Experimental Approach for Bacteriophage Susceptibility Testing of Planktonic and Sessile Bacterial Populations—Study Protocol
Abstract
Introduction
Aims of the study
Methods
Study design
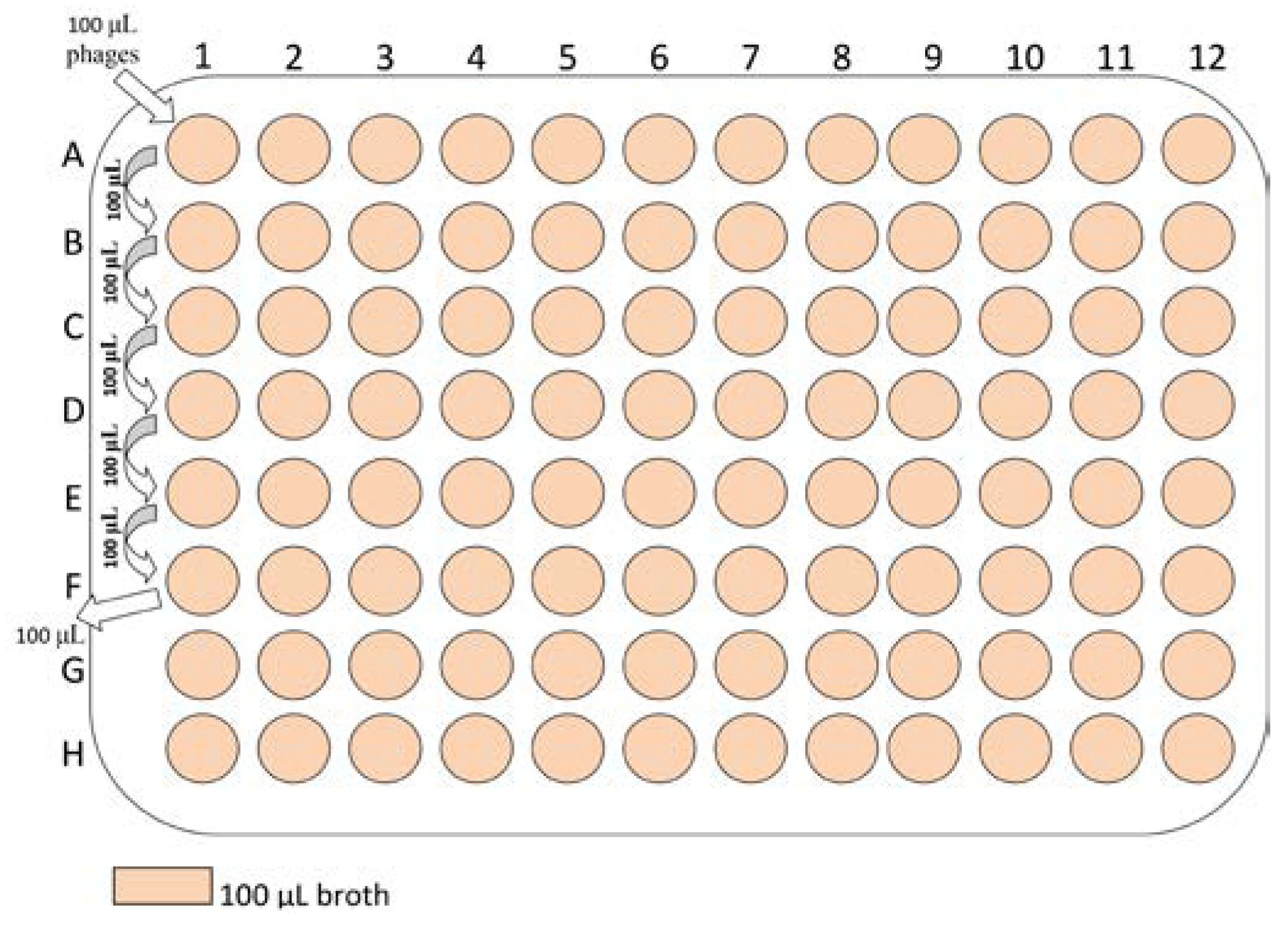
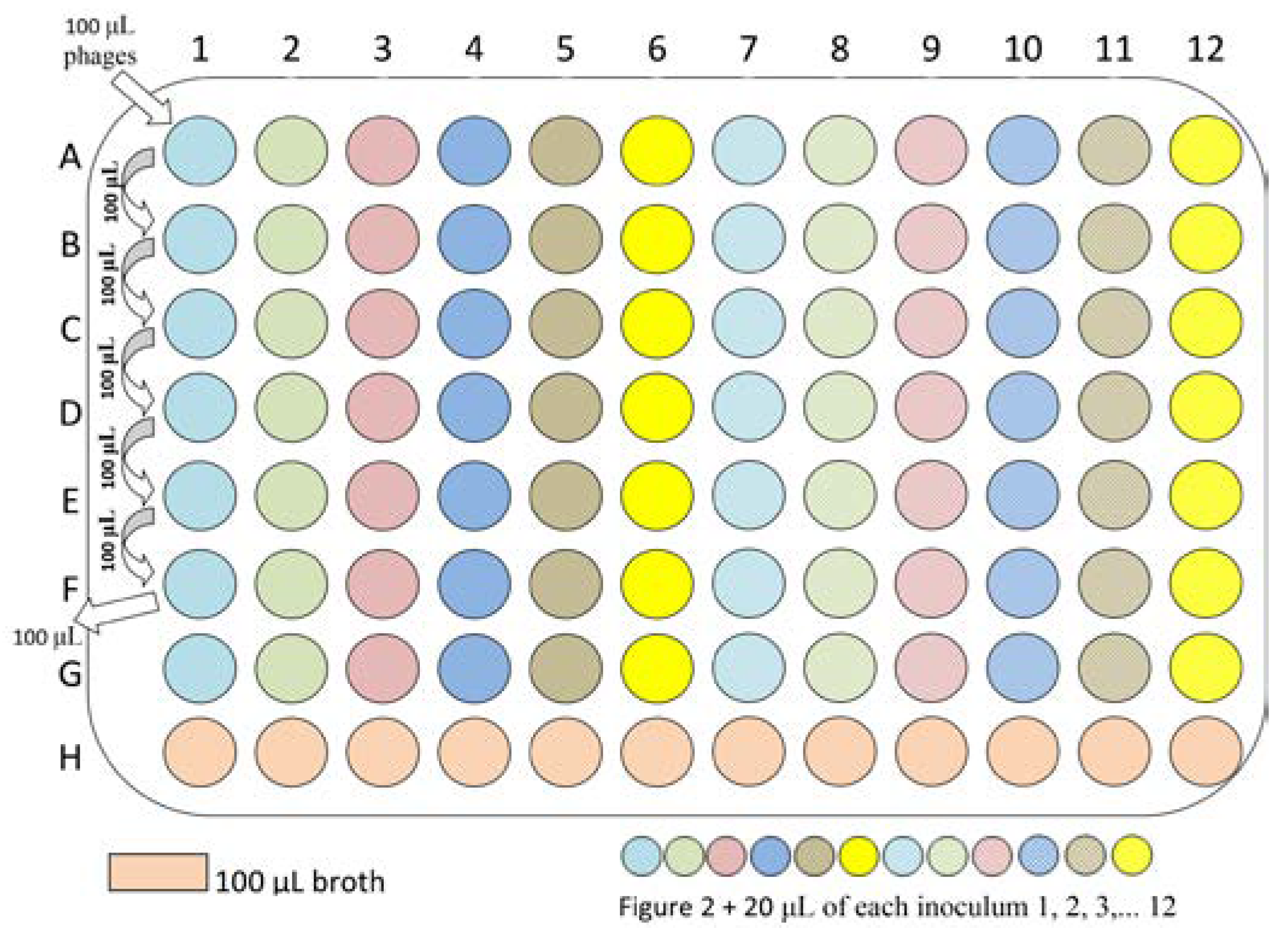
Laboratory procedures
Expected results
Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, E.; Weil, D.E.; Raviglione, M.; Nakatani, H. World Health Organization World Health Day Antimicrobial Resistance Technical Working Group. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ 2011, 89, 390–392. [Google Scholar] [CrossRef] [PubMed]
- ECDC/EMEA Joint Working Group. The bacterial challenge: Time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and development of new antibacterial agents. Stockholm: European Centre for Disease Prevention and Control. 2009.
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol J Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Phage renaissance: New hope against antibiotic resistance. Environ Health Perspect 2013, 121, a48–a53. [Google Scholar] [CrossRef] [PubMed]
- Zilișteanu, C.; Mintzer-Morgenstern, L.; Ghyka, G. Influence des bactériophages homologues sur la résistance aux antibiotiques de certaines souches d’Enterobacteriaceae et de staphylocoques. Etude “in vitro”. Arch Roum Pathol Exp Microbiol 1965, 24, 1029–1040. [Google Scholar]
- Zilișteanu, C.; Mintzer-Morgenstern, L.; Ionesco, H.; Ionesco-Dorohoi, T. Further data on the association of bacteriophage with antibiotic therapy for the purpose of sterilizing carriers of dysentery bacilli. Arch Roum Pathol Exp Microbiol 1969, 28, 1073–1080. [Google Scholar] [PubMed]
- Zilișteanu, C.; Ionescu, H.; Ionescu-Dorohoi, T.; Mintzer, L. Treatment of urinary infections with bacteriophage-autovaccine-antibiotics. Arch Roum Pathol Exp Microbiol 1971, 30, 195–207. [Google Scholar] [PubMed]
- Coulter, L.B.; McLean, R.J.; Rohde, R.E.; Aron, G.M. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 2014, 6, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.K.; Erwin, T.C.; McLean, R.J.; Aron, G.M. Bacteriophage ecology in Escherichia coli and Pseudomonas aeruginosa mixed-biofilm communities. Appl Environ Microbiol 2011, 77, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Nag, D.; Bansal, S. Inhibiting biofilm formation by Klebsiella pneumoniae B5055 using an iron antagonizing molecule and a bacteriophage. BMC Microbiol 2013, 13, 174. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2014.
Share and Cite
Neguț, A.C.; Săndulescu, O.; Popa, M.; Streinu-Cercel, A.; Alavidze, Z.; Berciu, I.; Bleotu, C.; Popa, M.I.; Chifiriuc, M.C.; Streinu-Cercel, A. Experimental Approach for Bacteriophage Susceptibility Testing of Planktonic and Sessile Bacterial Populations—Study Protocol. GERMS 2014, 4, 92-96. https://doi.org/10.11599/germs.2014.1062
Neguț AC, Săndulescu O, Popa M, Streinu-Cercel A, Alavidze Z, Berciu I, Bleotu C, Popa MI, Chifiriuc MC, Streinu-Cercel A. Experimental Approach for Bacteriophage Susceptibility Testing of Planktonic and Sessile Bacterial Populations—Study Protocol. GERMS. 2014; 4(4):92-96. https://doi.org/10.11599/germs.2014.1062
Chicago/Turabian StyleNeguț, Alina Cristina, Oana Săndulescu, Marcela Popa, Anca Streinu-Cercel, Zemphira Alavidze, Ioana Berciu, Coralia Bleotu, Mircea Ioan Popa, Mariana Carmen Chifiriuc, and Adrian Streinu-Cercel. 2014. "Experimental Approach for Bacteriophage Susceptibility Testing of Planktonic and Sessile Bacterial Populations—Study Protocol" GERMS 4, no. 4: 92-96. https://doi.org/10.11599/germs.2014.1062
APA StyleNeguț, A. C., Săndulescu, O., Popa, M., Streinu-Cercel, A., Alavidze, Z., Berciu, I., Bleotu, C., Popa, M. I., Chifiriuc, M. C., & Streinu-Cercel, A. (2014). Experimental Approach for Bacteriophage Susceptibility Testing of Planktonic and Sessile Bacterial Populations—Study Protocol. GERMS, 4(4), 92-96. https://doi.org/10.11599/germs.2014.1062



