Efficacy and Safety of Darunavir (Prezista®) with Low-Dose Ritonavir and Other Antiretroviral Medications in Subtype F HIV-1 Infected, Treatment-Experienced Subjects in Romania: A Post-Authorization, Open-Label, One-Cohort, Non-Interventional, Prospective Study
Abstract
Introduction
Methods
Study design
Patients
Medication
Study assessments
Statistical analyses
Results
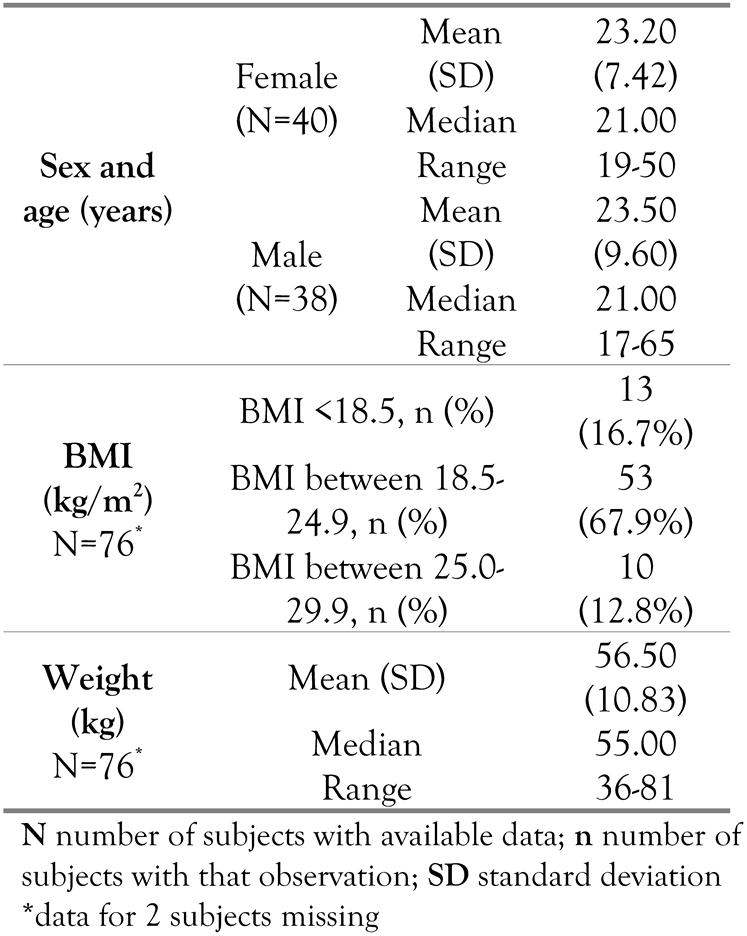
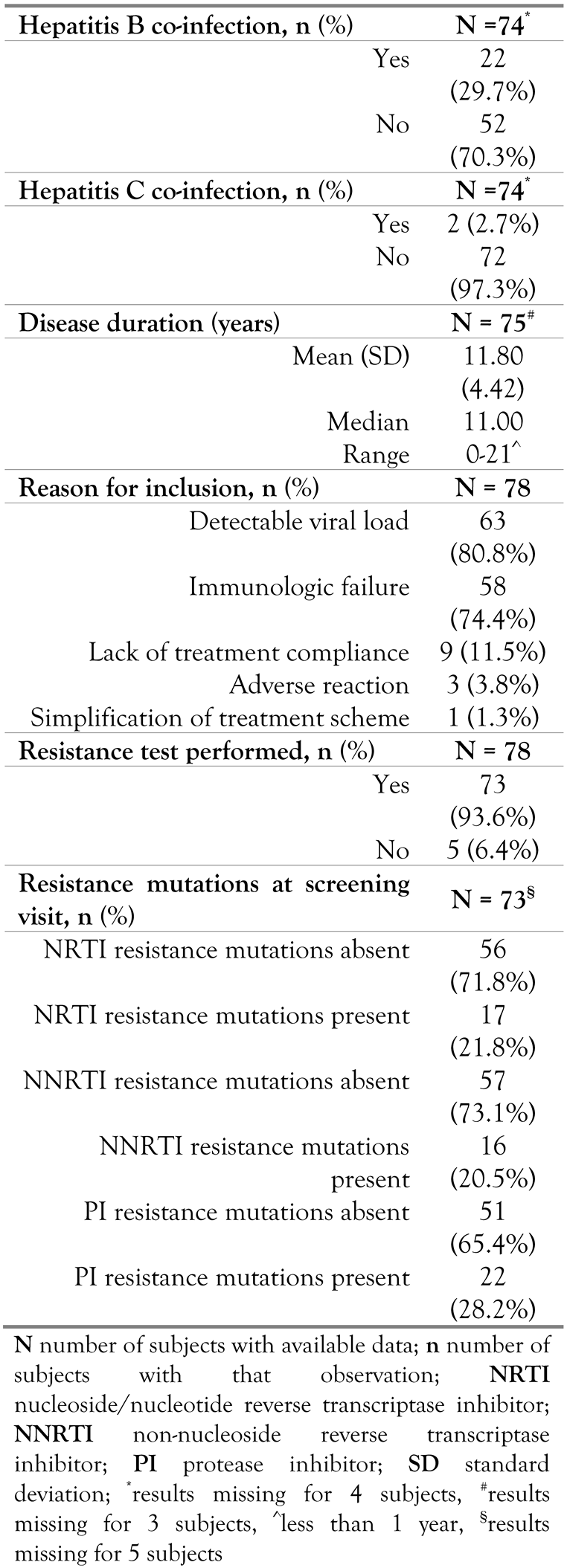
Baseline characteristics
Efficacy
CD4 cell count
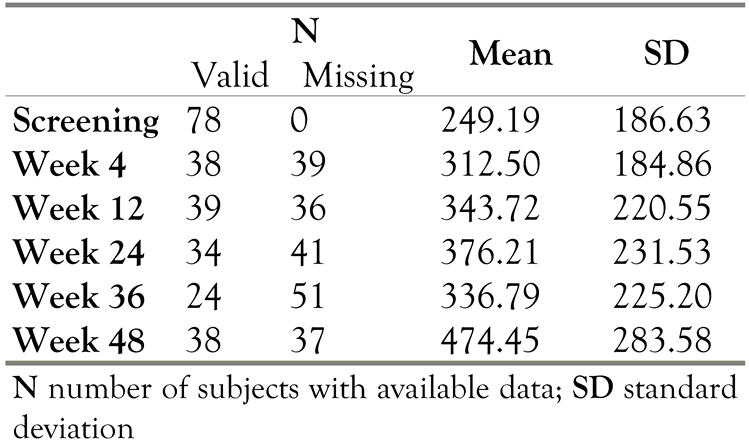
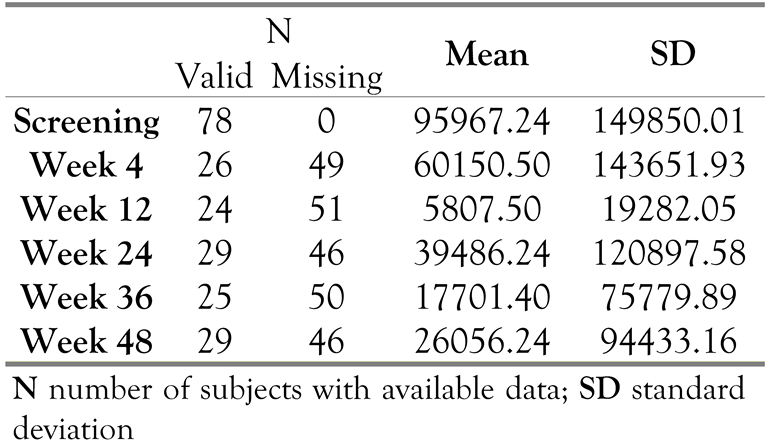
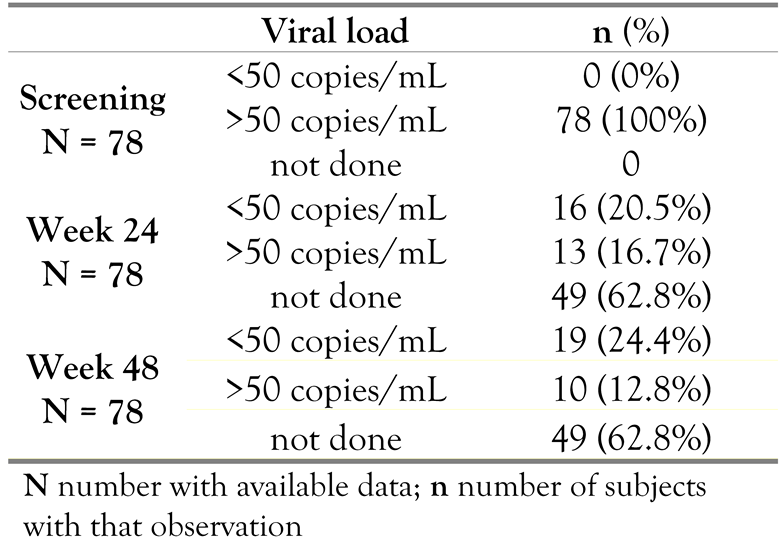
Viral load
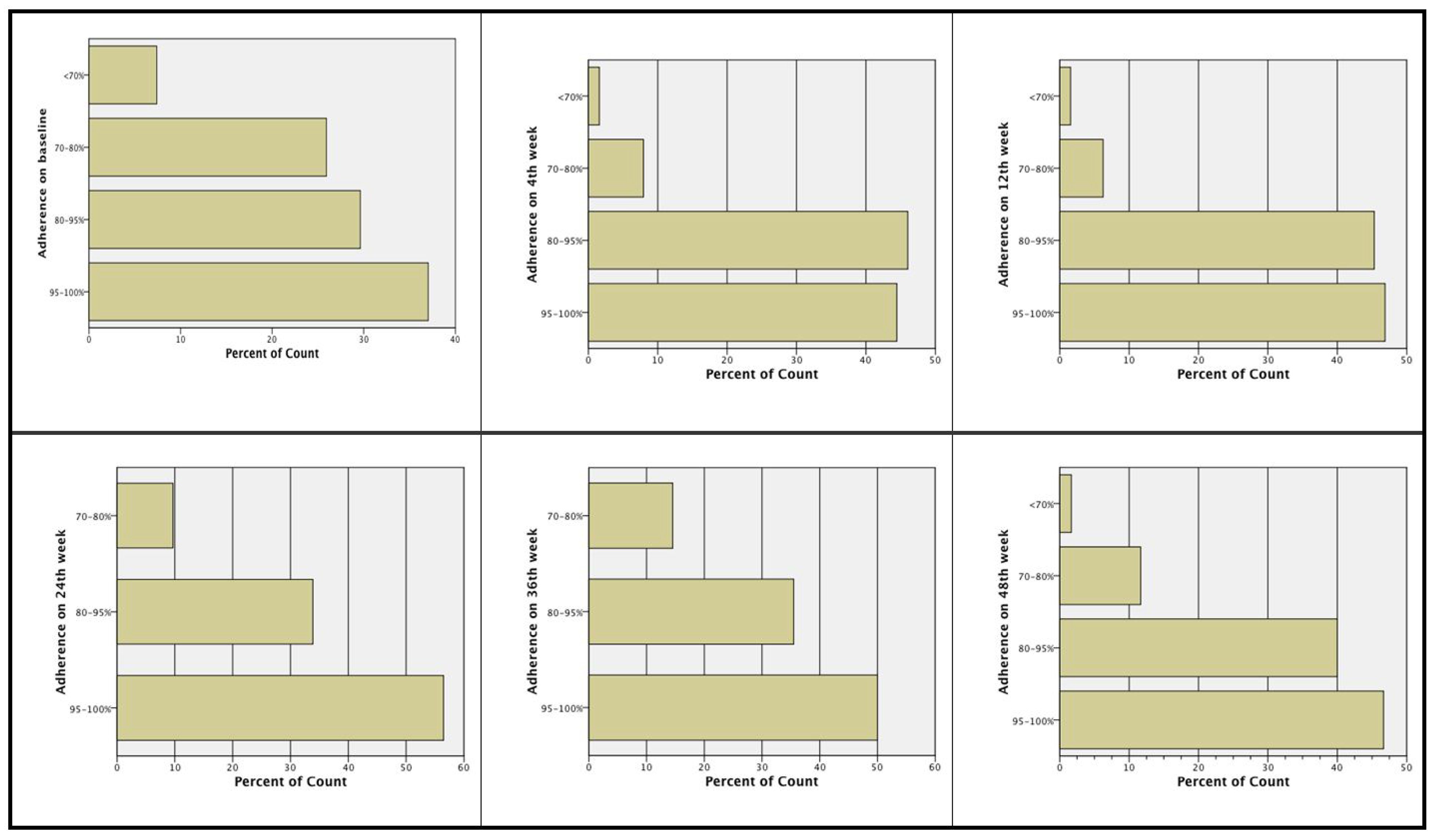
Treatment adherence at Week 24 and Week 48
Safety
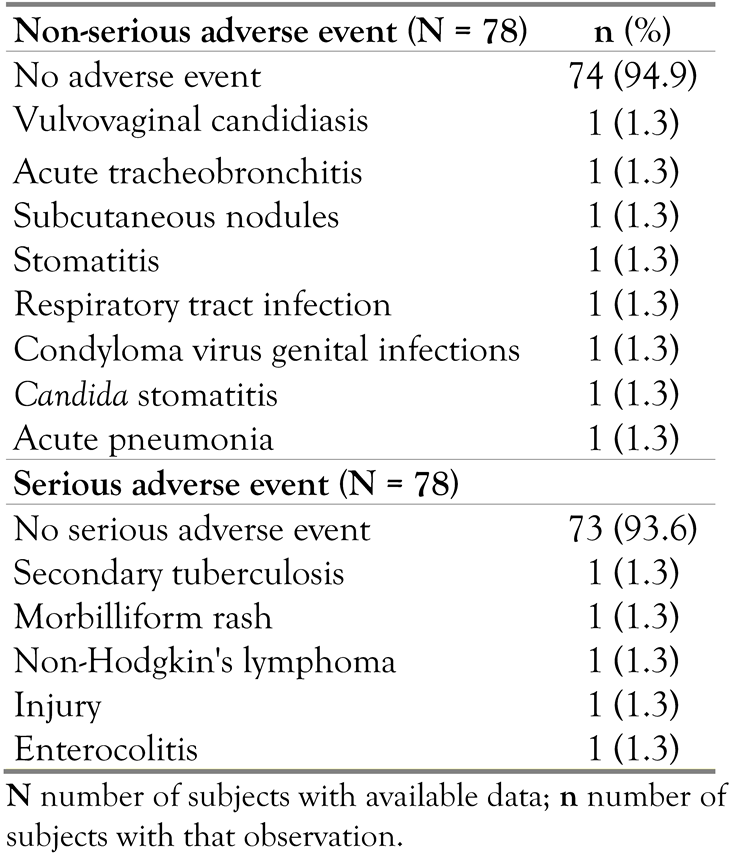
Adverse events
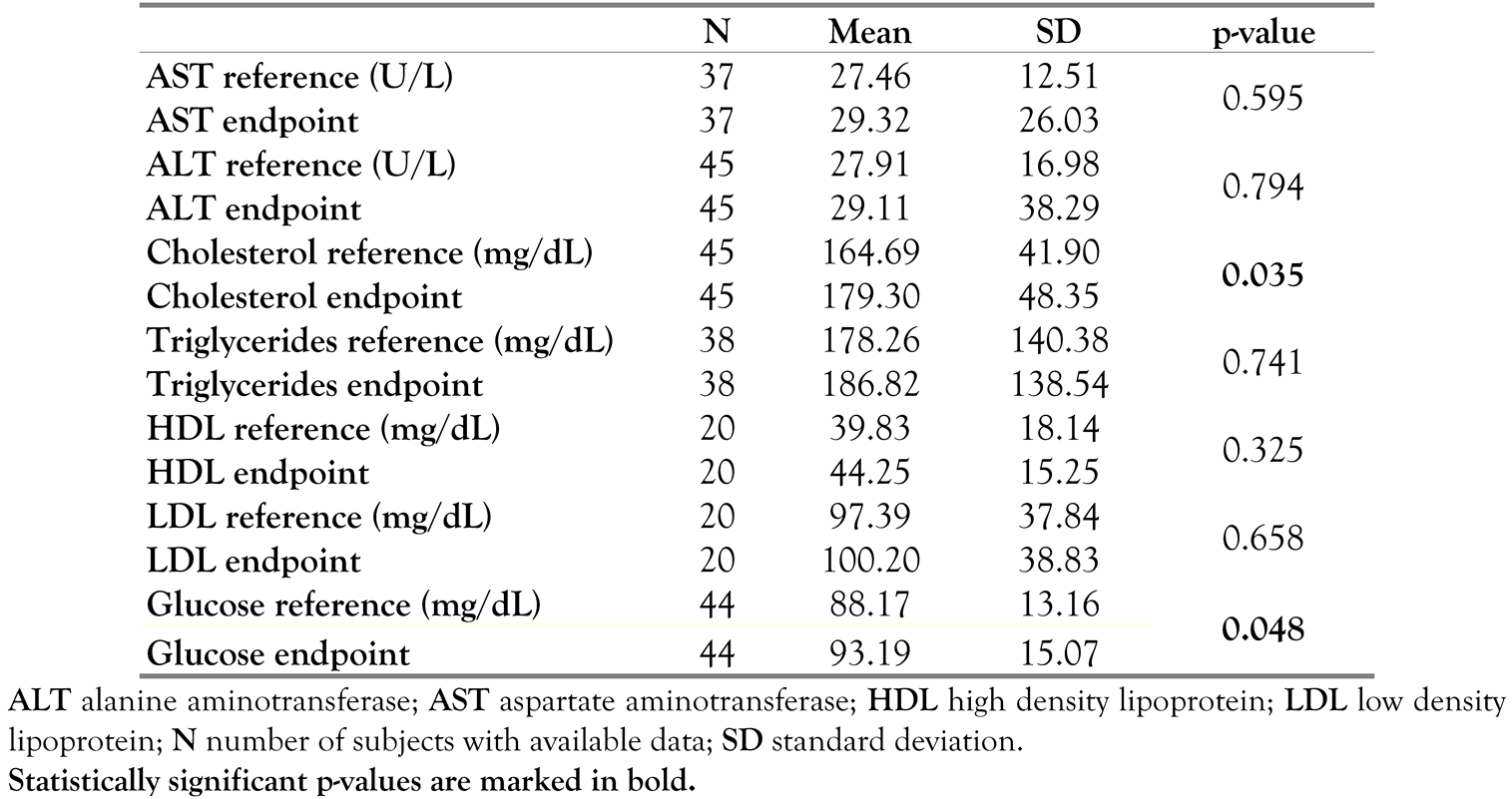
Clinical laboratory evaluation
Vital signs
Resistance pattern
Discussion
Conclusion
Author Contributions
Acknowledgments and role of the funding source:
Trial: registration
Conflicts of Interest
References
- Detels, R.; Munoz, A.; McFarlane, G.; et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 1998, 280, 1497–1503. [Google Scholar] [CrossRef]
- Hogg, R.S.; Yip, B.; Kully, C.; et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 1999, 160, 659–665. [Google Scholar] [PubMed]
- Palella FJJr Delaney, K.M.; Moorman, A.C.; et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Department of Health and Human Services (DHHS). Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (accessed on 28 December 2012).
- Perno, C.F.; Moyle, G.; Tsoukas, C.; Ratanasuwan, W.; Gatell, J.; Schechter, M. Overcoming resistance to existing therapies in HIV-infected patients: The role of new antiretroviral drugs. J Med Virol 2008, 80, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M.; Nelson, M.; Jayaweera, D.; et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 2009, 23, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004, 170, 229–238. [Google Scholar]
- d’Arminio, M.A.; Lepri, A.C.; Rezza, G.; et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS 2000, 14, 499–507. [Google Scholar]
- Lucas, G.M.; Chaisson, R.E.; Moore, R.D. Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Ann Intern Med 1999, 131, 81–87. [Google Scholar] [CrossRef]
- Crespo-Fierro, M. Compliance/adherence and care management in HIV disease. J Assoc Nurses AIDS Care 1997, 8, 43–54. [Google Scholar] [CrossRef]
- Carr, A.; Cooper, D.A. Adverse effects of antiretroviral therapy. Lancet 2000, 356, 1423–1430. [Google Scholar] [CrossRef]
- Koh, Y.; Nakata, H.; Maeda, K.; et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chemother 2003, 47, 3123–3129. [Google Scholar] [CrossRef]
- Clotet, B.; Bellos, N.; Molina, J.M.; et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: A pooled subgroup analysis of data from two randomised trials. Lancet 2007, 369, 1169–1178. [Google Scholar] [CrossRef]
- Katlama, C.; Esposito, R.; Gatell, J.M.; et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS 2007, 21, 395–402. [Google Scholar] [CrossRef]
- Haubrich, R.; Berger, D.; Chiliade, P.; et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS 2007, 21, F11–F18. [Google Scholar] [CrossRef] [PubMed]
- PREZISTA (darunavir) Summary of Product Characteristics. 2013. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000707/human_med_000988.jsp&mid=WC0b01ac058001d124 (accessed on 28 December 2012).
- McCoy, C. Darunavir: A nonpeptidic antiretroviral protease inhibitor. Clin Ther 2007, 29, 1559–1576. [Google Scholar] [CrossRef]
- Carr, A.; Cooper, D.A. Images in clinical medicine. Lipodystrophy associated with an HIV-protease inhibitor. N Engl J Med 1998, 339, 1296. [Google Scholar] [CrossRef]
- van der Valk, M.; Gisolf, E.H.; Reiss, P.; et al. Increased risk of lipodystrophy when nucleoside analogue reverse transcriptase inhibitors are included with protease inhibitors in the treatment of HIV-1 infection. AIDS 2001, 15, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Mateo, M.G.; Pruvost, A.; et al. Polymorphisms of Pyrimidine Pathway Enzymes Encoding Genes and HLA-B*40:01 Carriage in Stavudine-Associated Lipodystrophy in HIV-Infected Patients. PLoS ONE 2013, 8, e67035. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Ko, W.S.; Kao, J.T.; et al. Association of single-nucleotide polymorphism 3 and c.553G>T of APOA5 with hypertriglyceridemia after treatment with highly active antiretroviral therapy containing protease inhibitors in HIV-infected individuals in Taiwan. Clin Infect Dis 2009, 48, 832–835. [Google Scholar] [CrossRef]
- Robles, D.T.; Olson, J.M.; Colven, R.M. Lipodystrophy in HIV. Medscape 2013. Available online: http://emedicine.medscape.com/article/1082199-overview (accessed on 10 February 2014).
- Hill, A.; Sawyer, W.; Gazzard, B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials 2009, 10, 1–12. [Google Scholar] [CrossRef]
- Barlow-Mosha, L.; Eckard, A.R.; McComsey, G.A.; Musoke, P.M. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc 2013, 16, 18600. [Google Scholar] [CrossRef]
- Feleke, Y.; Fekade, D.; Mezegebu, Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J 2012, 50, 221–230. [Google Scholar] [PubMed]
- Mallolas, J.; Podzamczer, D.; Milinkovic, A.; et al. Efficacy and safety of switching from boosted lopinavir to boosted atazanavir in patients with virological suppression receiving a LPV/r-containing HAART: The ATAZIP study. J Acquir Immune Defic Syndr 2009, 51, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Hoy, J.; Beatty, G.; Vangeneugden, T.; Lefebvre, E. Body mass change and anthropometric-related adverse events at week 24 in treatment-experienced HIV-infected patients receiving TMC114/r or control PIs in POWER 1, 2 and 3. 8th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. 24–26 September 2006, San Francisco, CA, USA. Antiviral Therapy 2006, 11, L21. [Google Scholar]
© GERMS 2014.
Share and Cite
Benea, O.E.; Streinu-Cercel, A.; Dorobăț, C.; Rugină, S.; Negruțiu, L.; Cupșa, A.; Duiculescu, D.; Chiriac, C.; Itu, C.; Prisăcariu, L.J.; et al. Efficacy and Safety of Darunavir (Prezista®) with Low-Dose Ritonavir and Other Antiretroviral Medications in Subtype F HIV-1 Infected, Treatment-Experienced Subjects in Romania: A Post-Authorization, Open-Label, One-Cohort, Non-Interventional, Prospective Study. GERMS 2014, 4, 59-69. https://doi.org/10.11599/germs.2014.1057
Benea OE, Streinu-Cercel A, Dorobăț C, Rugină S, Negruțiu L, Cupșa A, Duiculescu D, Chiriac C, Itu C, Prisăcariu LJ, et al. Efficacy and Safety of Darunavir (Prezista®) with Low-Dose Ritonavir and Other Antiretroviral Medications in Subtype F HIV-1 Infected, Treatment-Experienced Subjects in Romania: A Post-Authorization, Open-Label, One-Cohort, Non-Interventional, Prospective Study. GERMS. 2014; 4(3):59-69. https://doi.org/10.11599/germs.2014.1057
Chicago/Turabian StyleBenea, Otilia Elisabeta, Adrian Streinu-Cercel, Carmen Dorobăț, Sorin Rugină, Lucian Negruțiu, Augustin Cupșa, Dan Duiculescu, Carmen Chiriac, Corina Itu, Liviu Jany Prisăcariu, and et al. 2014. "Efficacy and Safety of Darunavir (Prezista®) with Low-Dose Ritonavir and Other Antiretroviral Medications in Subtype F HIV-1 Infected, Treatment-Experienced Subjects in Romania: A Post-Authorization, Open-Label, One-Cohort, Non-Interventional, Prospective Study" GERMS 4, no. 3: 59-69. https://doi.org/10.11599/germs.2014.1057
APA StyleBenea, O. E., Streinu-Cercel, A., Dorobăț, C., Rugină, S., Negruțiu, L., Cupșa, A., Duiculescu, D., Chiriac, C., Itu, C., Prisăcariu, L. J., & Iosif, I. (2014). Efficacy and Safety of Darunavir (Prezista®) with Low-Dose Ritonavir and Other Antiretroviral Medications in Subtype F HIV-1 Infected, Treatment-Experienced Subjects in Romania: A Post-Authorization, Open-Label, One-Cohort, Non-Interventional, Prospective Study. GERMS, 4(3), 59-69. https://doi.org/10.11599/germs.2014.1057




