Rotavirus Gastroenteritis in Children Less than Five Years of Age in Primary Care Settings in Bulgaria: An Observational Study
Abstract
Background
Methods
Study Design
Study Population and Case Definition
Data Collection
Diagnosis of RV
Statistical Analysis
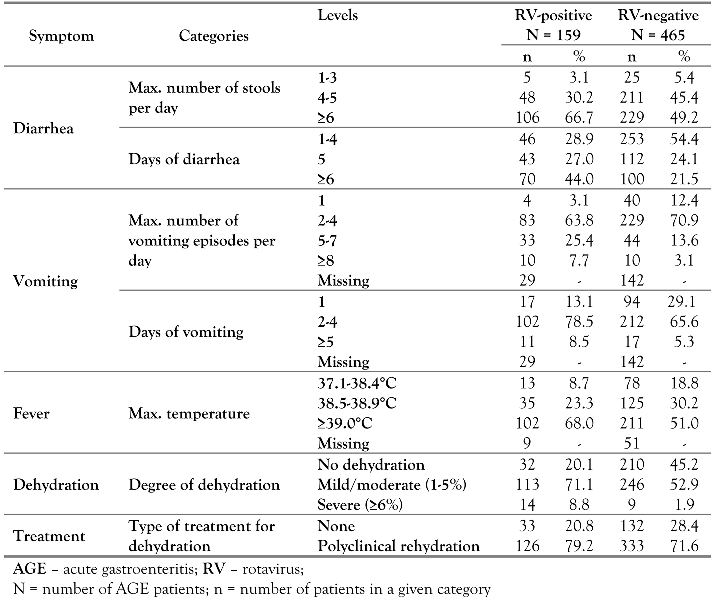
Results
Demographic Characteristics
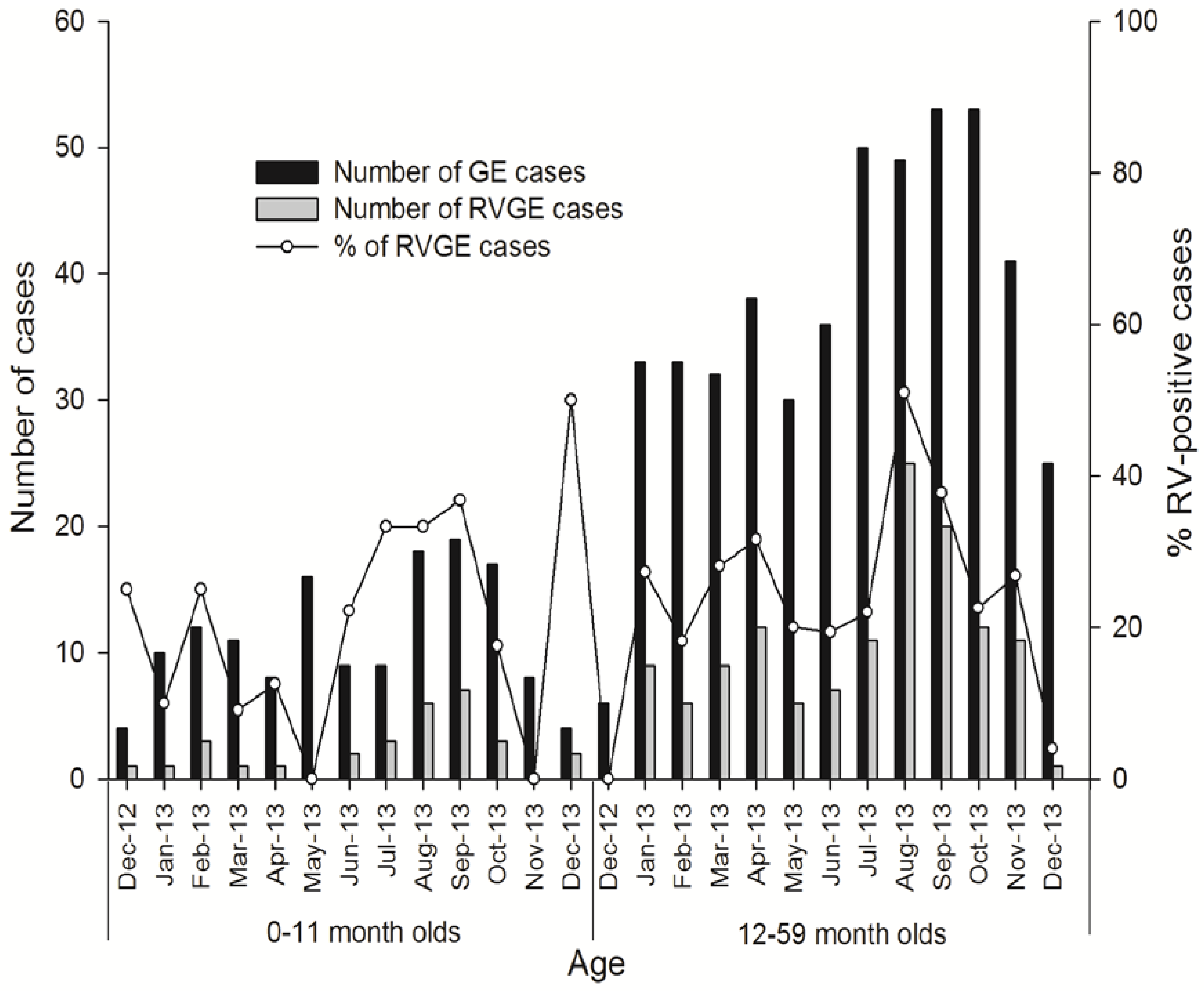
Proportion of RVGE Cases
| RV-Positive N = 159 | RV-Negative N = 465 | |||||
|---|---|---|---|---|---|---|
| Age (months) | Severity | n | % | n | % | p-Values |
| Mild (1-6) | 0 | 0.0 | 9 | 20.0 | ||
| 0-5 | Moderate (7-10) | 1 | 16.7 | 19 | 42.2 | 0.160 |
| Severe (≥11) | 5 | 83.3 | 17 | 37.8 | ||
| Mild (1-6) | 1 | 4.2 | 10 | 14.3 | ||
| 6-11 | Moderate (7-10) | 2 | 8.3 | 26 | 37.1 | 0.003 |
| Severe (≥11) | 21 | 87.5 | 34 | 48.6 | ||
| Mild (1-6) | 1 | 2.1 | 18 | 12.1 | ||
| 12-23 | Moderate (7-10) | 9 | 18.8 | 45 | 30.2 | 0.017 |
| Severe (≥11) | 38 | 79.2 | 86 | 57.7 | ||
| Mild (1-6) | 5 | 6.2 | 23 | 11.4 | ||
| 24-59 | Moderate (7-10) | 10 | 12.3 | 61 | 30.3 | 0.001 |
| Severe (≥11) | 66 | 81.5 | 117 | 58.2 | ||
| Mild (1-6) | 1 | 3.3 | 19 | 16.5 | ||
| 0-11 | Moderate (7-10) | 3 | 10.0 | 45 | 39.1 | < 0.001 |
| Severe (≥11) | 26 | 86.7 | 51 | 4.3 | ||
| Mild (1-6) | 6 | 4.7 | 41 | 11.7 | ||
| 12-59 | Moderate (7-10) | 19 | 14.7 | 106 | 30.3 | < 0.001 |
| Severe (≥11) | 104 | 80.6 | 203 | 58.0 | ||
| Mild (1-6) | 7 | 4.4 | 60 | 12.9 | ||
| Overall | Moderate (7-10) | 22 | 13.8 | 151 | 32.5 | < 0.001 |
| Severe (≥11) | 130 | 81.8 | 254 | 54.6 | ||
Proportion of Patients Hospitalized for AGE
Seasonal Distribution of RVGE
Discussion
Conclusions
Authors’ Contributions Statement
Funding
Acknowledgments
Conflicts of Interest
References
- Tate, J.E.; Patel, M.M.; Steele, A.D.; et al. Global impact of rotavirus vaccines. Expert Rev Vacc 2010, 9, 395–407. [Google Scholar]
- Rotavirus vaccines. WHO position paper—January 2013. Wkly Epidemiol Rec. 2013, 88, 49–64. [Google Scholar]
- Вuttery, J.P.; Kirkwood, C. Rotavirus vaccines in developed countries. Curr Opin Infect Dis 2007, 20, 253–258. [Google Scholar]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis 2012, 12, 136–141. [Google Scholar]
- Van Damme, P.; Van der Wielen, M.; Ansaldi, F.; et al. Rotavirus vaccines: Considerations for successful implementation in Europe. Lancet Infect Dis 2006, 6, 805–812. [Google Scholar]
- PATH. Rotavirus vaccine access and delivery. Frequently Asked Questions. Available online: http://sites.path.org/rotavirusvaccine/rotavirus faq/ (accessed on 15 October 2015).
- Meeting of the Strategic Advisory Group of Experts on immunization—Conclusions and recommendations. Wkly Epidemiol Rec 2009, 84, 517–532.
- Lepage, P.; Vergison, A. Impact of rotavirus vaccines on rotavirus disease. Expert Rev Anti Infect Ther 2012, 10, 547–561. [Google Scholar] [PubMed]
- Rotavirus vaccine and intussusception: Report from an expert consultation. Wkly Epidemiol Rec 2011, 86, 317–321.
- Aballéa, S.; Millier, A.; Quilici, S.; Carroll, S.; Petrou, S.; Toumi, M. A critical leterature review of health economic evaluations of rotavirus vaccination. Hum Vaccin Immunother 2013, 9, 1272–1288. [Google Scholar]
- Frühwirth, M.; Heininger, U.; Ehlken, B.; et al. International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially aquired infection. Pediatr Infec Dis J 2001, 20, 784–791. [Google Scholar]
- Forster, J.; Guarino, A.; Parez, N.; et al. Hospital-based survey to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009, 123, e393–e400. [Google Scholar] [CrossRef]
- Giaquinto, C.; Van Damme, P.; Huet, F.; et al. Clinical consequences of acute gastroenteritis in Europe 2004-2005: The REVEAL study. J Infect Dis 2007, 195 (Suppl. 1), S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Lai, P.; Valle, L.; et al. Burden of rotavirus- associated and non-rotavirus-associated diarrhea among nonhospitalized individuals in central Italy: A 1-year sentinel-based epidemiological and virological surveillance. Clin Infect Dis 2008, 46, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Mészner, Z.; Anca, I.; André, F.; et al. Rotavirus vaccination in central Europe. J Pediatr Gastroenterol Nutr 2013, 56, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, Z.; Iturriza-Gómara, M.; Esona, M.D.; Gray, J.; Korsun, N. Genetic characterization of Bulgarian rotavirus isolates and detection of rotavirus variants: Challenges for the rotavirus vaccine program? J Med Virol 2011, 83, 348–356. [Google Scholar] [CrossRef]
- Mladenova, Z.; Korsun, N.; Geonova, T.; Iturriza-Gómara, M.; Rotavirus Study Group. Molecular epidemiology of rotaviruses in Bulgaria: Annual shift of the predominant genotype. Eur J Clin Microbiol Infect Dis 2010, 29, 555–562. [Google Scholar] [CrossRef]
- Ogilvie, I.; Khoury, H.; El Khoury, A.C.; Goetghebeur, M.M. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: Serotype distribution and burden of illness. Hum Vaccin 2011, 7, 523–533. [Google Scholar] [CrossRef]
- Lewis, K. Vesikari Clinical Severity Scoring System Manual; PATH (Program for Appropriate Technology in Health): Seattle, WA, USA, 2011. [Google Scholar]
- Pitzer, V.E.; Viboud, C.; Simonsen, L.; et al. Demographic variability, vaccination and the spatiotemporal dynamics of rotavirus epidemics. Science 2009, 325, 290–294. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Viboud, C.; Lopman, B.A.; Patel, M.M.; Parashar, U.D.; Grenfell, B.T. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J R Soc Interface 2011, 8, 1584–1593. [Google Scholar] [CrossRef]
- Patel, M.M.; Pitzer, V.E.; Alonso, W.J.; et al. Global seasonality of rotavirus disease. Pediatr Infect Dis J 2013, 32, e134–e147. [Google Scholar] [CrossRef]
- Giaquinto, C.; Dominiak-Felden, G.; Van Damme, P.; et al. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: A systematic review of the experience in industrialized countries. Human Vaccines 2011, 7, 734–748. [Google Scholar] [CrossRef]

 |
| RV-Positive N = 159 | RV-Negative N = 465 | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (months) | N | n | % | 95% CI | N | n | % | 95% CI |
| 0-5 | 6 | 1 | 16.7 | 0.4-64.1 | 45 | 2 | 4.4 | 0.5-15.1 |
| 6-11 | 24 | 7 | 29.2 | 12.6-51.1 | 70 | 1 | 1.1 | 0.0-7.7 |
| 12-23 | 48 | 11 | 22.9 | 12.0-37.3 | 149 | 1 | 0.7 | 0.0-3.7 |
| 24-59 | 81 | 13 | 16.0 | 8.8-25.9 | 201 | 3 | 1.5 | 0.3-4.3 |
| All | 159 | 32 | 20.1 | 14.2-27.2 | 465 | 7 | 1.5 | 0.6-3.1 |
© GERMS 2016.
Share and Cite
Tiholova, M.; Gopala, K.; Berberova, M.; Strokova-Stoilova, M.; Tafalla, M. Rotavirus Gastroenteritis in Children Less than Five Years of Age in Primary Care Settings in Bulgaria: An Observational Study. Germs 2016, 6, 97-105. https://doi.org/10.11599/germs.2016.1095
Tiholova M, Gopala K, Berberova M, Strokova-Stoilova M, Tafalla M. Rotavirus Gastroenteritis in Children Less than Five Years of Age in Primary Care Settings in Bulgaria: An Observational Study. Germs. 2016; 6(3):97-105. https://doi.org/10.11599/germs.2016.1095
Chicago/Turabian StyleTiholova, Mayda, Kusuma Gopala, Magda Berberova, Margarita Strokova-Stoilova, and Monica Tafalla. 2016. "Rotavirus Gastroenteritis in Children Less than Five Years of Age in Primary Care Settings in Bulgaria: An Observational Study" Germs 6, no. 3: 97-105. https://doi.org/10.11599/germs.2016.1095
APA StyleTiholova, M., Gopala, K., Berberova, M., Strokova-Stoilova, M., & Tafalla, M. (2016). Rotavirus Gastroenteritis in Children Less than Five Years of Age in Primary Care Settings in Bulgaria: An Observational Study. Germs, 6(3), 97-105. https://doi.org/10.11599/germs.2016.1095




