Abstract
Introduction: Sexually transmitted diseases (STDs) are a major public health issue in Europe. Numerous outbreaks of syphilis have been described recently and an increased prevalence of high-risk sexual practices has raised concern about the transmission of HIV and other STDs. Similarly, an increase in sexually transmitted infections has been recorded in Northern Greece. Methods: This report describes a recent outbreak of syphilis in people living with HIV. The demographic, clinical, and serologic data of HIV patients diagnosed with syphilis were recorded and analyzed. Data on syphilis incidence from the general population was also compared to HIV patients’ data. Results: Fifty-eight HIV-patients of the Infectious Diseases Unit of a tertiary hospital (5.2%) were diagnosed with syphilis during a three-year period (2008-2010). Highly active antiretroviral therapy (HAART) and coexistence of other STDs were independent predictors of syphilis (OR: 2.4, 95CI%: 1.26, 4.63, p=0.008; OR: 9.4, 95%CI: 4.49, 19.64, p<0.001, respectively). Origin from a country other than Greece (p=0.005), and homosexual contact (p=0.003), were separate risk factors for syphilis in the general population in the same area. Conclusions: Diagnosis of a sexually transmitted disease in an HIV patient is a crucial clinical event that should trigger the clinician’s suspicion for high-risk sexual behavior. Sexual health assessments should be a routine process for HIV patients.
Introduction
Syphilis is a sexually transmitted infection progressing in several stages. Symptoms in primary syphilis may vary, however this stage is considered highly infectious. Without treatment the infection progresses to the second stage, which often manifests with systemic symptoms. Transmission may also occur at this stage and symptoms usually resolve with or without treatment. If untreated, the patient advances to latent and late (tertiary) syphilis which can affect the majority of organs [1].
Syphilis may augment the rate of HIV acquisition between two- and four-fold and the risk for HIV transmission between two- to nine- fold [2]. A causal relation between the two diseases can be explained by behavioral factors as well as pathogenic mechanisms, such as facilitation of HIV transmission caused by genital ulceration and inflammation [3]. HIV and sexually transmitted disease (STD) coinfection has raised concern as a public health issue in the last decade. Studies have indicated multiple facets of this synergistic relationship: people infected with STDs are more likely to acquire HIV, and people coinfected with HIV and STDs are more likely to transmit HIV [4,5].
During the 1980s a reduction in the incidence of STDs was seen in many countries likely due to behavioral changes occurring in response to the emergence of HIV/AIDS [6,7]. The rise in high-risk sexual practices and migration has contributed to the increased incidence of STDs since 2000. Moreover, advances in epidemiologic surveillance and patient detection methods have led to higher numbers of reported cases [8]. In Western Europe the reappearance of syphilis was mainly attributed to outbreaks among men who have sex with men (MSM) with novel sexual networks and high-risk sexual behaviours [9].
The overall rate of syphilis cases in the European Union was 8.2 per 100,000 in 2000. In 2009, 18,279 syphilis cases were reported from 28 European Union countries, with an overall rate of 4.5 per 100,000 population. This decline in rates might be attributed to a significant decrease of cases in numerous countries previously reporting extremely high incidence of syphilis, due to prevention measures [10,11]. Syphilis was reported almost three times more frequently in males than in females [11]. In 2012, nearly 48% of the syphilis cases were reported in MSM [12,13]. More recent trends (2008–2012) show sharp increase of syphilis, particularly in Western Europe. Increases over 50% were observed in Denmark, Greece and Portugal during 2008–2012 [10]. The increase is probably due to the introduction of a new surveillance system and the inclusion of more reporting centers [10]. During 2006–2010 Greece has reported rising rates of syphilis (0.6 to 2.3) [10].
HIV infection still affects MSM disproportionately in the European Union, and a remarkable increase in the number of new HIV diagnoses in MSM has been recorded since 2000. Meanwhile, STDs have also re-emerged in this population and the occurrence of several outbreaks especially in HIV-infected MSM [9], underlines the high rate of HIV and STD coinfection in this group. Undoubtedly, the existence of other STDs increases the possibility of transmitting HIV infection [12,13]. Since STDs may represent a cofactor for HIV transmission and on the other hand HIV may alter the clinical presentation of syphilis, their coexistence in specific sexual networks may increase the spread of both infections [14,15].
Outbreaks of syphilis in high-risk groups, especially among MSM, drug users and sex workers, are frequent in many countries [16]. Episodic STD outbreaks among HIV seropositive people indicate intermittent risky sexual behavior, concerning STD transmission [16].
Data on syphilis epidemics in Greece have been provided until 2010, with only 11 hospitals providing source data on syphilis cases to the two national reference centers for syphilis (Southern and Northern Greece Reference Centers). Evidence also suggests an increase in syphilis cases recorded in the general population from 2008 to 2010 in Northern Greece [17].
Methods
All adult HIV seropositive patients in the Infectious Diseases Division of AHEPA University Hospital in Thessaloniki Greece, which comprises the National HIV/AIDS Reference Centre of Northern Greece, are routinely screened for syphilis once a year. Those diagnosed as having syphilis from 1st of January 2008 through 31st of December 2010 were studied (demographic characteristics, history, physical examination, clinical data, serologic screening, cerebrospinal fluid [CSF] examination) to assess the stage of their infection. Patient demographics, such as age, nationality, possible route of acquisition for sexually transmitted diseases (including HIV and syphilis), HAART use, years of HIV seropositivity and laboratory parameters such as CD4 count and HIV RNA viral load were recorded. Existence of other sexually transmitted diseases (genital herpes infection, gonorrhea, Chlamydia infection, donovanosis) was also investigated. Due to an increase in syphilis cases in 2008, the following two years (2009-2010), patients were encouraged to be tested twice a year and testing was also provided to their sexual partners. Moreover further prevention actions were taken to control the outbreak. Already diagnosed syphilis positive patients were excluded from the incident cases of next years, if they reappeared to be syphilis positive. By the time syphilis was diagnosed, repeat tests from the same individual were not included in the analysis.
Syphilis screening was performed using two non-treponemal tests, namely the venereal disease laboratory (VDRL) and rapid plasma reagin (RPR) tests. A positive VDRL was confirmed with the fluorescent treponemal antibody–absorption (FTA-ABS) test. Patient assessment included cardiovascular, ophthalmologic and neurologic evaluation, and cerebrospinal fluid examination (cell profile, VDRL, and FTA-ABS). Both VDRL and RPR test were performed for each patient. Disease stage was determined based on history, clinical findings, and laboratory results. All positive patients are encouraged to get a CSF analysis, especially when their CD4 cell count is below 400 cells/cmm. All patients included in the study had signed an informed consent.
Adults over 18 years old can be tested in the Syphilis Reference Centre of General Population of Northern Greece, maintaining their anonymity. Data from the National Syphilis Reference Centre of General Population of Northern Greece were reviewed for the same time period (2008-2010). These data concerned the gender, age, origin, possible way of transmission, existence of symptoms and results of serologic tests. Sex workers (which are tested frequently twice a year) were excluded from the analysis as well as all HIV positive patients that joined the National Syphilis Reference Centre for syphilis testing. The new surveillance system for STDs, established in 2009, actively collects data on cases of syphilis and congenital syphilis. Data is collected from hospitals, laboratories and clinicians, in the public and private sector. The EU-2008 case definitions are used. The new system is intended to be comprehensive but does not yet provide national coverage. Underreporting may still exist in some Greek regions. So it was decided that the data from the National Syphilis Reference Centre of General Population of Northern Greece would better describe the trend of syphilis in the population of Northern Greece, to juxtapose syphilis incidence in the general population and in HIV-infected individuals throughout the study period.
Statistical Analysis
The odds of syphilis were considered as the dependent variable in logistic regression and its association with other variables was examined. First, univariate analyses were conducted and all significant variables with p<0.05 were considered for inclusion in the multivariable model by forced entry. The odds ratios and corresponding 95% confidence intervals (95%CI) are reported. All reported p-values are two-tailed at a 0.05 significance level; analyses were conducted using SPSS, version 18 (SPSS Inc, Chicago, IL, USA).
Results
HIV Population
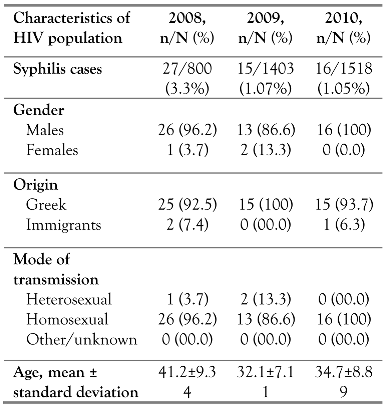
Of the 1119 HIV patients followed up at our hospital during the study period, 58 (5.2%) were positive for syphilis (Table 1). Τhe entire cohort was serologically tested for syphilis. The vast majority of syphilis patients were MSM (55 patients, 94.82%) with a mean age ± standard deviation (SD) of 35.65±10.94 years. The mean duration of HIV infection was 3.32±4.35 years and the possible route of syphilis transmission was sexual contact. At the time of syphilis seropositivity, mean CD4 cells count was 440.3±238.9 cells/cmm and mean plasma HIV RNA viral load was 201,903±382730 copies/cmm. Of the 58 patients, 45 (77.7%) were receiving HAART at the time of syphilis diagnosis, and the mean time on HAART was 2.68±4.26 years. HIV and syphilis coinfected patients’ demographics per year of the outbreak are illustrated in Table 1.

Table 1.
Demographics of HIV population coinfected with syphilis.
Regarding syphilis stage, the distribution in the HIV population was 26% primary syphilis, 33% secondary syphilis, 37% latent syphilis and 4% neurosyphilis. Most HIV patients were diagnosed with latent, secondary or primary infection during the outbreak period. All 58 syphilis positive patients, consented to undergo a CSF analysis. Of them, only 4% suffered from confirmed central nervous system syphilis infection at diagnosis.
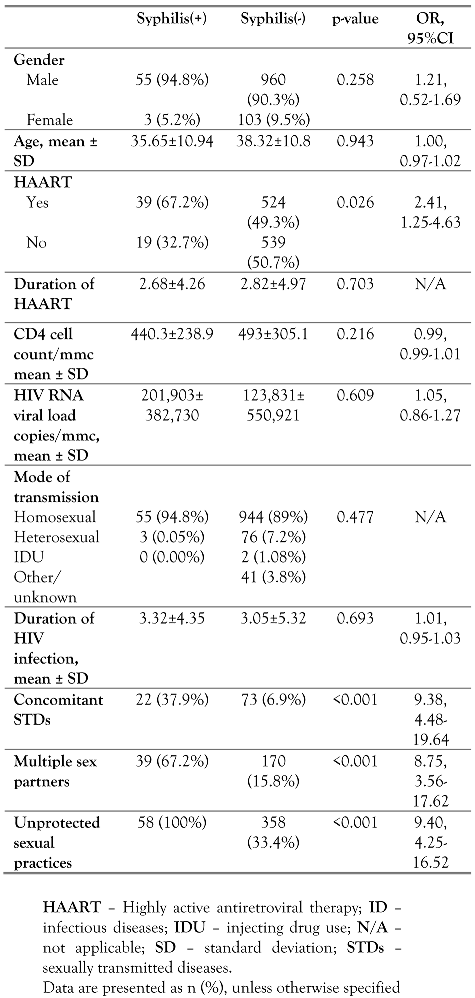
A significantly greater proportion of patients with syphilis were on HAART than patients without syphilis (OR 2.41, 95%CI: 1.25-4.63, p=0.026). Furthermore, a significantly greater proportion of patients with syphilis had concomitant STDs than patients without syphilis (OR 9.38, 95%CI: 4.48-19.64, p<0.001). There was no association between syphilis and CD4 cell count (p=0.216), viral load (p=0.609), age (p=0.943), mode of transmission (p=0.484) or duration of HIV infection (p=0.693). In the multivariate logistic model, both HAART and existence of STDs were independent predictors of syphilis, showing that the odds of syphilis were 2.4 times higher in patients with HAART than in patients not receiving HAART (95CI%: 1.26, 4.63, p=0.008) and it was 9.4 times higher in patients with STDs than in patients without STDs (95% CI: 4.49, 19.64, p<0.001)—Table 2.

Table 2.
Multivariate analysis of all variables in the HIV study population of the ID Unit.
High risk sexual behaviors such as unprotected sexual contacts or multiple sex partners, were remarkably prevalent in the HIV syphilis-positive population. All of the syphilis patients mentioned no use of protection (i.e., condom use), which was statistically significant when compared to the respective proportion of the syphilis-negative adults. Furthermore, the vast majority of syphilis positive patients mentioned exchange of multiple sex partners during the last 6 months compared to negative individuals (67.2% vs. 15.8%) (Table 2). Five out of the 58 (8.6%) syphilis positive patients were re-infected during the follow up study, but these were excluded from the analysis.
All HIV patients who were found positive for syphilis were offered behavioral counseling from a clinical psychologist. Screening for HIV and syphilis was also offered to their sexual partners. Forty two sexual partners were tested for syphilis and 11 were found to be syphilis positive (26.1%). Twelve of the sexual partners were not HIV positive (28.5%). Subsequently, serosorting between those couples reaches approximately a quarter. Individuals exposed to syphilis within 90 days preceding the diagnosis of primary, secondary, or early latent syphilis in their sex partners were treated. A letter informing that there is an outbreak of syphilis among patients of our HIV unit, providing information about safe sexual practices was administered to all patients during their follow-up visits. Starting with 2009, syphilis testing was offered twice a year.
General Population
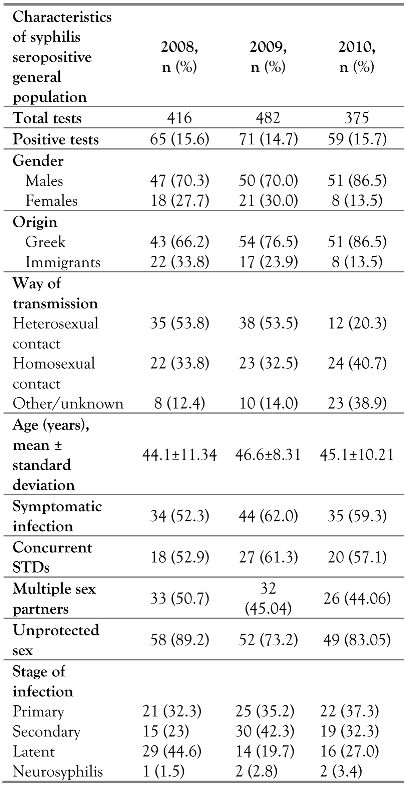
During the study period, there was also an increased prevalence of syphilis in the general population. An average 15% of the 1273 adults tested were found to be syphilis positive during the same three years of observation. Sex workers and HIV positive adults were not included in the analysis. The characteristics of this population differed from the HIV population in terms of sex, country of origin, age and mode of transmission. More than half of the syphilis positive adults of the general population tested were diagnosed with concomitant STDs (genital herpes infection, gonorrhea, Chlamydia infection, donovanosis) throughout the study period (Table 3). The vast majority admitted adoption of risky sexual behavior for syphilis acquisition (unprotected sex), while nearly half of them mentioned more than one sex partner during the previous months (Table 3).

Table 3.
Demographics of syphilis infected patients in the general population in Greece.
Male gender and patient age did not correlate with the incidence of syphilis in the general population. On the other hand, origin from a country other than Greece (HR 4.6, 95%CI: 1.1- 18.5, p=0.005), and homosexual contact (HR 3.1, 95%CI 0.8-9.7, p=0.003), both were individual risk factors for syphilis in the general population. Additionally, as well as in the HIV population, high risk sexual behaviors such as history of multiple sex partners (HR 8.1, 95%CI: 2.8-26.2) and unprotected sex predicted syphilis infection (HR 7.9, 95%CI: 2.6-25.2, overall p<0.001) [data not shown].
Discussion
The aim of this study was to identify the epidemiological traits of a syphilis outbreak among HIV patients in our geographic area compared to the simultaneous outbreak in the general population. This study was based on data from the Reference Center of HIV and Infectious Diseases of Northern Greece and the Reference Centre of General Population. It is the first time that such a study is performed in our country and we believe it may contribute beneficially to the enhancement of existing national infectious diseases surveillance systems, especially during the current refugee and economic crisis that Greece suffers.
Multiple sexual partners, unprotected sex, being on HAART and coinfection with other STDs were risk factors for acquiring syphilis in this epidemic in our HIV population. Declining trends in some countries may be due to alterations in healthcare systems, diagnostic tests and recording rather than real changes of the incidence [11]. In other countries, dramatic increases were noted and could probably be attributed (based on the male-to-female ratio) to the recent rise in syphilis among MSM [12]. A significantly greater proportion of patients with syphilis had a history of multiple sexual partners and did not use protective measures during sexual contact (p<0.001). The overall high rate of unprotected sex is probably attributed to HAART-related treatment optimism and reduced fear of transmitting STDs [18]. In the HAART era, HIV infections continue to rise [19], which suggests that many seropositive patients continue to engage in unprotected sexual intercourse post HIV diagnosis, leading to acquisition and transmission of additional STDs and exposure of their partners to infections. The coexistence of STDs has also been linked to multiple anonymous sex partners, lack of condom use, web use for meeting sex partners, and more widespread use of recreational drugs and sildenafil [14]. Moreover, the lateral effect of highly active antiretroviral therapy (HAART) to augmented syphilis transmission rates among HIV-infected individuals has also been reported [15]. HAART improves immunological status in the setting of HIV infection, which makes patients less vulnerable to other infections, and at the same time it leads to HIV RNA virological suppression. The latter reduces the possibility of HIV transmission to other individuals. This fact has been associated with risky sexual practices due to strong confidence and reassurance of patients on HAART for low risk of HIV transmission and other STDs and consequently the rejection of condom use.
The use of HAART has been associated with reduced HIV transmission in sero-discordant couples [20,21], it has been associated with decreased use of safe practices and STDs prevention measures. Serosorting is also an important issue raised from our study, in which a significant proportion (more than a quarter) of syphilis patients were sexual partners of syphilis infected adults, irrespectively of HIV status and derived from risky sexual practices. It is confirmed that coexistence of other STDs is a predisposing factor for transmitting other STDs [1,2,3,5]. HAART administration is not a reason for closer management of these patients, except for the first three months of administration which is the necessary time period for detecting possible adverse events (renal, marrow toxicity, allergic reactions, jaundice) of HAART initiation. So we should not imply that HAART intake is associated with better surveillance of STDs or more frequent regular visits of HIV patients to healthcare services.
A change in sexual behavior with more risk- taking practices is likely to have contributed to the recent increase in STDs in Europe, making the need for enhanced preventive actions urgent [22].
Our results highlight the increased rate of syphilis, especially among MSM and HIV- infected MSM. Among people living with HIV followed at the HIV reference center of Northern Greece, MSM seem to be the major risk group for acquiring new STDs. Moreover, most of these MSM were not newly diagnosed HIV patients. Therefore, clinician alertness intensified testing, prompt diagnosis, early treatment and contact tracing are essential measures in order to control a syphilis outbreak in an HIV population.
Our study is subject to certain limitations. First, by not discriminating separately each sexually transmitted infection, we may underestimate the full spectrum of STDs emerging among people living with HIV and the most risky one for acquiring syphilis [5]. However, syphilis was chosen due to its sudden and increased incidence, comprising a moderate but still uncontrolled outbreak in the HIV population in Northern Greece. Second, our attempt to identify specific high-risk factors was limited by a significant proportion of missing data in our database. Missing data may be attributed to the discomfort in asking more personal information especially in the general population or to clinician time constraints. Third, no data are available on the role of social media applications to find partners in this study. Though, their use was not as wide during the outbreak as it currently is.
The rise of syphilis incidence in the general population during this period is remarkable. The population tested consisted of normal adults at high risk of acquiring an STD or having a symptomatic condition. To control testing bias we excluded HIV coinfected persons and sex- workers from this population. Literature shows that increased frequency of testing increases syphilis detection. Moreover, symptomatic individuals are more likely to be syphilis positive. The most significant point is that MSM are the main source of recycling STDs including syphilis in the adult population, irrespectively of HIV status. This fact confirms high risk behaviors among MSM, despite HAART intake and prevention campaigns.
The results of our study underline the importance of regular screening for STDs among HIV-positive people. Moreover, our findings show a need for improved sexual health promotion among MSM. Our study also suggests that the use of safe sexual practices to prevent STDs has decreased and it is important to emphasize the use of barrier methods also during oral sex, given that this is an usual route for syphilis transmission [23]. Further studies are needed to better understand the epidemiology of HIV/syphilis coinfection in MSM and most certainly HIV-positive individuals and immigrants should be a priority for STD prevention actions [6,24,25,26]. Additionally, closer surveillance of the general population is needed even in high-income countries since the epidemiology of syphilis and other STDs may change throughout the years [27].
It is also worth mentioning that HIV patients in Northern Greece are described mainly as late presenters [28] (delayed HIV diagnosis) and in many cases, HIV diagnosis was simultaneous with syphilis diagnosis. This fact may justify the delayed stage of syphilis identified in coinfection cases (latent vs. secondary vs. primary syphilis). The classification of syphilis cases refers to the incidence of syphilis (new cases during 2008- 2010), which was determined post completing the testing and diagnostic procedure for each patient (blood serology testing, lumbar puncture). Previous screening campaigns were also implemented before 2008, but the incident cases were fewer among HIV patients served in our Infectious Diseases Unit. Screening was intensified during 2009-2010 to assess the outbreak risk factors and to prevent expansion of the infection.
Conclusions
In summary, diagnosis of a sexually transmitted disease in an HIV patient is a crucial clinical event that should trigger the clinician’s suspicion for high-risk sexual behavior [29]. Sexual health assessments should be a routine process for HIV patients during follow-up visits [30]. For high-risk patients, screening for STDs should be done at least annually or more frequently as recommended by many experts [30,31,32,33,34]. Our study also suggests that the use of protection methods against STDs has dropped and it is important to emphasize the specific use of barrier methods during oral sex, given that this is a route for syphilis transmission [23].
Further studies are needed to better understand the epidemiology of HIV/syphilis coinfection in MSM and definitely HIV-positive people and immigrants should be a priority for STD prevention actions [24,25,26,27,32,33,34]. Even currently, syphilis remains a huge public health issue and this underlines the importance of adding further data to investigate risk factors [35]. Surveillance of STDs in AIDS Reference Centers could identify risk groups and detect different STDs emerging among the HIV population in a specific region. These data are useful for healthcare professionals, for the implementation of screening strategies and prevention efforts to reduce HIV transmission [16].
Author Contributions
OT contributed to the initial idea and design of the manuscript, the interpretation of data, drafting and revising the article, and approved the final version of the draft for publication. LS interpreted the laboratory results, contributed to the design of the study, edited the manuscript and approved its final version. EC contributed to the idea, concept and design of the manuscript, the analysis and interpretation of the data, drafting and revising of the article and approved the final version of the manuscript for publication. PK contributed to the design of the manuscript, drafting and revising of the manuscript, and approved the final version of the manuscript. ES performed the laboratory tests and contributed to the concept and design of the manuscript, the interpretation of data, drafting and revising the article, and approved the final version of the manuscript for publication. PZ contributed to the idea and design of the manuscript, the interpretation of data, drafting and revising the article, approved the final version of the manuscript and performed part of the statistical analysis. EV examined the participants, interpreted the results and contributed to the concept of the manuscript, revised the article, and approved the final version of the draft for publishing. AM performed the laboratory tests and wrote part of the manuscript, revised it and approved its final version. SM contributed to the organization of the study, performed statistical analysis, revised the manuscript and approved its final version. All authors read and approved the final version of the manuscript.
Conflicts of Interest
All authors—none to declare.
References
- Fleming, D.T.; Wasserheit, J.N. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999, 75, 3–17. [Google Scholar] [CrossRef]
- Huhn, G.D.; McIntyre, A.F.; Broad, J.M.; et al. Factors associated with newly diagnosed HIV among persons with concomitant sexually transmitted diseases. Sex Transm Dis 2008, 35, 731–737. [Google Scholar]
- Centers for Disease Control and Prevention (US). HIV Surveillance in Men Who Have Sex with Men (MSM). Available online: https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-slides-msm.pdf (accessed on 19 August 2016).
- Buchacz, K.; Greenberg, A.; Onorato, I.; Janssen, R. Syphilis epidemics and human immunodeficiency virus (HIV) incidence among men who have sex with men in the United States: Implications for HIV prevention. Sex Transm Dis 2005, 32, S73–S79. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Rompa, D.; Cage, M. Sexually transmitted infections among HIV seropositive men and women. Sex Transm Infect 2000, 76, 350–354. [Google Scholar] [CrossRef][Green Version]
- Zetola, N.M.; Klausner, J.D. Syphilis and HIV Infection: An update. Clin Infect Dis 2007, 44, 1222–1228. [Google Scholar] [CrossRef]
- Savage, E.J.; Hughes, G.; Ison, C.; European Surveillance of Sexually Transmitted Infections network. Syphilis and gonorrhoea in men who have sex with men: A European overview. Euro Surveill 2009, 14, pii–19417. [Google Scholar] [CrossRef]
- Fenton, K.A.; Lowndess, C.M. Recent trends in the epidemiology of sexually transmitted infection in the European Union. Sex Transm Infect. 2004, 80, 255–263. [Google Scholar] [CrossRef]
- Heffelfinger, J.D.; Swint, E.B.; Berman, S.M.; Weinstock, H.S. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health 2007, 97, 1076–1083. [Google Scholar] [CrossRef]
- Amato-Gauci, A.J. Sexually Transmitted Infections in Europe 2012. Available online: http://ecdc.europa.eu/en/publications/Publications/sexually-transmitted-infections-europe-surveillance-report-2012.pdf (accessed on 15 October 2015).
- European Centre for Disease Prevention and Control. Sexually Transmitted Infections in Europe 1990–2009; ECDC: Stockholm, 2011. [Google Scholar]
- Marcus, U.; Voss, L.; Kollan, C.; Hamouda, O. HIV incidence increasing in MSM in Germany: Factors influencing infection dynamics. Euro Surveill 2006, 11, 157–160. [Google Scholar] [CrossRef]
- Vall Mayans, M.; Sanz Colomo, B.; Armengol, P.; Loureiro, E. Outbreaks of infectious syphilis and other STIs in men who have sex with men in Barcelona, 2002–2003. Euro Surveill 2004, 8, pii–2578. [Google Scholar]
- Golden, M.R.; Marra, C.M.; Holmes, K.K. Update on syphilis: Resurgence of an old problem. JAMA 2003, 290, 1510–1514. [Google Scholar] [CrossRef]
- Katz, M.H.; Schwarcz, S.K.; Kellogg, T.A.; et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health 2002, 92, 388–394. [Google Scholar] [CrossRef]
- Defraye, A.; Van Beckhoven, D.; Sasse, A. Surveillance of sexually transmitted infections among persons living with HIV. Int J Public Health 2011, 56, 169–174. [Google Scholar] [CrossRef]
- Ministry of Health, Hellenic Center for Disease Control and Prevention. HIV/AIDS Surveillance in Greece. Available online: http://www.keelpno.gr/Portals/0/%CE%91%CF%81%CF%87%CE%B5%CE%AF%CE%B1/HIV/2014/Epidimiologiko_2013_final.pdf (accessed on 15 October 2015).
- Crepaz, N.; Hart, T.A.; Marks, G. Highly active antiretroviral therapy and sexual risk behavior: A meta- analytic review. JAMA 2004, 292, 224–236. [Google Scholar] [CrossRef]
- Weinhardt, L.S.; Kelly, J.A.; Brondino, M.J.; et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immun Defic Syndr 2004, 36, 1057–1066. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Kelley, C.F.; Haaland, R.E.; Patel, P.; et al. HIV-1 RNA rectal shedding is reduced in men with low plasma HIV-1 RNA viral loads and is not enhanced by sexually transmitted bacterial infections of the rectum. J Infect Dis 2011, 204, 761–767. [Google Scholar] [CrossRef]
- Fenton, K.A. A multilevel approach to understanding the resurgence and evolution of infectious syphilis in Western Europe. Euro Surveill 2004, 9, 3–4. [Google Scholar] [CrossRef]
- Vilar, J.; Dehesa, L.; Gómez-Duaso, A.J. [Epidemiological study of an epidemic outbreak of syphilis in Palmas de Gran Canaria]. Actas Dermosifiliogr 2007, 98, 466–469. [Google Scholar] [CrossRef]
- Williamson, L.M.; Dodds, J.P.; Mercey, D.E.; Hart, G.J.; Johnson, A.M. Sexual risk behaviour and knowledge of HIV status among community samples of gay men in the UK. AIDS 2008, 22, 1063–1070. [Google Scholar] [CrossRef]
- Jin, F.; Prestage, G.; Zablotska, I.; et al. High rates of sexually transmitted infections in HIV positive homosexual men: Data from two community based cohorts. Sex Transm Infect 2007, 83, 397–399. [Google Scholar] [CrossRef]
- Bremer, V.; Marcus, U.; Hofmann, A.; Hamouda, O. Building a sentinel surveillance system for sexually transmitted infections in Germany, 2003. Sex Transm Infect 2005, 81, 173–179. [Google Scholar] [CrossRef][Green Version]
- Read, P.; Fairley, C.K.; Chow, E.P. Increasing trends of syphilis among men who have sex with men in high income countries. Sex Health 2015, 12, 155–163. [Google Scholar] [CrossRef]
- Metallidis, S.; Pilalas, D.; Skoura, L.; et al. Time trends and correlates of late presentation for HIV care in Northern Greece during the decade 2000 to 2010. J Int AIDS Soc 2012, 15, 17395. [Google Scholar] [CrossRef]
- Klausner, J.D.; Wong, W. Sexually transmitted diseases in men who have sex with men: A clinical review. Curr Infect Dis Rep 2003, 5, 135–144. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), Health Resources and Services Administration; National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2003, 52, 1–24. [Google Scholar]
- Workowski, K.A.; Levine, W.C. Sexually transmitted diseases treatment guidelines—2002. MMWR Morb Mortal Wkly Rep 2002, 51, 1–80. [Google Scholar]
- California STD Controllers Association, California Coalition of Local AIDS Diectors. Guidance for STD clinical preventive services for persons infected with HIV. Sex Transm Dis 2001, 28, 460–463. [Google Scholar] [CrossRef][Green Version]
- Hu, Q.H.; Xu, J.J.; Zou, H.C.; et al. Risk factors associated with prevalent and incident syphilis among an HIV-infected cohort in Northeast China. BMC Infect Dis 2014, 14, 658. [Google Scholar] [CrossRef]
- Callegari, F.M.; Pinto-Neto, L.F.; Medeiros, C.J.; Scopel, C.B.; Page, K.; Miranda, A.E. Syphilis and HIV co-infection in patients who attend an AIDS outpatient clinic in Vitoria, Brazil. AIDS Behav 2014, 18 (Suppl. 1), S104–S109. [Google Scholar] [CrossRef][Green Version]
- Clement, M.E.; Hicks, C.B. Syphilis on the rise: What went wrong? JAMA 2016, 315, 2281–2283. [Google Scholar] [CrossRef]
© GERMS 2016.