Abstract
Introduction: The aim of the study was to assess the safety and efficacy of darunavir (Prezista®) used in subtype F human immunodeficiency virus—type 1 (HIV-1) infected, antiretroviral therapy (ART)-experienced patients in Romania in routine clinical practice. Methods: This was a post-authorization, open-label, one-cohort, non-interventional, prospective study conducted at multiple sites in Romania to assess efficacy (CD4 cell count, viral load, and treatment compliance) and safety ([serious] adverse events, clinical laboratory evaluation, and vital signs) of darunavir in combination with low-dose ritonavir (DRV/r) and other antiretroviral (ARV) medications in subtype F HIV-1 infected subjects in naturalistic settings. Seventy-eight subjects were recruited by 9 investigational sites and received 600/100 mg DRV/r twice daily. Results: Treatment with DRV/r administered with other ARV medications resulted in the expected, statistically relevant improvement of CD4 cell count and viral load in subjects eligible for such treatment. In addition, adherence to treatment was high and the treatment-emergent safety profile observed during this study was consistent with the established safety profile of darunavir. Conclusion: DRV/r administered in combination with other ARV medications in subtype F HIV-1 infected subjects in naturalistic settings proved to be an effective and safe treatment in Romania. Trial registration: NCT01253967
Introduction
The introduction of highly active antiretroviral therapy (HAART) resulted in significant reduction in morbidity and mortality in HIV-infected patients [1,2,3]. Inhibitors of HIV protease have become cornerstones in the treatment of HIV disease. Current options for the treatment of HIV-1-infected adult subjects consist of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), entry inhibitors, and an integrase inhibitor. A triple regimen is considered standard of care (SoC) and when effective, it results in suppression of the virus below the detection limits of the current tests, thereby strongly reducing the emergence of resistance [4,5,6].
In Romania, the national guide to HIV/acquired immune deficiency syndrome (AIDS) therapy describes that a triple regimen of three antiretroviral (ARV) therapies is SoC for treatment-experienced patients.
For treatment of naïve HIV-infected patients (patients who did not take ARV therapy before) the first-line ARV therapy should be selected carefully to overcome treatment failure.
Treatment adherence to HAART is often compromised because of treatment failure (inability to suppress HIV viral replication to below the current limit of detection, i.e., 50copies/mL), adverse effects, significant complexity of the available drug regimens, or noncompliance within the first eight months [5,7]. The concern of this noncompliance to the HAART therapy is that it ultimately will result in multi-drug resistance, afflicting the classes of oral ARVs, and will compromise future treatment options [5,8,9]. Especially for PIs maintenance of the therapeutic level is important, otherwise resistance to therapy is bound to emerge [10].
Adverse effects caused by ART are widespread throughout the human body. Most ARV drugs cause mild adverse effects including gastrointestinal effects such as bloating, nausea, and diarrhea. Serious adverse effects have also been reported: lactic acidosis, hepatic steatosis, and/or body fat redistribution (for NRTIs); rash, Stevens-Johnson syndrome and toxic epidermal necrolysis (for NNRTIs); hepatotoxicity, gastrointestinal and metabolic abnormalities including dyslipidemia, hyperglycemia, insulin resistance, and lipodystrophy (for PIs) [11].
A new PI has received marketing authorization in Romania, the Unites States of America (USA), and the European Union; it is known as Prezista® (darunavir [DRV]). This is an inhibitor of HIV protease with potent in vitro activity against wild-type and resistant HIV-1 (type1) and is indicated in combination with low-dose ritonavir (DRV/r) and with other ARV products for the treatment of patients with HIV-1 infection. Darunavir is very effective for therapy-experienced patients with limited options due to its impressive potency in the presence of PI-resistance mutations [12]. Two randomized, controlled, phase IIb studies, POWER-1 (USA) and -2 (Europe), brought darunavir to the forefront of attention and led to an accelerated approval of darunavir for use in combination with ritonavir in adults with HIV strains that are resistant to other PIs [13,14,15].
In Romania the need for safety and efficacy data for darunavir used in subtype F HIV-1 ART-experienced patients was the starting point of this project. The primary aim of the current national multi-center prospective study was to evaluate the efficacy (CD4 cell count, viral load, and treatment compliance) of DRV/r and other ARV medications under marketed conditions in subtype F HIV-1 infected, treatment-experienced subjects in routine clinical practice in Romania. Secondary objectives were to evaluate the safety (adverse events [AEs] and serious adverse events [SAEs], clinical laboratory evaluation, and vital signs).
Methods
Study design
This was a post-authorization, open-label, one-cohort, non-interventional, prospective study conducted at multiple sites in Romania to assess effectiveness and safety of DRV/r administered with other ARV drugs in subtype F HIV-1 infected subjects in Romania. The target population were HIV-1 infected individuals who required treatment with DRV/r containing regimens according to the summary of product characteristics (SmPC) [16]. The treatment duration for each patient was 48 weeks or less, in case of treatment failure.
Patients
All patients with a documented subtype F HIV-1 infection, who were male or female over 18 years of age, who were in need of DRV and fulfilled the criteria according to the approved indication for highly-treatment-experienced patients (with plasma HIV-1 RNA <100,000copies/mL and CD4+ cell count ≥100x106 cells/L), who had failed at least one previous ARV therapeutic regimen, and who had voluntarily signed the Agreement for Medical Information Disclosure Form before initiation of study procedures were eligible for inclusion in this study [16]. In total, 78patients were recruited by nine investigational sites in Romania.
Patients were excluded from the study if they were recently (i.e., not having received any ARV therapy) diagnosed with HIV-1 infection, had a contraindication to DRV regimen administration according to the SmPC, had any conditions which in the opinion of the investigator compromised the subject’s safety or adherence to the study protocol, used disallowed concomitant therapy according to the SmPC, or had participated in another clinical trial or program, within less than 30 days prior to the current study [16].
Medication
Patients were treated with darunavir according to daily practice. Dosage and administration of darunavir were prescribed as per SmPC, i.e., 600 mg twice daily for treatment-experienced patients. Concomitant medication, including 100 mg ritonavir as booster, was prescribed in compliance with the SmPC [16].
Study assessments
The data were collected throughout the study during the routine hospital healthcare treatment and assessments. The following parameters were collected during the study: demographic data, anthropometric measurements for body composition assessments, vital signs, clinically significant medical and surgical history, hepatitis B and/or C co-infection status, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, lipid profile (total cholesterol, high density lipoprotein [HDL] cholesterol, low density lipoprotein [LDL] cholesterol, triglycerides), serum glucose level, absolute CD4 count, HIV-1 plasma viral load, previous concomitant ARV therapy, current concomitant ARV therapy, currently used concomitant treatment (for diseases other than HIV infection), optimized background ARV therapy used in combination with DRV/r and any changes during the study, the physician’s opinion about the patient’s adherence to ARV therapy, AEs and SAEs (both related and not related to the study treatment), pregnancies and outcomes, concomitant treatment for (S)AEs. Treatment compliance was assessed by the physician’s appreciation about patient’s adherence to treatment as follows: 95-100% (the patient stated he/she had forgotten to take the medication one to maximum two days from the total 30 days of the month); 80-95% (patient stated he/she had forgotten to take the medication two to maximum six days from the total 30 days of the month); 70-80% (patient stated he/she had forgotten to take the medication six to maximum nine days from the total 30 days of the month); 70% (patient stated he/she had forgotten to take the medication more than nine days from the total 30 days of the month).
Subjects were evaluated according to the hospital’s routine practice and data were collected at the following time points after starting DRV/r and other ARVs: baseline, Week4 (±3 days), Week 12 (±3 days), Week 24 (±3 days), Week36 (±3 days), Week 48 (±3 days): end of the study or early withdrawal visit in case of early discontinuation.
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and was consistent with Good Clinical Practices and applicable regulatory requirements.
Statistical analyses
Due to the explorative character of the study, the sample size was not calculated. The statistical analysis was merely descriptive in nature. All subjects who received at least one dose of darunavir were included in the analysis of the efficacy, safety, demographic, and baseline characteristics data. An analysis of treatment-emergent adverse events was performed. Statistical analysis was performed in SPSS v20.
Descriptive statistics were used to describe the basic features of the data, including the main indicators of central tendency (mean, median, standard deviation [SD] and mode). In order to evaluate the efficacy and safety of darunavir, some parametric or non-parametric tests were used (depending of the data distribution): Chi2 test, T-test, Fisher’s exact test, analysis of variance (ANOVA) or Mann-Whitney and other non-parametric tests.
Results
Baseline characteristics
In total, 78 subjects were recruited by nine investigational sites in Romania. Thereof, 57(76.0%) subjects completed the 48-week observation period and 18 (24.0%) subjects were lost to follow-up. Two (2.7%) subjects discontinued the study after 12 weeks. The reason for discontinuation was a lack of treatment compliance.
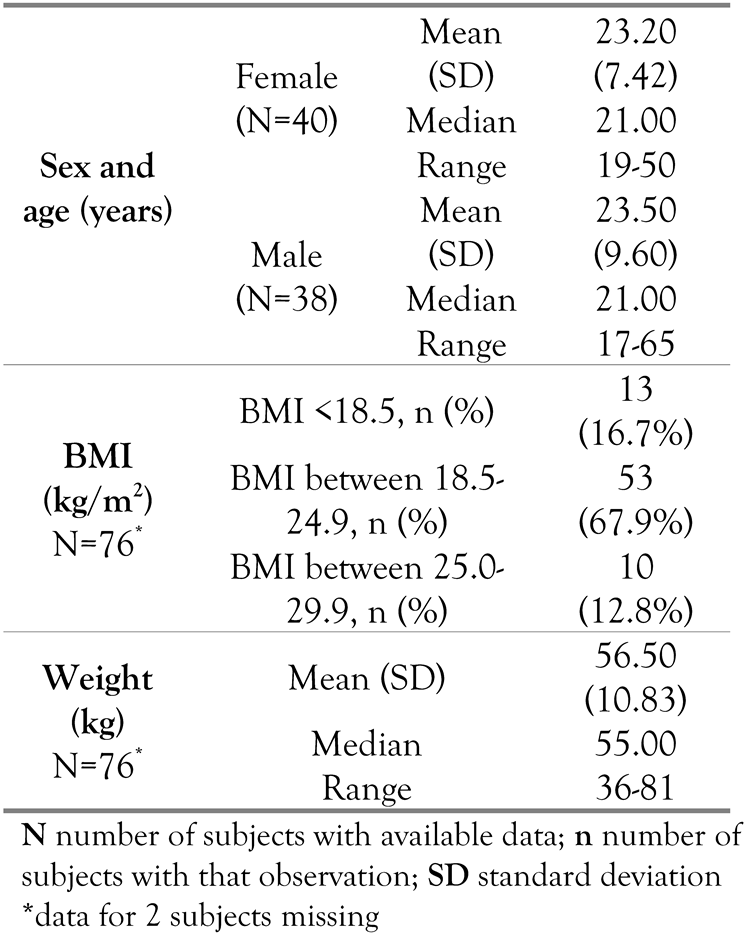
Forty (51.3%) subjects were female. The subjects’ median age was 21 years (overall range 19-50years for females and 17-65 years for males). Two male subjects of 17 years were included in the study (one subject was 17 years and 10 months and one subject was about to turn 18years old in 22 days). Although subjects below 18 years of age were to be excluded from this study, these subjects were considered to be 18years old because both dates exceeded with more than 6months the age of 17 (according to the Romanian National Institute of Statistics). Table 1 presents the demographic and baseline disease data for the patients included in this study.

Table 1.
Demographic characteristics.
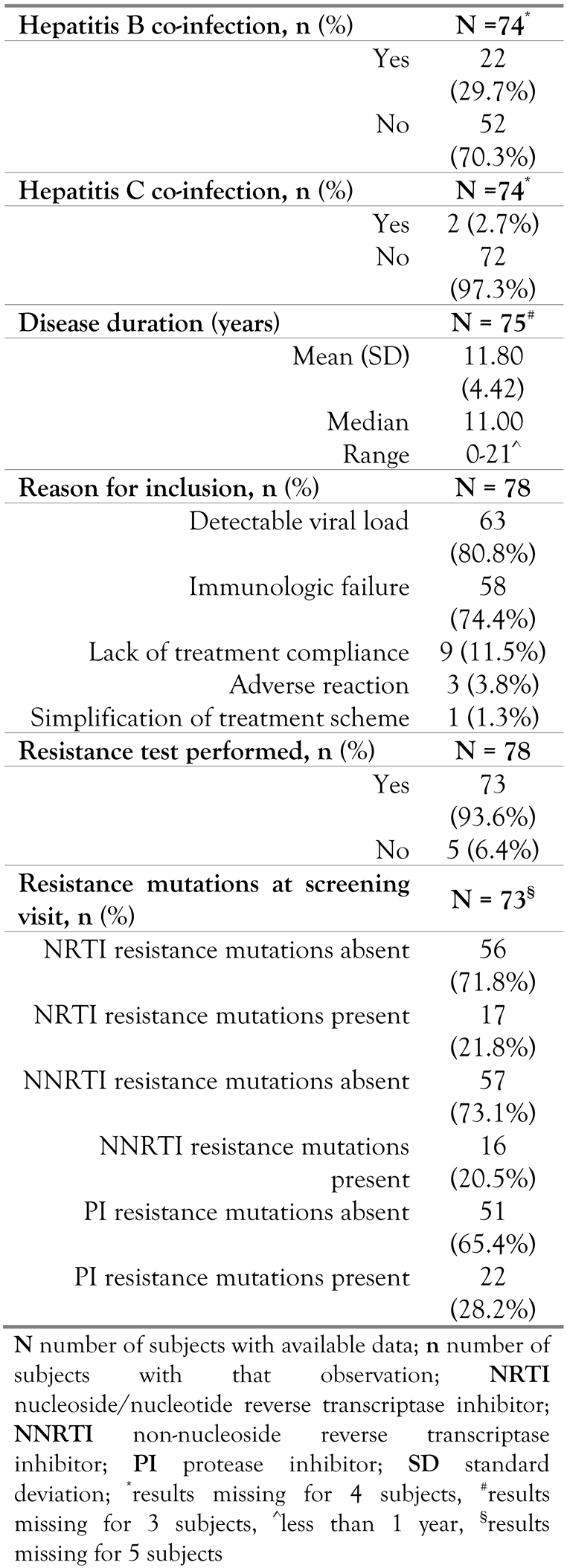
The most common reasons for inclusion of subjects in the study were detectable viral load for 63(80.8%) subjects and immunologic failure for 58 (74.4%) subjects. The mean (SD) duration of HIV infection at time of inclusion was 11.8 (4.42) years. Table 2 summarizes the baseline disease characteristics.

Table 2.
Baseline disease characteristics.
In all subjects, previous ARV therapy was recorded at baseline. The most frequent (≥30subjects) previous ARV therapies administered were NRTIs and NNRTIs. All subjects used ritonavir (NNRTI) as concomitant ARV therapy as indicated per SmPC [16].
Efficacy
CD4 cell count
Descriptive statistics of CD4 cell count are provided per visit in Table3. The mean values of the CD4 cell count increased at every visit compared to reference (screening). There was a statistically significant increase in mean CD4 cell count (p<0.001) from reference (252.39 cells/µL) to endpoint, i.e., the last available value for each patient, (376.27cells/µL) and thus an improved immune response in the patients, using a t-test for paired comparison.

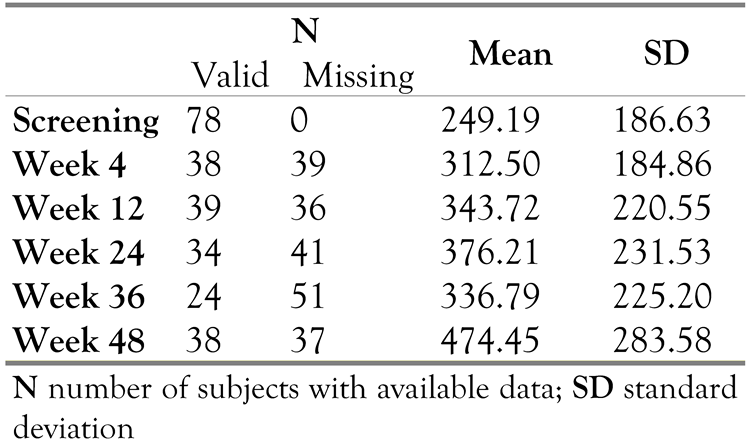

Table 3.
CD4 cell count (cells/µL).
Table 3.
CD4 cell count (cells/µL).

Viral load
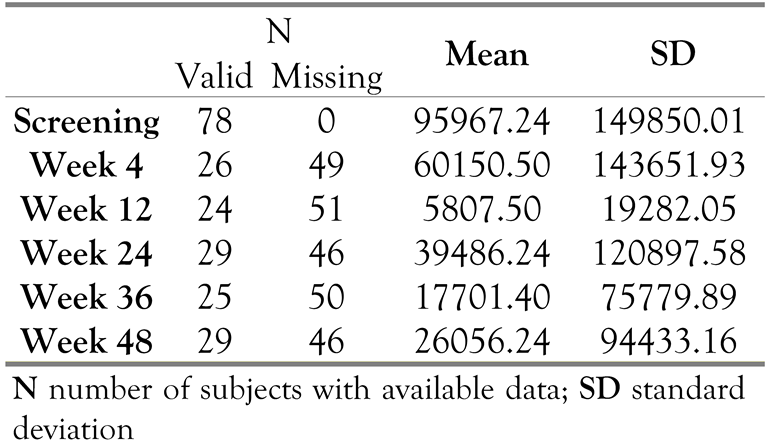
Descriptive statistics of plasma viral load (VL) count are provided in Table 4. The mean values of plasma viral load decreased at every visit compared to reference (screening). There was a statistically significant decrease in mean plasma viral load count (p=0.010) from reference (screening) (86,430.91 RNA copies/mL) to endpoint (58,771.06RNA copies/mL) i.e., the last available value for each patient, using a t-test for paired comparison.

Table 4.
Viral load (RNA copies/mL).
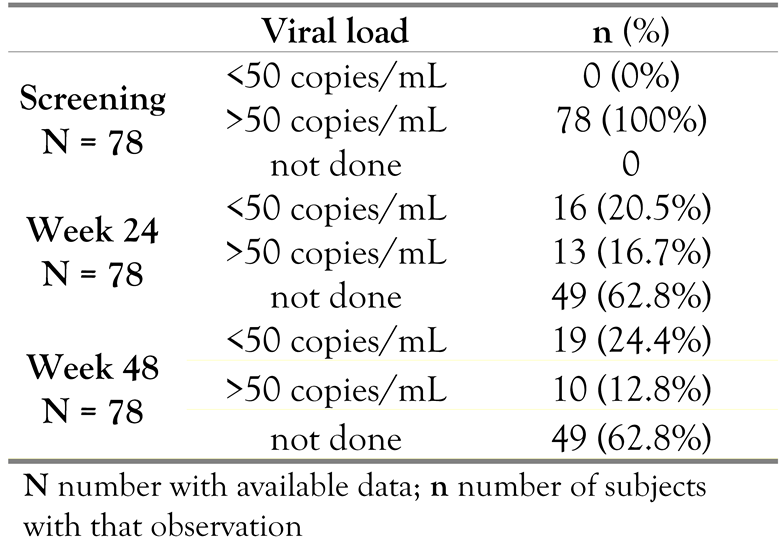
Mean log10 values of plasma VL decreased from 4.03 log10copies/mL at reference (screening) to 3.25log10 copies/mL at endpoint, which was statistically significant (p=0.0001). Table 5 shows the percentage of subjects with plasma HIV-1 RNA above or below 50copies/mL at the reference point (screening), Week 24, and Week 48. At the reference point (screening), all 78subjects had a viral load of >50 copies/mL. A VL<50copies/mL was observed in 16(20.5%)subjects at Week 24 and in 19 (24.4%) subjects at Week 48. Note that the viral load was not determined in the majority of the subjects at Week 24 and Week 48 (in 49 [62.8%] subjects). Virologic response rates (<50copies/mL) using the observed case method were noted in 16(55.2%) subjects at Week 24 and in 19 (65.5%) subjects at Week 48.

Table 5.
Viral load (RNA copies/mL) at screening, Week 24, and Week 48.
Treatment adherence at Week 24 and Week 48
Only 2 subjects discontinued DRV treatment after Week 12 due to lack of treatment compliance. One subject died after she stopped treatment. Sixty-six subjects completed the Week 24 visit and 9 subjects were lost to follow-up. Fifty-seven subjects completed the Week48 visit and 18 subjects were lost to follow-up (see Table 1).
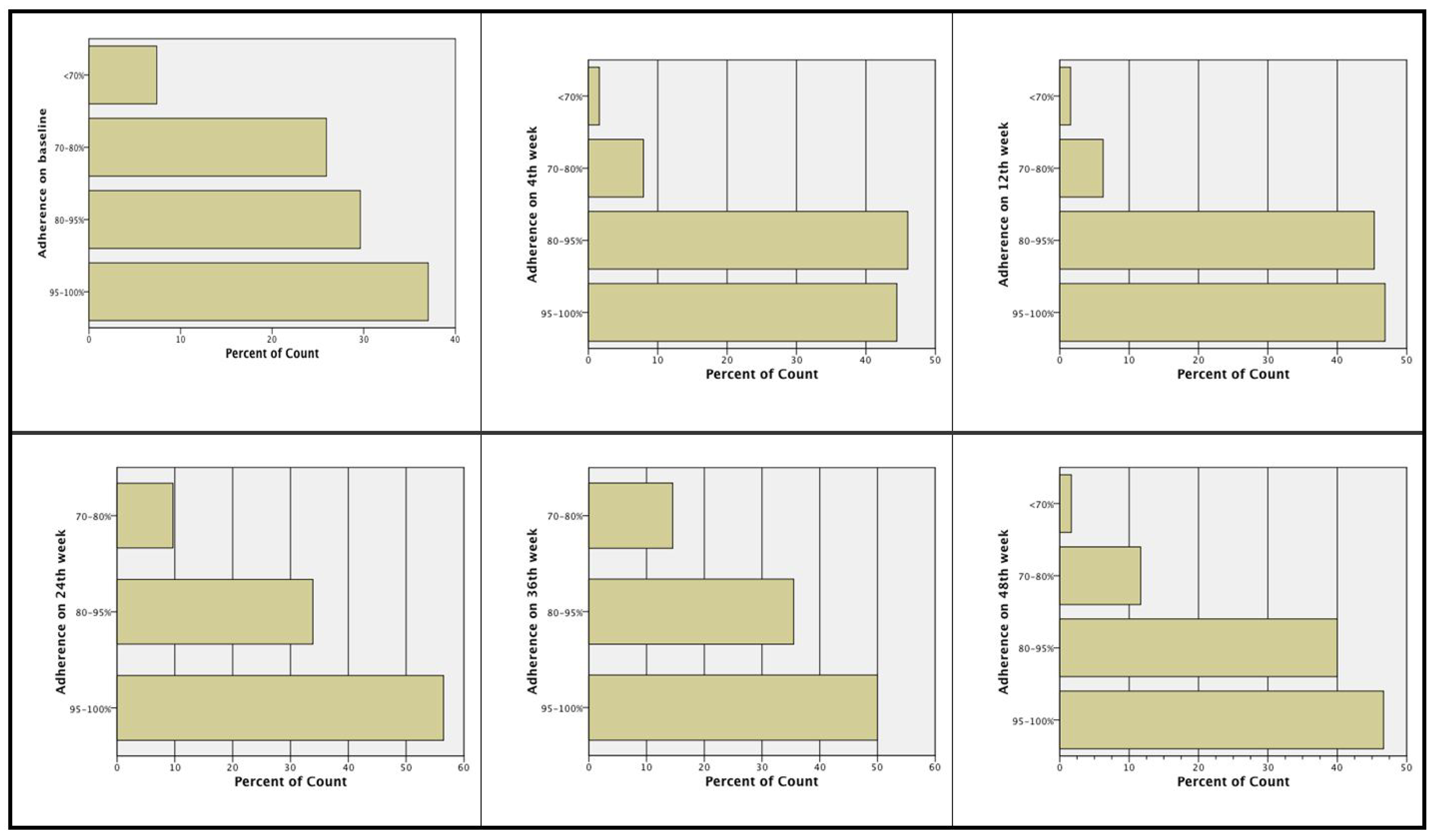
At screening, 69.2% of the subjects had been more than 80% adherent to prior treatment, i.e., subjects stated they had forgotten to take the medication one to maximum six days from the total 30 days of the month. The majority of the subjects were more than 80% adherent to treatment: baseline (66.2%), Week 4 (89.8%), Week 12 (91.8%), Week 24 (89.8%), Week 36 (84.7%), and Week 48 (86.4%). At Week 24 and Week 48, more subjects had an adherence rate >80% compared to baseline, i.e., 89.8% and 86.4% of the subjects at Week 24 and Week48, respectively, compared to 66.2% of the subjects at baseline (see Figure 1).
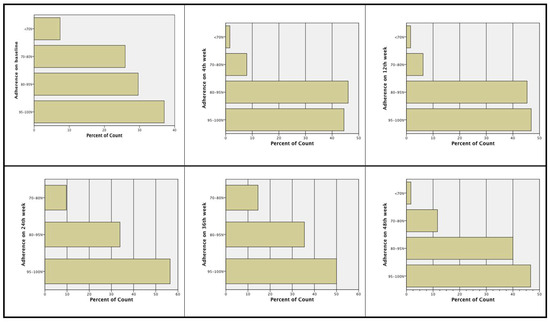
Figure 1.
Treatment adherence during the study.
Safety
Adverse events
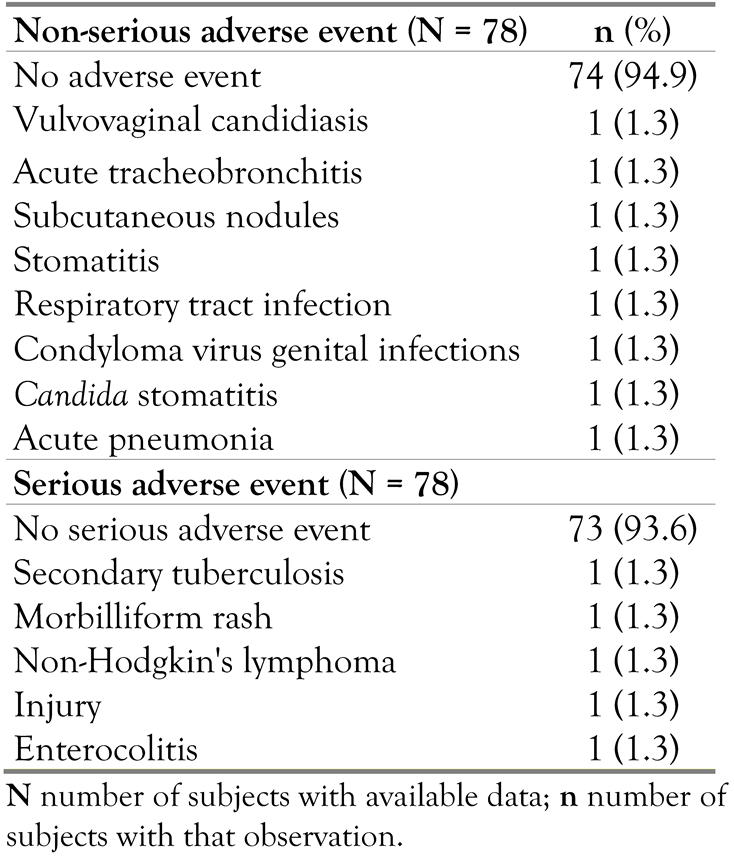
Four (5.1%) subjects experienced a non-serious AE after administration of study medication. The (S)AEs are presented by preferred term in Table 6. The non-serious treatment-emergent AEs (TEAEs) were reported in at most one(1.3%) subject. The reported non-serious TEAEs were considered not related to darunavir by the investigator. Five (6.4%) subjects were reported with an SAE, which were considered not related to darunavir by the investigator. As described above, one subject died due to progression of non-Hodgkin lymphoma after the subject ended her treatment with darunavir. There were no non-serious AEs leading to discontinuation of the study medication.

Table 6.
Serious and non-serious adverse events by preferred term.
Clinical laboratory evaluation
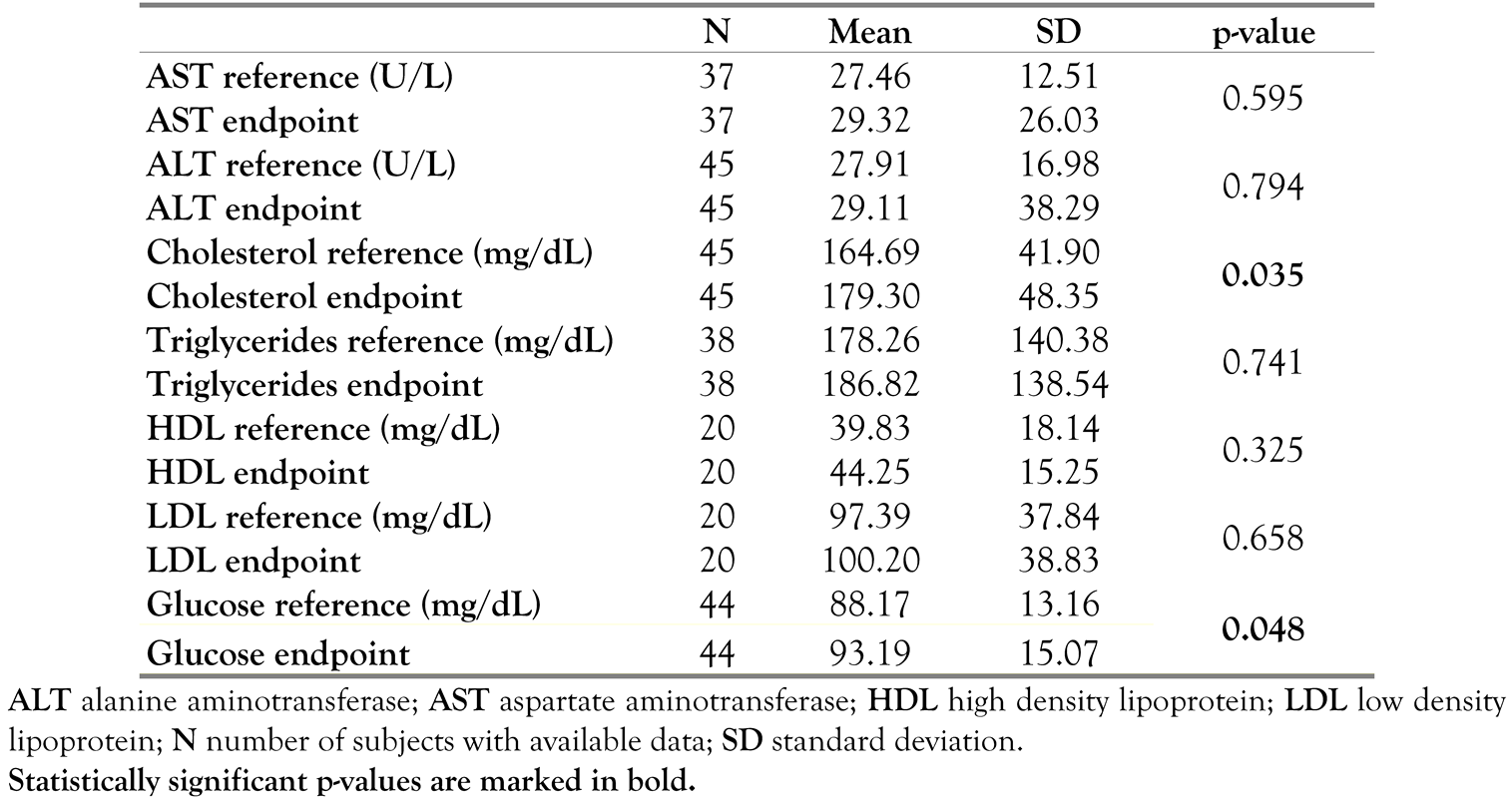
Table 7 summarizes the pairwise comparisons for laboratory assessments at endpoint versus reference (screening).

Table 7.
T-test for paired comparison for laboratory assessments.
No relevant changes were observed for the mean values over time for ALT, AST, triglycerides, HDL, and LDL. A slight increase for the mean/median serum glucose and for the mean/median cholesterol values was observed. No significant changes at endpoint were observed in mean laboratory values for ALT, AST, HDL, LDL, and triglycerides, compared to reference (screening). The increase in mean glucose values and total cholesterol values at Week 48 (93.19 mg/dL and 179.30 mg/dL, respectively) compared to reference (screening) (88.17 mg/dL and 164.69mg/dL, respectively) was statistically significant (p=0.048 and p=0.035, respectively). No (S)AEs related to laboratory assessments were reported.
Vital signs
The mean (SD) systolic blood pressure (SBP) and diastolic blood pressure (DBP) at reference (baseline) were 113.56 mmHg (9.66) and 68.62mmHg (9.57 mmHg), respectively, and the median SBP and DBP after 48 weeks of treatment were 112.56 mmHg (10.67) and 68.18 mmHg (11.20), respectively.
Pairwise comparison for weight showed a significant (p=0.0001) mean increase of 2.53±4.5kg after 48 weeks of treatment with darunavir.
Resistance pattern
A resistance test was performed at reference, screening (reason for changing the ARV therapy scheme), see Table 2, for 73 (93.6%) subjects. The strains from 17 (21.8%) subjects displayed NRTI resistance mutations, 16 (20.5%) displayed NNRTI resistance mutations, and 22 (28.2%) PI resistance mutations. The subjects included in the study were treatment multi-experienced (first treatment started in the early ’90s). From the total of 78 subjects enrolled, 73subjects were assessed at reference (screening) with a resistance test for DRV. Fifty-four (74.0%) subjects displayed sensitivity to DRV treatment and 12 (16.4%) subjects had an intermediary sensitivity to DRV treatment. For seven subjects the results of sensitivity testing were not available.
Discussion
Darunavir is an inhibitor of HIV protease with potent in vitro activity against wild-type and resistant HIV-1 and is indicated in combination with low-dose ritonavir (DRV/r) and with other ARV products for the treatment of patients with HIV-1 infection. In particular, therapy- experienced patients with limited options are a target population for this drug due to its proven efficacy in the presence of PI-resistance mutations [17].
This was a post-authorization, open-label, one-cohort, non-interventional, prospective study conducted at multiple sites in Romania to assess effectiveness and safety of darunavir with low-dose ritonavir and other ARV medications in subtypeF HIV-1 infected subjects in naturalistic settings.
The study was performed in a difficult timeframe of economic crisis resulting in the enrollment of a small number of patients, i.e., 78 subjects instead of the planned 300 subjects. Also, monitoring during treatment was not performed according to the protocol in all cases. Consequently, there was a lack of results for many patients included in this study (CD4, VL and resistance test at virologic failure).
In total, 78 subjects were enrolled in the study, of which 57(76.0%) subjects completed the 48-week observation period and 18 (24.0%) subjects were lost to follow-up. The most common reasons for inclusion of subjects in the study were detectable viral load for 63(80.8%) subjects and immunologic failure for 58 (74.4%) subjects. In this study, there was an equal gender distribution, i.e., 51.3% females versus 48.7% males, with a mean age of 23years.
The primary objective of the study was to evaluate the efficacy of darunavir in connection with the different patterns of virus resistance, when darunavir is used in combination with low-dose ritonavir and other ARV medications in subtype F HIV-1 infected, treatment-experienced subjects in naturalistic settings.
Results of CD4 and VL were missing for many subjects from Week 4 onwards for the reasons described above. Despite that, the available results showed a statistically significant increase in mean CD4 cell count from reference to endpoint and thus an improved immune response in the subjects. Also, a statistically significant decrease in mean log10 plasma VL count was observed from reference to endpoint. Virologic response rates (<50copies/mL) were observed in 16 (55.2%) subjects at Week 24 and in 19 (65.5%) subjects at Week 48, using the observed case method.
Adverse effects resulting from darunavir treatment may often compromise treatment adherence, as is the case with other ARV therapies, especially for long-term treatment [8,9]. Interestingly, this study showed high adherence rates of >80% at Week 24 and Week48, i.e., 89.8% and 86.4% of the subjects at Week24 and Week 48, suggesting that DRV/r did not compromise the compliance. Only two subjects discontinued treatment with darunavir after Week 12 due to lack of treatment compliance. Subjects who had any conditions that in the opinion of the investigator could compromise the subject’s safety or adherence to the study protocol were excluded from this study, thus implying adherence to darunavir treatment in the current study. The included patients already had experience with ARV therapy, and this could have facilitated the routine of the treatment. Also, counselling of the patients about the proven efficacy of a new drug (darunavir) might have motivated the patients to be compliant to the treatment. The high treatment adherence can also be a consequence of a permanent and complex monitoring of the ARV treatment in the national system, i.e., national centers with active tracing, repeated therapeutic counselling for consolidation and monthly reinforcement, control of treatment release in specialized centers, targeted discussion.
The safety findings in both healthy subjects and HIV-1 infected subjects demonstrate that treatment with all studied dose regimens of DRV/r was generally safe and well-tolerated.
This study evaluated the safety of darunavir used in combination with low-dose ritonavir and other ARV agents and showed a good safety profile. Only 5.1% of subjects experienced a non-serious AE after the administration of study medication. The reported non-serious AEs were vulvovaginal candidiasis, acute tracheobronchitis, subcutaneous nodules, stomatitis, respiratory tract infection, condyloma virus genital infections, Candidastomatitis, and acute pneumonia. Five (6.4%) subjects were reported with an SAE: secondary tuberculosis, morbilliform rash, non-Hodgkin’s lymphoma, injury, and enterocolitis. All reported non-serious AEs and SAEs were considered not related to DRV by the investigator. Thus, the reported non-serious AEs and SAEs were not a direct consequence of DRV treatment. One subject died after the subject completed the treatment with darunavir (progression of non-Hodgkin lymphoma). A possible explanation of this low frequency of (non) serious AEs in this study might be related to an underreporting of the (non) serious AEs by the patients to the investigator. Because the patients’ well-being improved under treatment with DRV compared to their previous therapy, this could lead them to consider small side effects to not be worth reporting.
Lipodystrophy syndrome is a common adverse effect in combination antiretroviral therapy. The syndrome comprises metabolic disorders, such as lipodystrophy, dyslipidemia, and insulin resistance, as well as body shape changes typically seen as subcutaneous lipoatrophy in the face, limbs and buttocks, and abdominal fat accumulation [18]. Although not entirely understood, there are several hypotheses regarding the pathogenesis of HIV-associated lipodystrophy: correlation with HIV-1 protease inhibitors, nucleoside reverse transcriptase inhibitors, and genetic factors. Long-term protease inhibitor use has been associated with lipodystrophy syndrome [17,19,20,21,22]. The choice of the protease inhibitor can affect lipid elevations. The finding that DRV/r has a favorable lipid profile is supported by a meta-analysis of 12 clinical trials examining the effect of different ritonavir-boosted protease inhibitors on lipid changes at 48 weeks in over 4000 treatment-naïve patients. Dyslipidemia and raised liver enzymes appeared not to be significant [23]. The current study showed no relevant changes for the mean values for ALT, AST, triglycerides, HDL, and LDL, over time after treatment with DRV.
Combination antiretroviral therapy has also been associated with metabolic abnormalities such as hypertriglyceridemia, hyper-cholesterolemia, insulin resistance, hyperglycemia and hyperlactatemia [7,24,25,26]. A slight increase for the mean serum glucose values and cholesterol values was observed in this study. The increase in mean glucose values and total cholesterol values at Week 48 compared to reference (screening) was statistically significant. Significant lipid abnormalities or insulin resistance resulting from treatment with DRV are known. The incidence of these lipid abnormalities are similar as for other protease inhibitors [17].
In this study, pairwise comparison for weight showed a significant mean increase of 2.53±4.5 kg after 48 weeks of treatment with darunavir. This is in line with the increase in mean body weight in the darunavir group (+2 kg) compared to the control group (-0.2 kg) of a pooled analysis of the 24-week data from 2 randomized controlled trials and the analysis of two non-randomized, open-label trials performed by Roberts et al. to examine the incidence of adverse events related to anthropometric measurements in treatment-experienced HIV patients [27].
Conclusion
Treatment with darunavir with low-dose ritonavir and other ARVs resulted in the expected, statistically relevant improvement of CD4 cell count and viral load in subjects eligible for such treatment. In addition, adherence to treatment was high and the treatment-emergent safety profile observed during this study was consistent with the established safety profile of darunavir. The results of this study proved a favorable metabolic profile in subjects treated with DRV/r.
Author Contributions
The authors of this publication were the investigators of the study and/or revised the publication critically for important intellectual content.
Acknowledgments and role of the funding source:
The study was designed by Johnson & Johnson Romania Medical Affairs and conducted by qualified investigators under the sponsorship of Johnson & Johnson Romania Medical Affairs. Data were gathered by the sponsor and evaluated jointly by the authors and the sponsor. All authors were involved in development and writing of the manuscript. The authors thank Liesbeth Backx, who provided medical writing services on behalf of Johnson & Johnson Romania Medical Affairs. The corresponding author takes responsibility for the integrity and the accuracy of the data analysis, and also had final responsibility for the decision to submit for publication.
Trial: registration
TMC114HIV4044.
Conflicts of Interest
Prof. Dr. Augustin Cupșa received honoraria as coordinating and principal investigator in the Speranțe study from Johnson & Johnson Romania Medical Affairs. He is occasionally consultant in advisory boards for Abbott, MSD, Roche, GSK, J&J, BMS Romanian local branches and sometimes speaker for the same groups.
References
- Detels, R.; Munoz, A.; McFarlane, G.; et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 1998, 280, 1497–1503. [Google Scholar] [CrossRef]
- Hogg, R.S.; Yip, B.; Kully, C.; et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 1999, 160, 659–665. [Google Scholar] [PubMed]
- Palella FJJr Delaney, K.M.; Moorman, A.C.; et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Department of Health and Human Services (DHHS). Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (accessed on 28 December 2012).
- Perno, C.F.; Moyle, G.; Tsoukas, C.; Ratanasuwan, W.; Gatell, J.; Schechter, M. Overcoming resistance to existing therapies in HIV-infected patients: The role of new antiretroviral drugs. J Med Virol 2008, 80, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M.; Nelson, M.; Jayaweera, D.; et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 2009, 23, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Montessori, V.; Press, N.; Harris, M.; Akagi, L.; Montaner, J.S. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 2004, 170, 229–238. [Google Scholar]
- d’Arminio, M.A.; Lepri, A.C.; Rezza, G.; et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS 2000, 14, 499–507. [Google Scholar]
- Lucas, G.M.; Chaisson, R.E.; Moore, R.D. Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Ann Intern Med 1999, 131, 81–87. [Google Scholar] [CrossRef]
- Crespo-Fierro, M. Compliance/adherence and care management in HIV disease. J Assoc Nurses AIDS Care 1997, 8, 43–54. [Google Scholar] [CrossRef]
- Carr, A.; Cooper, D.A. Adverse effects of antiretroviral therapy. Lancet 2000, 356, 1423–1430. [Google Scholar] [CrossRef]
- Koh, Y.; Nakata, H.; Maeda, K.; et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chemother 2003, 47, 3123–3129. [Google Scholar] [CrossRef]
- Clotet, B.; Bellos, N.; Molina, J.M.; et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: A pooled subgroup analysis of data from two randomised trials. Lancet 2007, 369, 1169–1178. [Google Scholar] [CrossRef]
- Katlama, C.; Esposito, R.; Gatell, J.M.; et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS 2007, 21, 395–402. [Google Scholar] [CrossRef]
- Haubrich, R.; Berger, D.; Chiliade, P.; et al. Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients. AIDS 2007, 21, F11–F18. [Google Scholar] [CrossRef] [PubMed]
- PREZISTA (darunavir) Summary of Product Characteristics. 2013. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000707/human_med_000988.jsp&mid=WC0b01ac058001d124 (accessed on 28 December 2012).
- McCoy, C. Darunavir: A nonpeptidic antiretroviral protease inhibitor. Clin Ther 2007, 29, 1559–1576. [Google Scholar] [CrossRef]
- Carr, A.; Cooper, D.A. Images in clinical medicine. Lipodystrophy associated with an HIV-protease inhibitor. N Engl J Med 1998, 339, 1296. [Google Scholar] [CrossRef]
- van der Valk, M.; Gisolf, E.H.; Reiss, P.; et al. Increased risk of lipodystrophy when nucleoside analogue reverse transcriptase inhibitors are included with protease inhibitors in the treatment of HIV-1 infection. AIDS 2001, 15, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Mateo, M.G.; Pruvost, A.; et al. Polymorphisms of Pyrimidine Pathway Enzymes Encoding Genes and HLA-B*40:01 Carriage in Stavudine-Associated Lipodystrophy in HIV-Infected Patients. PLoS ONE 2013, 8, e67035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, S.Y.; Ko, W.S.; Kao, J.T.; et al. Association of single-nucleotide polymorphism 3 and c.553G>T of APOA5 with hypertriglyceridemia after treatment with highly active antiretroviral therapy containing protease inhibitors in HIV-infected individuals in Taiwan. Clin Infect Dis 2009, 48, 832–835. [Google Scholar] [CrossRef]
- Robles, D.T.; Olson, J.M.; Colven, R.M. Lipodystrophy in HIV. Medscape 2013. Available online: http://emedicine.medscape.com/article/1082199-overview (accessed on 10 February 2014).
- Hill, A.; Sawyer, W.; Gazzard, B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials 2009, 10, 1–12. [Google Scholar] [CrossRef]
- Barlow-Mosha, L.; Eckard, A.R.; McComsey, G.A.; Musoke, P.M. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc 2013, 16, 18600. [Google Scholar] [CrossRef]
- Feleke, Y.; Fekade, D.; Mezegebu, Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J 2012, 50, 221–230. [Google Scholar] [PubMed]
- Mallolas, J.; Podzamczer, D.; Milinkovic, A.; et al. Efficacy and safety of switching from boosted lopinavir to boosted atazanavir in patients with virological suppression receiving a LPV/r-containing HAART: The ATAZIP study. J Acquir Immune Defic Syndr 2009, 51, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Hoy, J.; Beatty, G.; Vangeneugden, T.; Lefebvre, E. Body mass change and anthropometric-related adverse events at week 24 in treatment-experienced HIV-infected patients receiving TMC114/r or control PIs in POWER 1, 2 and 3. 8th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. 24–26 September 2006, San Francisco, CA, USA. Antiviral Therapy 2006, 11, L21. [Google Scholar]
© GERMS 2014.