH) in Jos, Nigeria. Methods: We performed a retrospective cohort study on 260 PTB-HIV-1 co-infected children, aged 2 months to 13 years, being treated for PTB and on ART from July 2005 to March 2013. The mortality rate and associated risk factors were determined using multivariate Cox proportional hazards modelling. Results: The mortality rate for the study cohort was 1.4 per 100 child-years of follow-up. Median follow-up time was 5.2 years (IQR, 3.5-6.0 years) with total study time being 1159 child-years. The median age of those who died was lower than that of survivors, 1.9 years (IQR, 0.6-3.6 years) versus 3.8 years (IQR, 1.8-6.0 years), p=0.005). The majority of the deaths occurred in males (13, 81.2%), those <5 years of age (14, 87.4%) and those who had severe immunosuppression (11, 68.8%). Risk factors for death were age (with the risk of dying decreasing by 25% for every 1 year increase in age, adjusted hazard ratio (AHR)=0.75 [0.58-0.98], p=0.032), male gender (AHR=3.80 [1.07-13.5], p=0.039) and severe immunosuppression (AHR=3.35 [1.16-9.66], p=0.025). Conclusion: In our clinic setting, mortality among our PTB-HIV co-infected children being treated for PTB and on ART was low. However, those presenting with severe immunosuppression and who are males and very young, should be monitored more closely during follow-up in order to further reduce mortality.
Introduction
In 2014, the World Health Organization (WHO) estimated that there were 1.2 million human immunodeficiency virus (HIV)-positive new tuberculosis (TB) cases globally, 74% of them living in sub-Saharan Africa. TB was the most common presenting disease in people living with HIV (PLWHA), including those on antiretroviral therapy (ART) [1]. At least one-third of the 37 million PLWHA worldwide are infected with TB, with TB disease being the leading cause of death, accounting for about 390,000 deaths from HIV-associated TB in 2014 [1]. This mortality data on TB-HIV co-infection did not specify the figures for children, although it is reported that of 2.6 million children with ages below 15 years living with HIV in 2014 [2], 15,000 died [3]. About 0.55 million children develop TB disease each year, 70-80% being pulmonary TB, of which 80,000 die each year [4].
In the African sub-region various risk factors for mortality have been identified in children with TB-HIV co-infection, before or during ART; some of these children had received anti-tuberculosis treatment (ATT) prior to ART initiation while others had not [4,5,6,7,8,9]. However, mortality data including the risk factors for mortality in HIV infected children with pulmonary TB (PTB), being treated for PTB and on ART, are lacking in Nigeria. Such data could be useful in the overall strategy of reducing the morbidity and mortality associated with TB-HIV co-infection in children, not only in Nigeria but also in the African sub-region.
In this study we determined the mortality rate and the risk factors for mortality among PTB-HIV co-infected children being treated for PTB and on ART, at the pediatric HIV clinic of the Jos University Teaching Hospital (JUTH) in Jos, Nigeria. We have previously described elsewhere [10] the prevalence and the risk factors for pulmonary tuberculosis in this cohort of PTB-HIV co-infected children at the time of diagnosis and enrollment into care. This present study now focuses on the factors that could impact their mortality as they are being followed-up while on ART and ATT.
Methods
Study Design
We performed a retrospective cohort study on PTB-HIV-1 co-infected children being treated for PTB and on ART from July 2005 to March 2013.
Study Subjects
There were 260 PTB-HIV-1 co-infected children, aged 2 months to 13 years, being treated for PTB and on ART during the study period. These study subjects were among a total of 707 children, aged 2 months to 13 years who were on ART during the study period. Children diagnosed with HIV-1 infection were routinely screened for PTB and other forms of TB and usually started on ATT before initiating ART.
Study Setting
The study site was a pediatric HIV clinic, supported by AIDS Prevention Initiative in Nigeria (APIN), at JUTH, which provides HIV care services for the city of Jos, in Jos North Local Government Area (LGA) of Plateau State. The state has an estimated population of 3,206,531 [11]. The clinic is one of the largest of its kind in North-Central Nigeria and has been previously described in detail.
Patient Care and Management
HIV diagnosis and the criteria for initiating ART in the children were based on the Nigerian National Guidelines for Pediatric HIV and AIDS Treatment and Care [12,13]. The techniques used for HIV serodiagnosis in children have been presented in detail elsewhere [10]. Most children had ART initiated within 2 to 4 weeks of HIV diagnosis. From the time of HIV and PTB diagnosis and initiation of ART, the children were monitored, initially every 1 or 2 weeks for one month then at 4-week intervals thereafter. The first-line ART the children were initiated on could be any one of the following combinations: zidovudine (AZT) + lamivudine (3TC) + nevirapine (NVP) or AZT + 3TC + efavirenz (EFV) or AZT + 3TC + abacavir (ABC). Those failing first line ART were placed on second line regimen: ABC + 3TC + lopinavir/ritonavir (LPV/r) or tenofovir (TDF) + 3TC + LPV/r [12,13].
At enrollment into the HIV care programme, all children were routinely screened for PTB and other forms of TB. All children diagnosed with PTB received 6 months of standard ATT based on the 2010 Nigerian National Tuberculosis and Leprosy Control Programme (NTLCP) guidelines [14]. The ATT consists of four drugs (rifampicin, isoniazid, pyrazinamide and ethambutol) given in the initial intense phase that lasts 2 months and two drugs (rifampicin and isoniazid) in the continuation phase that lasts 4 months. Recommendations on the timing of ATT in relation to ART initiation underwent several changes during the study period: in some cases ART was initiated 2 months or more after starting ATT [15] (the study period 2005-2008), and in others as early as 2-8 weeks after starting ATT [13] (the study period 2009-2013). The children were also placed on co-trimoxazole (CTX) for opportunistic infection (OI) prophylaxis at some point after HIV diagnosis—in some cases before initiating ART and in others afterwards, depending on the existing national guidelines at the time [12,13].
As part of routine clinical care, all patients had laboratory tests performed at baseline, and repeated every 6 months to monitor adherence and adverse drug reactions. Laboratory evaluations included viral load, CD4+ count, full blood count, blood chemistry (liver and kidney function tests).
At each clinic visit, patients and their caregivers received adherence counseling by trained nurses, and were taught how to use alarm clocks daily as reminders on when their next ARV dose was due.
Prompt treatment for any identified intercurrent infections was also provided to the patients. Any associated malnutrition was managed with ‘plumpy nuts’ in some cases, in addition to nutrition education. Caregivers were encouraged to engage in the HIV support group.
Ethics Approval
For use of the data for this research, parents/ guardians of the children gave a written informed consent and the research was approved by the Ethics Committee of the Jos University Teaching Hospital, AIDS Prevention Initiative in Nigeria (APIN) Ltd., Abuja and Harvard T. H. Chan School of Public Health, Boston, USA.
Source of Data
For our analyses, we retrieved patient data from our electronic medical records systems (EMRS). Patients’ data were maintained in electronic databases, data having been entered into the EMRS from paper-based records that had been completed at the point of care.
Data were obtained at the time of enrollment into care and included baseline data such as: demographic (age, gender, year enrolled), clinical (height, weight, WHO HIV clinical stage, oral thrush, prophylaxis with co-trimoxazole for opportunistic infections), and laboratory (hemoglobin level, viral load and CD4+ cell count). Outcome data including death and cause of death, as well as failing first line ART were also obtained.
Case Definitions
A case of PTB was a child, either clinically diagnosed/ suspected with PTB (smear negative) or bacteriologically confirmed (smear positive) or both [14] and a child with both PTB and HIV at the time of HIV diagnosis was regarded as a case of PTB-HIV co-infection [15]. A clinically diagnosed case of PTB was defined as a child with persistent cough >2 weeks and a chest radiograph suggestive of PTB, plus one or more of the following: history of close contact with a TB case, fever, progressive weight loss or failure to thrive, poor appetite, night sweats, reduced playfulness or fatigue, wasting, pallor, chest signs. Chest radiograph features suggestive of PTB include one or more of the following: perihilar and/or paratracheal nodular shadows, streaky and nodular shadows, diffuse bilateral patchy opacities, homogenous opacities in any of the lung fields with or without features of pleural effusion, and miliary shadows. A bacteriologically confirmed case of PTB was a child with smear positive microscopy on Ziehl-Neelsen (Z-N) staining of sputum specimen for the presence of acid fast bacilli (AFB) on light microscopy. Gastric aspiration for smear microscopy is rarely done in our clinic setting.
Severe malnutrition was defined by a WHO weight-for-age Z score (WAZ) of < -3 [16], the Z-scores adjusted for age and sex having been determined from subjects’ weight and height using the WHO AnthroPlus software (WHO, Geneva, Switzerland) [17]. Severe immunosuppression (SI) was determined from the absolute CD4+ cell count using the CDC definition for SI in children <13 years [18] as follows: counts of <750/cmm for children <1 year, 500/cmm for those 1-5 years of age and <200/cmm for those >5 years old. Anemia as defined by the WHO referred to Hb <8 g/dL [19].
Outcome Variable
The outcome was death and this was categorized as resulting from HIV-related causes, ARV drug toxicity, or unknown causes. The cause of death was obtained from hospital records or through verbal autopsy from family members. Children who voluntarily withdrew from care or transferred to other health care facilities were considered as lost to follow-up (LTFU). Each child was followed up from the time they started ATT until death or to the end of the study period on 31st March 2013 or until censored at the time of being lost to follow-up.
Statistical Analysis
The association between each independent variable and death was first examined using the Wilcoxon-Mann-Whitney test for continuous variables and the Chi squared or Fisher’s exact test for categorical variables. Incidence based mortality was calculated with deaths as the numerator divided by total child-years. The cumulative probability of death was estimated using Kaplan-Meier survival methods and the survival curves were compared between groups using the log-rank test.
Associations between the independent variables and death were examined using unadjusted Cox proportional hazards regression modeling. Variables that were associated with death in the unadjusted Cox regression at p<0.05 were fit into a multivariate Cox regression model, followed by a forward step-wise modeling strategy where variables with p<0.05 remained in the model and those with p>0.10 exited the model, at each step of the modeling process. Results were presented as Hazard Ratios (HRs) along with 95% confidence intervals (CIs). All analyses were performed using Stata software version 10.0 (Stata Corporation, College Station, Texas, USA). All tests were two-sided with a p-value of <0.05 considered statistically significant.
To test for the potential impact of those that were LTFU, we repeated our multivariate modelling treating those that were LTFU as having died.
Results
Characteristics of Patients
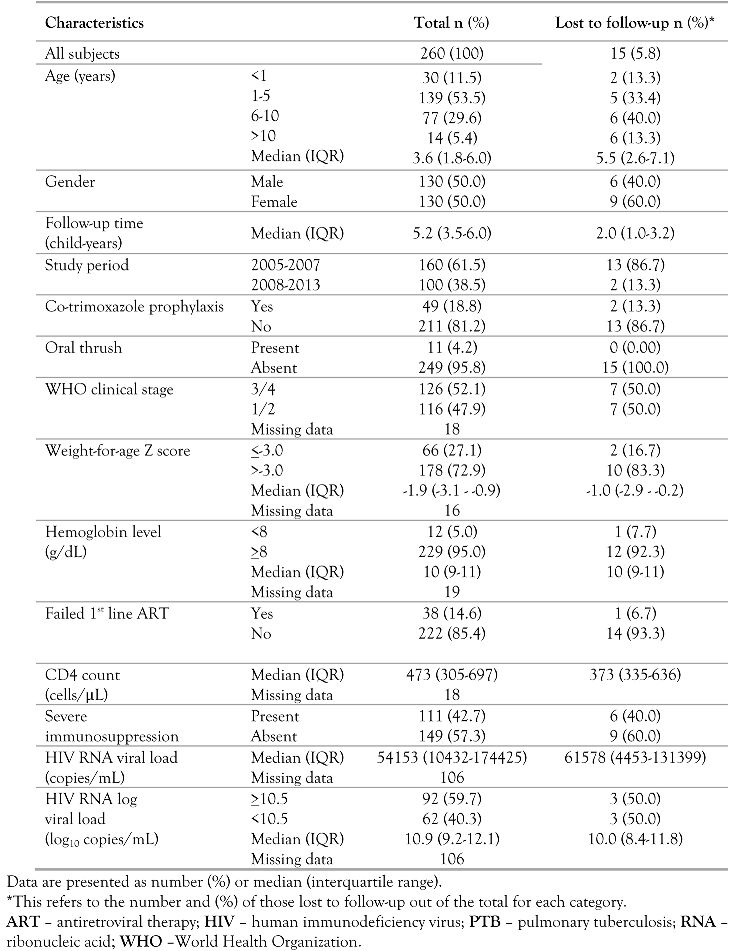
From July 2005 to March 2013, 260 children aged 2 months to 13 years with PTB-HIV co-infection being treated for PTB and on ART were studied, of which 245 (94.2%) had a known outcome and 15 (5.8%) were LTFU. The median follow-up time of the study cohort was 5.2 years (IQR, 3.5-6.0) and the total study time they contributed was 1159 child-years. Their median age was 3.6 years (IQR, 1.8-6.0) and 130 (50.0%) were males. The median time to starting ATT from the time of HIV diagnosis and enrollment into care was 1.0 month (IQR, 0.5–2.1). Most of the children (160, 61.5%) were enrolled in the years 2005-2007 when initiation of ART was deferred until a 6-month course of ATT was completed or until completion of the 2-month initiation phase of ATT. The majority were started on ATT (225/260, 86.5%) before being initiated on ART, most of them starting ATT within 2 months before ART initiation (160/260, 61.5%). The median time between starting ATT and initiating ART was 1.9 months (IQR, 0.6-3.8). Most children were in WHO clinical stage 3 or 4 (126, 52.1%) and not receiving CTX prophylaxis prior to initiating ART (211, 81.2%). Only a few had oral thrush (11, 4.2%), severe malnutrition (66, 27.1%) and severe anemia (12, 5.0%). There were 111 (42.7%) with severe immunosuppression. The median baseline CD4+ cell count and CD4% were 473 cells/cmm (IQR, 305-697) and 15% (IQR, 11-21) respectively, while the median HIV RNA viral load was 10.9 log10 copies/mL (IQR, 9.2-12.1) (Table 1). The majority of those with severe immunosuppression (75/111, 67.6%) were children <5 years of age.

Table 1.
Characteristics of PTB-HIV co-infected children being treated for PTB and on ART.
Mortality in the Study Cohort
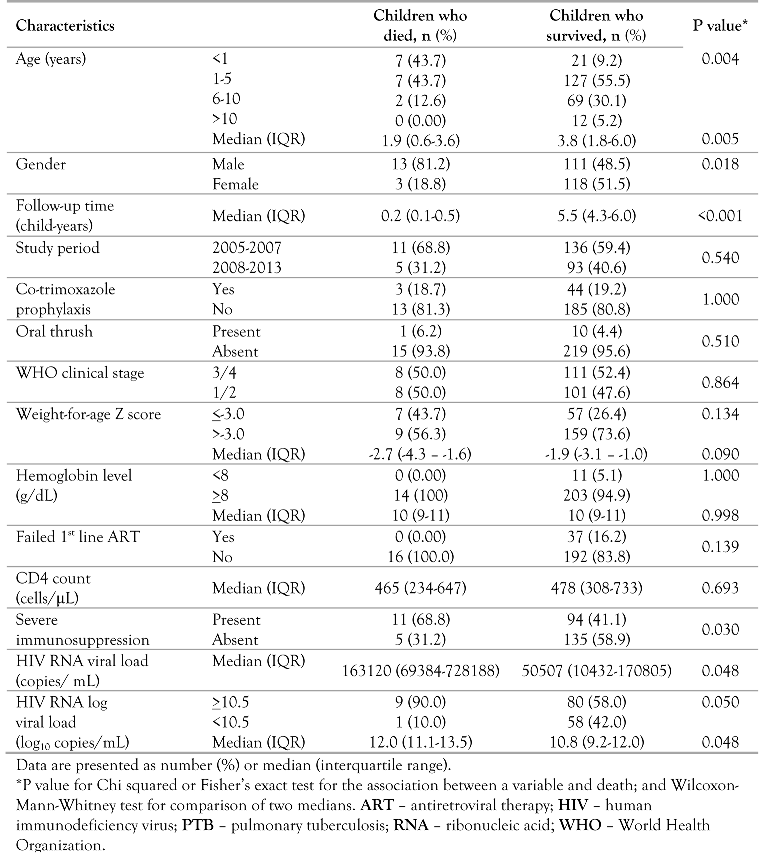
Out of the 260 PTB-HIV co-infected children being treated for PTB and on ART, 16 (6.2%) had died by the end of the follow-up period. The overall mortality rate was 1.4 per 100 child-years of follow-up (95% CI, 0.8-2.3) for the study cohort. For those who died, the median follow-up time was 0.2 years (IQR, 0.1-0.5) and for those who survived it was 5.5 years (IQR, 4.3–6.0). The median age of those who died was two years lower than that of survivors: 1.9 years (IQR, 0.6-3.6) versus 3.8 years (IQR, 1.8-6.0), p=0.005) (Table 2).

Table 2.
Association between patient characteristics and mortality.
The deaths were attributed to: HIV-related causes (14, 87.5%), ARV toxicity (1, 6.3%), other causes (1, 6.2%) and none to unknown causes. Most of the deaths occurred within the first 6 months of the follow-up period (14, 87.5%). The majority of the children who died were: males (13, 81.2%), <5 years of age (14, 87.4%), had severe immunosuppression (11, 68.8%) and did not receive CTX prophylaxis prior to initiating ART (13, 81.3%). Among the children that died none failed first line ART (Table 2). All the deaths (16, 100%) occurred in children who were still on first line ART. More deaths (11, 68.8%) occurred in the study period 2005-2007 when ART was initiated 2 months or more after starting ATT compared to fewer deaths (5, 31.2%) in the study period 2008-2013 when ART was initiated as early as 2-8 weeks after starting ATT.
The following Kaplan-Meier survival probabilities are shown in Figure 1: overall, by gender, severe immunosuppression and age group. In unadjusted analyses, the log rank tests showed significant associations between gender (p=0.012), as well as severe immunosuppression (p<0.031) and mortality risk from the time of ATT commencement.
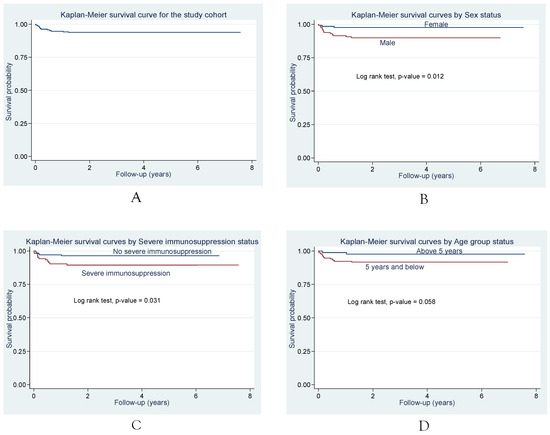
Figure 1.
Survival following initiation of ATT in PTB-HIV co-infected children being treated for PTB and on ART. Kaplan Meier survival curves: plot A shows the survival probability for the study cohort while plots B, C and D show the unadjusted associations between Sex, Severe immunosuppression, Age group status and mortality risk.
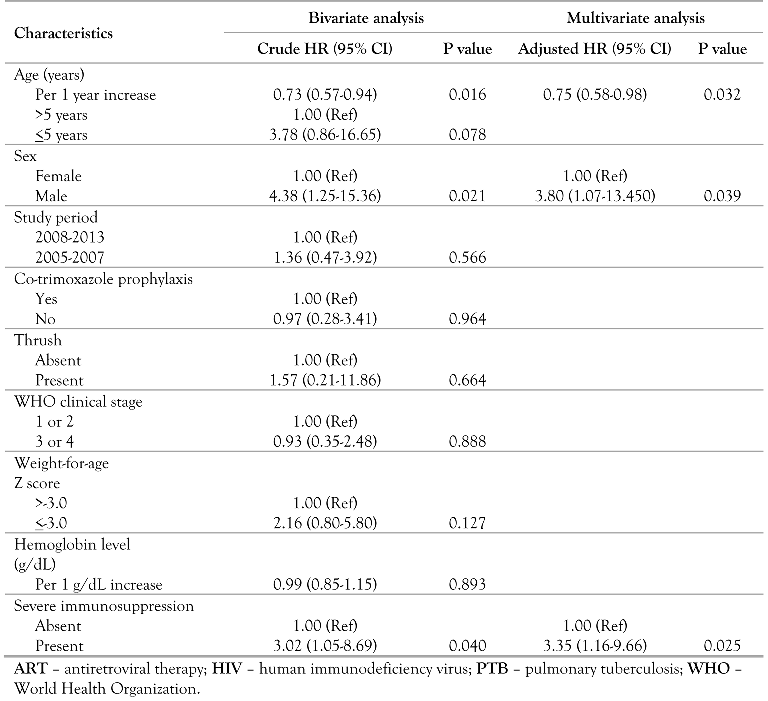
In the unadjusted Cox regression analysis, the risk of dying decreased by 27% for every 1 year increase in age (unadjusted hazard ratio (HR) = 0.73 [0.57-0.94], p=0.016) and the risk of dying was about 3½ times higher in children <5 years of age compared to those >5 years (HR=3.78 [0.86-16.65], p=0.078), while the risk of dying was about 4 times higher in males compared to females (HR=4.38 [1.25-15.36], p=0.021) and 3 times higher in patients with severe immunosuppression compared to those without severe immunosuppression (HR=3.02 [1.05-8.69], p=0.040) (Table 3). Following multivariate Cox regression modeling, the risk factors for death were: age, sex and severe immunosuppression, with the risk of dying decreasing by 25% for every 1 year increase in age (adjusted hazard ratio (AHR) = 0.75 [0.58–0.98], p=0.032), and the risk of dying increased by 3.8 fold in males compared to females (AHR=3.80 [1.07-13.50], p=0.039) and 3.3 fold in those with severe immunosuppression compared to those without severe immunosuppression (AHR=3.35 [1.16-9.66], p=0.025), Table 3.

Table 3.
Risk factors for mortality among PTB-HIV co-infected children being treated for PTB and on ART.
Over the study period, 15 children were considered LTFU as they voluntarily withdrew from care or transferred to other health care facilities. A sensitivity analysis was performed whereby all LTFU children were considered deaths with a higher mortality rate of 2.7 per 100 child-years of follow-up.
Discussion
The overall mortality rate was 1.4 per 100 child-years of follow-up, the proportion of deaths in the study population was 6.2% and the risk factors for mortality were age, male gender and severe immunosuppression among the study cohort.
The mortality rate of 1.4 per 100 child-years and the proportion of deaths (16/260, 6.2%) observed among our study patients was lower compared to the proportion of deaths among HIV-TB co-infected children treated for TB and on ART in a Malawian study [5] (92% had PTB) where 11.05% (123/1113) died, but slightly higher than that of a South African study [7] (74.5% had PTB) where mortality was 4.8%. Another study from the West Indies [20] in children with HIV-TB co-infection (87% had PTB) receiving ATT and on ART, showed a higher mortality figure of 20% compared to the 6.2% in our study. In agreement with our study, it is well established that mortality is generally lower in HIV-infected children on ART compared to those not on ART, with or without TB co-infection [5,21,22]. Our lower mortality figure may be attributed to our HIV programme’s policy of prompt screening for TB of all children diagnosed with HIV, and followed by the immediate commencement of ATT prior to early initiation of ART. Our sensitivity analysis examined the worst-case scenario where all LTFU patients were considered to have died. This yielded a higher mortality rate of 2.7 per 100 child-years of follow-up and 11.9% mortality. Our median time to starting ATT from the time of HIV diagnosis and enrollment into care was 1.0 month (IQR, 0.5-2.1). Early initiation of ART, within 2-8 weeks of starting ATT in the majority (61.5%) of our patients, may have contributed to the lower mortality in our study. We observed that fewer deaths (5/16, 31.2%) were recorded among children in the study period 2008-2013 when ART was initiated within 2-8 weeks of starting ATT, which was comparable to the Malawian study [5] where fewer deaths (44/225, 19.5%) occurred in children with TB-HIV co-infection initiating ART within 0-2 months of starting ATT. There were fewer children with severe malnutrition (27.1%) in our study and this also could have contributed to the lower mortality. As part of our HIV care, children with any associated malnutrition at enrollment into care were managed with ‘plumpy nuts’ in some cases, in addition to nutrition education and this could have contributed to the prevention of subsequent malnutrition at follow-up. It has been shown that malnutrition, especially severe malnutrition, in TB-HIV co-infected children is associated with a higher mortality [9,23].
The mortality in children <5 years of age in Nigeria in 2013 was reported as 2.6%, with 4% of all the deaths due to HIV-AIDS [24]. The mortality of 6.2% in our study, where the majority (65%) of the children were <5 years, was higher than the overall under-five deaths reported in that study. This was as expected, since HIV-infected children with TB as comorbidity are usually at a higher risk of death than children without HIV or HIV-TB.
Our study showed that younger children with PTB-HIV co-infection, aged <5 years, were more likely to die than older children and also mortality was 43.7% in children <1 year of age, with the risk of dying decreasing by 25% for every 1 year increase in age. This was similar to the findings in Peruvian children treated for tuberculosis (42% had PTB, although only 0.1% had HIV co-infection), where younger age was a predictor of mortality, with mortality highest in children <1 year of age (46.9%), and then decreasing to 3.2% by 14 years of age [25]. The mortality pattern with respect to age in this study was similar to that of a cohort study among HIV-infected Zambian children [26] receiving ART and ATT (5.7% TB co-infected) where mortality was higher in younger children and reduced by 10% for each additional year of age.
In our study male gender was a risk factor for mortality and we also observed that the proportion of those who died was higher than that of survivors (43.7% versus 9.2%) in the younger age group (< 1 year) in contrast to the older age groups, and that the proportion of deaths in the under-five age group was higher compared to the other age groups combined (87.4% versus 12.6%). Our findings are in conformity with several reports that infant mortality is higher in boys than it is in girls in most parts of the world [27,28,29] which has been explained by genetic and biological differences, and with the observation by Sawyer et al. [28] that on average, mortality in boys <5 years in the 2000s was about 2% higher than that seen in girls in less developed regions such as Africa. However, our finding contrasts with that of a South African study (42.5% had TB) where Zanoni et al. [30] reported that female gender associated increased mortality in HIV infected children initiated on ART and that of another study in children in North America where mortality rate was higher in female children, this being attributed to gender differences in HIV viral load [31]. Zanoni et al. concluded that it is not clear whether this disparity is due to biological differences between genders, or to social differences such as delayed presentation for care, differences in parental supervision of boys and girls, or devaluation of females that could place girls at higher risk of mortality after ART initiation in South Africa.
We found that severe immunosuppression in our study cohort at HIV diagnosis was a risk factor for mortality in our TB-HIV co-infected children, and this finding was similar to that of the Malawian study [5]. The severe immunosuppression seen in TB-HIV co-infection has been attributed to CD4+ T-cell depletion [32,33].
One limitation of this study was the possible overestimation of the number of PTB cases and hence a higher proportion of PTB-HIV co-infection cases due to our inability to routinely provide bacteriological confirmation (culture and/or smear microscopy) of PTB in most cases; even though it is well-recognized that bacteriological confirmation is often difficult in childhood TB because of their paucibacillary nature. Only 1.2% (3/260) of our study cohort were bacteriologically confirmed (sputum smear positive). Another limitation was that we used only baseline measurements of CD4+ counts, viral loads and other baseline variables in our analyses, not taking into consideration repeat measurements of these variables, all of which could have influenced subsequent mortality in the study cohort; and similarly, the modifications of these variables during the treatment for either TB or HIV could also have influenced mortality. Our sensitivity analysis indicated that LTFU in our patient population may have resulted in an under-estimate of the mortality.
Since our study was only limited to Jos, in Nigeria, even though our pediatric HIV clinic is one of the largest in North-Central Nigeria, it may be difficult to generalize our findings to all Nigerian children. We therefore recommend that, as a line of future research, similar studies, but prospective cohort studies, should be carried out to find out how clinical and laboratory parameters as well as patient clinical care could impact mortality in PTB-HIV co-infected children undergoing TB and HIV treatment.
Conclusion
In our clinic setting, mortality among our PTB-HIV co-infected children being treated for PTB and on ART was low. However, those of them presenting with severe immuno-suppression and who are males and very young, should be monitored more closely during follow-up in order to further reduce mortality.
Author Contributions
AOE contributed to study design, statistical analysis and drafting of the manuscript. AOE, SO, OOA, ASS, PIO, JAI, PJK. contributed to data acquisition and interpretation, as well as critical revision for intellectual content. All authors reviewed and approved the final version of the manuscript.
Funding
This publication was facilitated, in part, by the US Department of Health and Human Services, Health Resources and Services Administration (U51HA02522-01-01) and the Centers for Disease Control and Prevention (PS 001058) which supported HIV/AIDS treatment and care services at APIN, JUTH, Jos. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Acknowledgments
We thank APIN, JUTH for permission to use the patients’ data.
Conflicts of Interest
All authors—none to declare.
References
- WHO TB/HIV Facts 2015. Available online: http://www.who.int/hiv/topics/tb/tbhiv_facts_2015/en/ (accessed on 2 April 2016).
- UNAIDS HIV Factsheet 2014: USA. Available online: http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet (accessed on 2 April 2016).
- UNAIDS Report 2015 How AIDS Changed Everything (p. 57). Available online: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf (accessed on 2 April 2016).
- WHO. Combating Tuberculosis in Children. Available online: http://www.who.int/tb/challenges/childtb_factsheet.pdf?ua=1 (accessed on 2 April 2016).
- Buck, W.C.; Olson, D.; Kabue, M.M.; et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis 2013, 17, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Bakeera-Kitaka, S.; Conesa-Botella, A.; Dhabangi, A.; et al. Tuberculosis in HIV-infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis 2011, 15, 1082–1086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walters, E.; Cotton, M.F.; Rabie, H.; Schaaf, H.S.; Walters, L.O.; Marais, B.J. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Jeena, P.M.; Pillay, P.; Pillay, T.; Coovadia, H.M. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int J Tuberc Lung Dis 2002, 6, 672–678. [Google Scholar] [PubMed]
- Hesseling, A.C.; Westra, A.E.; Werschkull, H.; et al. Outcome of HIV infected children with culture confirmed tuberculosis. Arch Dis Child 2005, 90, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Ebonyi, A.O.; Oguche, S.; Ejeliogu, E.U.; et al. Prevalence of and risk factors for pulmonary tuberculosis among newly diagnosed HIV-1 infected Nigerian children. Germs 2016, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- National Population Commission. Population Distribution by Sex, State, LGA & Senatorial District. 2006 Population and Housing Census: Priority Table Volume III. National Population Commission, of Abuja, Nigeria. Available online: http://catalog.ihsn.org/index.php/catalog/3340/download/48521 (accessed on 26 August 2015).
- Federal Ministry of Health. National Guidelines for Paediatric HIV and AIDS Treatment and Care; 2007. Federal Ministry of Health, Abuja, Nigeria. Available online: http://www.who.int/hiv/amds/Nigeria_paediatric_2007.pdf (accessed on 18 April 2016).
- Federal Ministry of Health. National Guidelines for Paediatric HIV and AIDS Treatment and Care; 2010. Federal Ministry of Health, Abuja, Nigeria. Available online: http://preventcrypto.org/wp-content/uploads/2015/10/NigeriaPaediatricARTguidelines 20101369045239.pdf (accessed on 28 February 2016).
- Federal Ministry of Health. National Tuberculosis and Leprosy Control Programme—Workers’ Manual, Revised 5th Edition; 2010. Federal Ministry of Health, Abuja, Nigeria, Department of Public Health. Available online: http://www.who.int/hiv/pub/guidelines/nigeria_tb.pdf (accessed on 26 August 2014).
- World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a Public Health Approach; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization (WHO). Global Database on Child Growth and Malnutrition. World Health Organization, Geneva, Switzerland. Available online: http://www.who.int/nutgrowthdb/about/introduction/en/index5.html (accessed on 20 April 2014).
- World Health Organization (WHO). Application Tools: WHO AnthroPlus Software. World Health Organization, Geneva, Switzerland. Available online: http://www.who.int/growthref/tools/en/ (accessed on 21 April 2014).
- Centers for Disease Control and Prevention (CDC). 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age; Official authorized addenda: Human immunodeficiency virus infection codes and official guidelines for coding and reporting ICD-9-CM. MMWR Morb Mortal Wkly Rep 1994, 43, 1–19. [Google Scholar]
- World Health Organization (WHO). Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Program Managers. 2001. Available online: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/ (accessed on 26 August 2014).
- Kumar, A.; Upadhyay, S.; Kumari, G. Clinical presentation, treatment outcome and survival among the HIV infected children with culture confirmed tuberculosis. Curr HIV Res 2007, 5, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.M.; Ramos Amador, J.T.; Fernández de Miguel, S.; et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J 2003, 22, 863–867. [Google Scholar] [PubMed]
- Gortmaker, S.L.; Hughes, M.; Cervia, J.; et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med 2001, 345, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Palme, I.B.; Gudetta, B.; Bruchfeld, J.; Muhe, L.; Giesecke, J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 2002, 21, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; WHO; The World Bank; UN Pop Div. Levels and Trends in Child Mortality. Report 2014.
- Drobac, P.C.; Shin, S.S.; Huamani, P.; et al. Risk factors for in-hospital mortality among children with tuberculosis: The 25-year experience in Peru. Pediatrics 2012, 130, e373–e379. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Moore, C.; Mubiana-Mbewe, M.; Cantrell, R.A.; et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007, 298, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Waldron, I. Sex differences in infant and early childhood mortality: Major causes of death and possible biological causes. In Too Young to Die: Genes or Gender? ST/ESA/SER.A/155; United Nations: New York, NY, USA, 1998; pp. 64–83. [Google Scholar]
- Sawyer, C.C. Child mortality estimation: Estimating sex differences in childhood mortality since the 1970s. PLoS Med 2012, 9, e1001287. [Google Scholar] [CrossRef] [PubMed]
- Pongou, R. Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 2013, 50, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, B.C.; Phungula, T.; Zanoni, H.M.; France, H.; Feeney, M.E. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS ONE 2011, 6, e22706. [Google Scholar] [CrossRef] [PubMed]
- Foca, M.; Moye, J.; Chu, C.; et al. Gender differences in lymphocyte populations, plasma HIV RNA levels, and disease progression in a cohort of children born to women infected with HIV. Pediatrics 2006, 118, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Geldmacher, C.; Zumla, A.; Hoelscher, M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS 2012, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Mak, J.W.; Maung, M.; Wong, S.F.; Kassim, A.I. Meta-analysis: The association between HIV infection and extrapulmonary tuberculosis. Lung 2013, 191, 27–34. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2016.