Introduction
Measles is a preventable viral disease which occurs worldwide and is a major cause of childhood mortality in developing countries, being targeted for elimination by the World Health Organization (WHO) [
1]. In 2003, the 56
th World Health Assembly adopted a resolution to reduce measles deaths by 50% by the end of 2005 compared to 1999 [
2]. In 2008 WHO announced that global measles death dropped from 750 000 in 2000 to 197 000 in 2007 (74% reduction) [
3]. In Africa, measles deaths dropped by 89% in the same period. Measles transmission has been terminated in large geographical areas for extended periods, particularly in Europe and America. Measles was declared non-endemic in the United States of America in 2000, with sporadic recognized or unrecognized import cases [
3] and in 2006 South Korea declared that they had put a stop to indigenous transmission of measles throughout the country [
4]. In this context, laboratory diagnosis for measles becomes critical for documenting the absence of measles transmission [
5].
In order to successfully eliminate and eradicate an infectious disease there is need for effective and practical diagnostic tools with sufficient sensitivity and specificity to detect levels of infection that can lead to transmission [
6]. WHO and United Nations Children's Fund (UNICEF) have a comprehensive strategy for measles elimination which includes strong routine immunization, supplementary immunization activities, surveillance and clinical management of measles cases [
2]. Routine immunization is the foundation of measles mortality reduction strategy and interruption of endemic measles transmission [
7]. The WHO recommends that at least 90% of children should be reached by routine immunization every year in every district [
8]. Surveillance enables prompt recognition and investigation of measles cases and outbreaks. It also provides information about the impact of measles elimination programs and appropriate outbreak response. Surveillance also contributes to the identification of groups in greatest need of vaccination and provides the basis for changes in prevention strategies as well as evaluating the protective efficacy of the vaccine [
9]. As countries attain and maintain measles elimination, laboratories play a pivotal role [
10] in confirming the clinical diagnosis of suspected cases, detecting and characterizing the circulating virus and, under certain circumstances, establishing measles seroprevalence or assisting in outbreak forecasting [
9]. Elimination of a certain disease cannot be declared without high quality laboratory-enhanced surveillance [
11].
WHO has developed specific laboratory indicators for quality case-based surveillance of measles and has a comprehensive system for monitoring performance indicators including proficiency testing and annual accreditation [
12]. A number of six quality indicators are monitored during the 12-month review period:
1. At least 90% of the serum specimens should arrive at the national measles virology laboratory (NML) in good condition (adequate volume, recommended temperature, not hemolyzed, not desiccated).
2. At least 80% of specimens should arrive at the NML within three days of collection.
3. At least 80% of the serology results should be sent to the national centers within seven days of receipt of the serum specimen. (This indicator monitors the timeliness of feedback of serology results to national centers).
4. At least 10% of serum specimens tested at the NML should be shared with the Regional Reference Laboratory (RRL). These representative samples are sent quarterly to the RRL for confirmation as part of the quality assurance program. [
13]
5. There should be at least 90% concordance of shared results between the NML and the RRL.
6. The NML should pass with at least 90% of WHO distributed serum panel samples. (This indicator ensures proficiency testing).
The indicators are accurately calculated to show if there is an adequate laboratory testing in a WHO accredited laboratory as well as a good reporting system. WHO recommends that at least 80% of all reported cases should have adequate investigation within less than 48 hours of notification [
12].
Adequate investigation requires the following minimum information to be included on the case investigation form: date of onset of measles symptoms, date of specimen collection, date specimen sent to laboratory, vaccination status, age of the patient and district of origin. These variables should be regularly monitored at different levels of the surveillance system and must be discussed at national and regional levels.
The main objective of this study was to assess and monitor measles laboratory-based surveillance progress in Zimbabwe according to the WHO predefined performance indicators. We analyzed NML data from 2004 (when the electronic laboratory surveillance database was introduced) to 2009.
The study’s specific objectives were: a) to monitor the quality of specimens sent to the laboratory; b) to assess timeliness in testing and communication of results; c) to assess adequacy of measles case investigations; d) to assess proficiency in measles laboratory diagnosis.
Results
During 2004-2009 the Zimbabwe national measles virology database recorded 3614 case investigation forms of measles reported from all over the country. An analysis of these case investigation forms revealed that 5.17% (187/3614) were measles laboratory-confirmed positive cases. The highest proportion (73.26%, 137/187) of positive cases were recorded in 2009 when there was an outbreak which started in Harare, followed by 2004 with 17.11% (32/187), when there were outbreaks in the Midlands and Manicaland provinces.
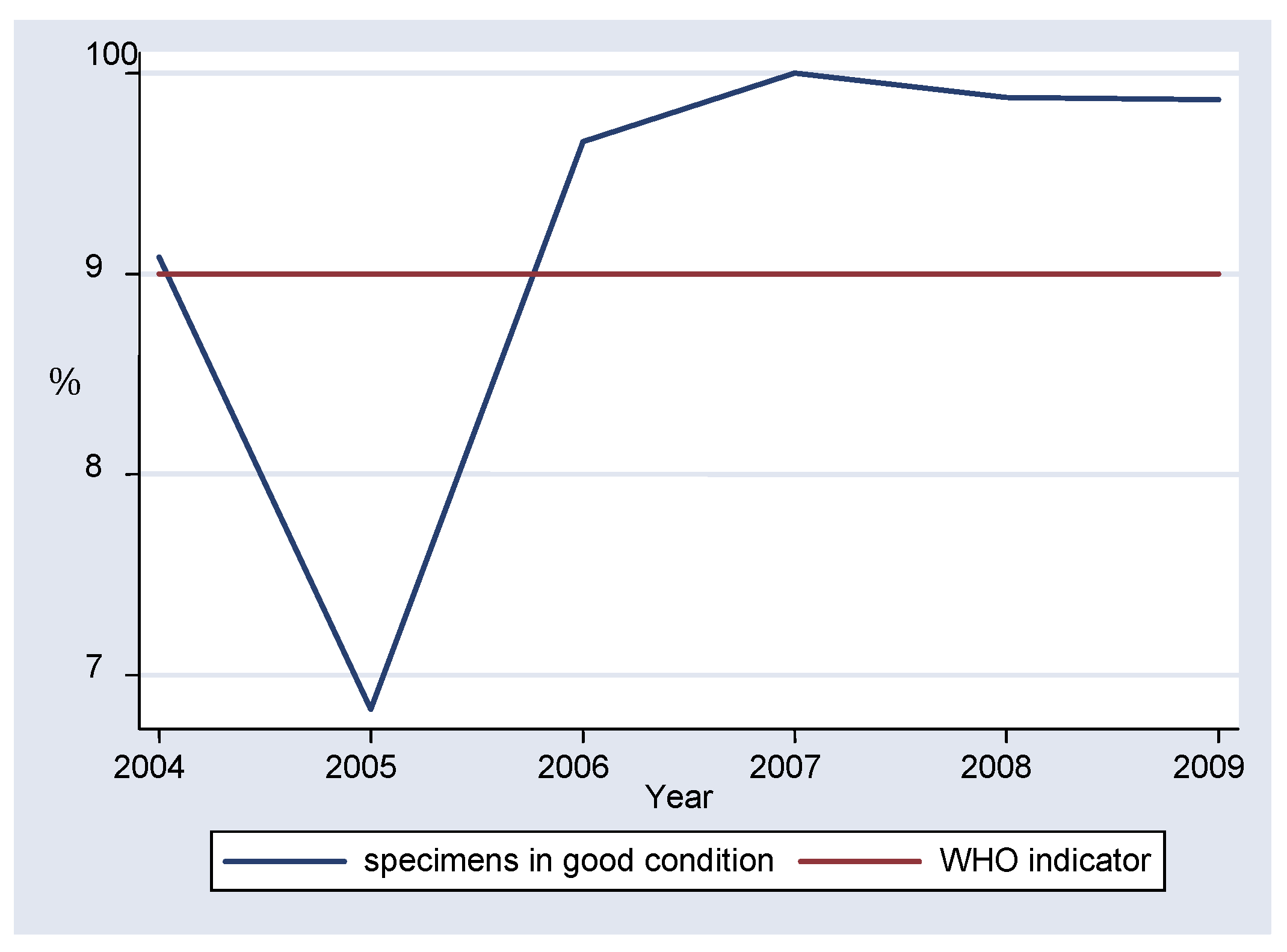
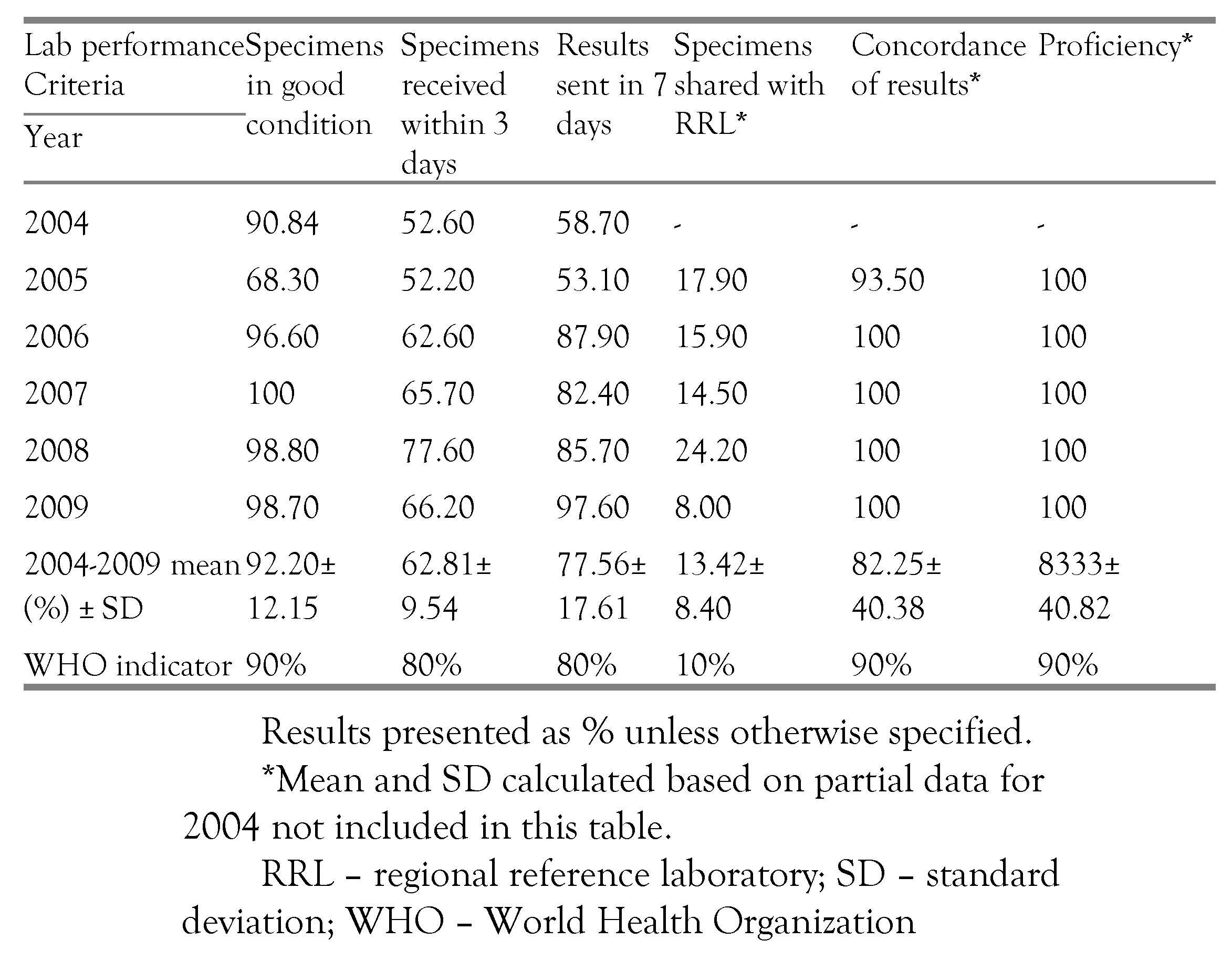
Data compiled from the period under review showed that an average of 92.20% of specimens arrived in the laboratory in good condition (
table I). During 2004-2005 there was a fall in the percentage of specimens arriving in the laboratory in good condition from 90.84% to 68.30%. After 2006, the values were constantly over 90%, with 100% recorded in 2007 (
figure 1). Chi-square test for comparing multiple proportions showed that the percentage of specimens received in good condition in 2005 differed significantly from the rest of the years (p<0.0001). However the difference between the rest of the years was not statistically significant, with 2008 and 2009 showing no difference at all.
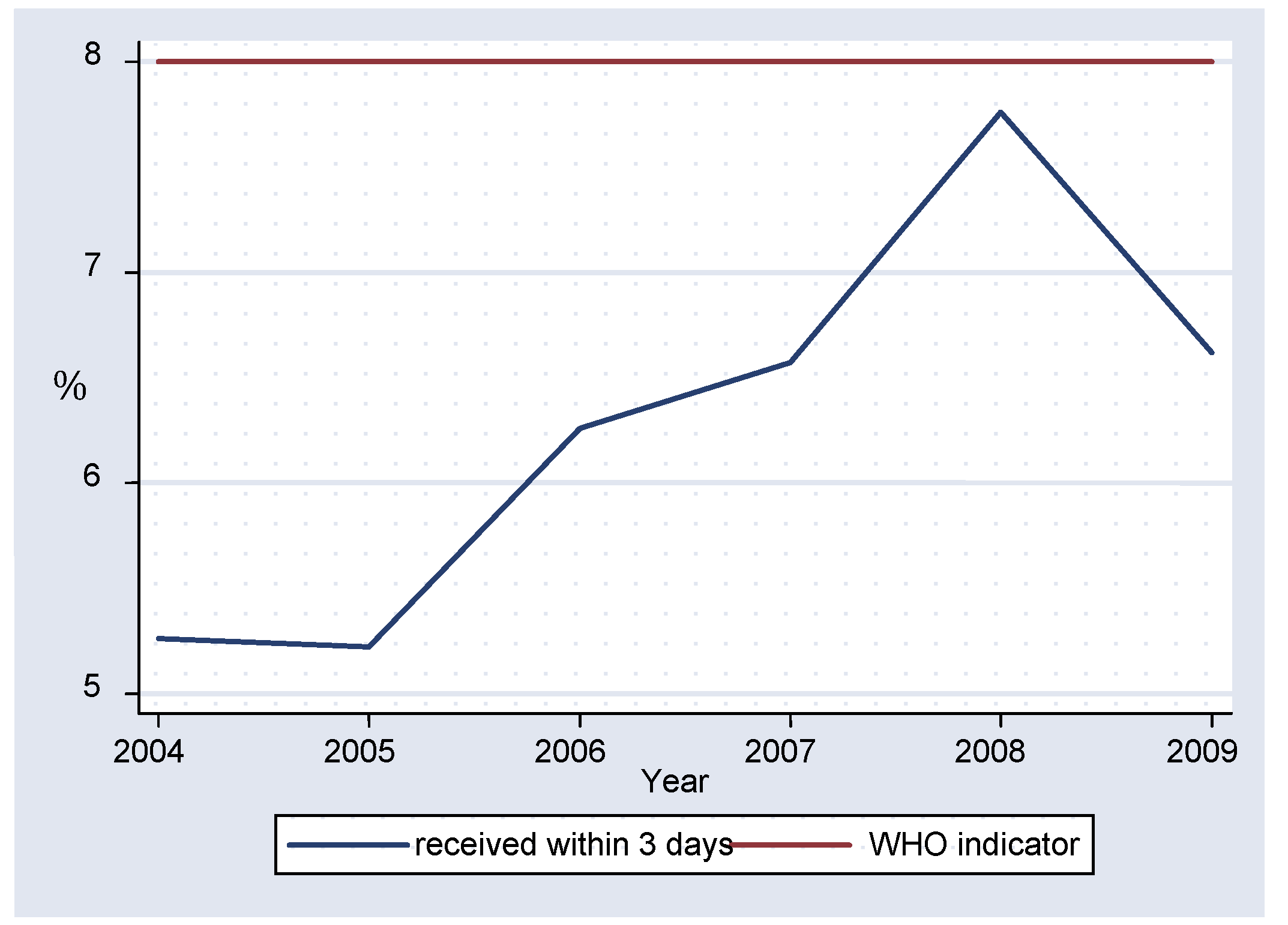
Between 2004-2009 an average of 62.81% of specimens reached the laboratory in the recommended three days, with a statistically significant difference between 2004 and 2005 (p=0.0114) and between 2004 and 2007 (p=0.0094). The proportion of specimens reaching the laboratory within three days fluctuated from 52.60% in 2004 to a peak of 77.60% in 2008 but all values were below the WHO target of 80% (
figure 2).
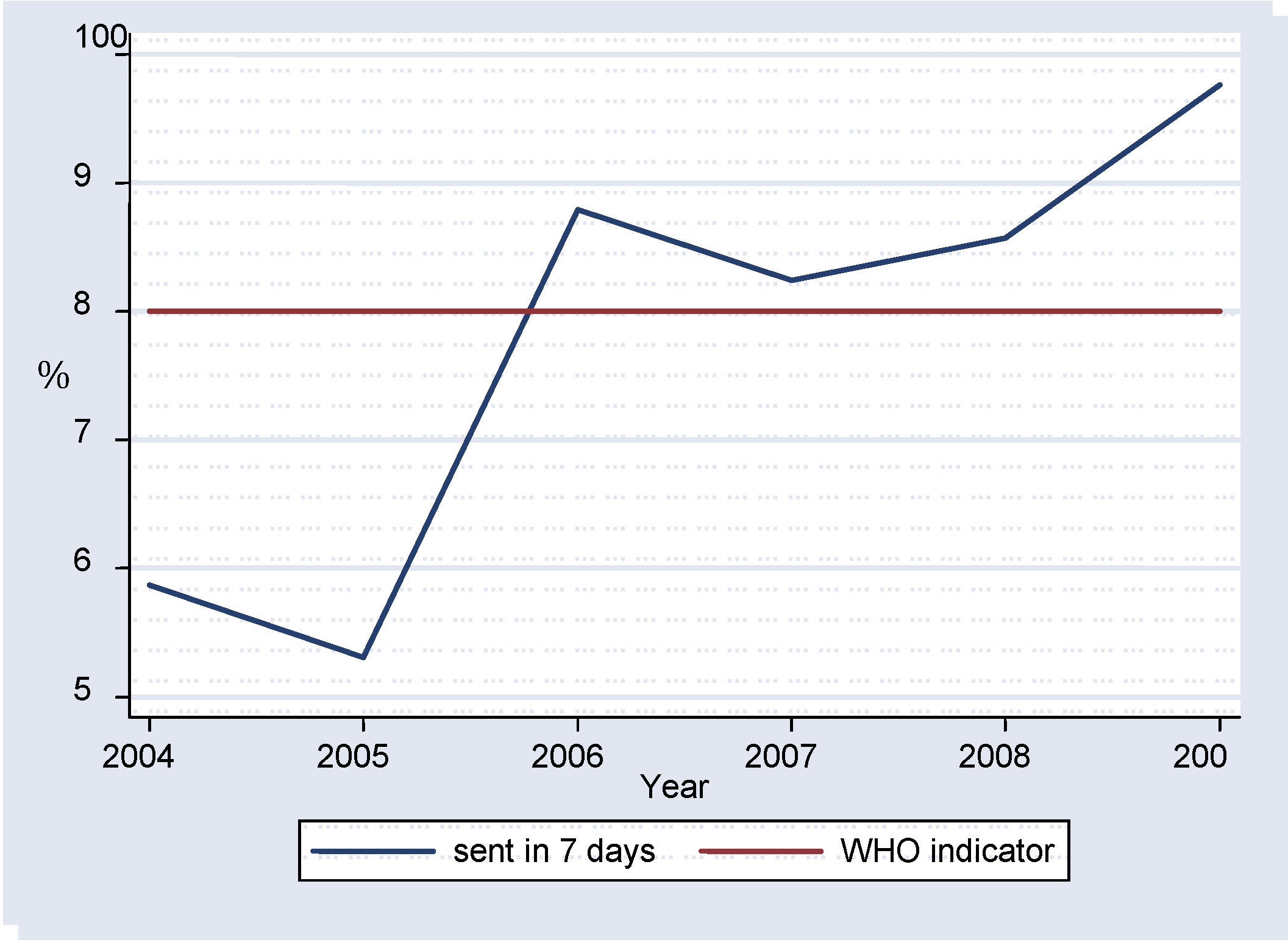
Results that were sent to the national centers for dissemination to primary health centers within seven days after reception ranged from 53.10% in 2005 to 97.60% in 2009 (
Table I,
figure 3). Statistically significant differences were noticed for 2004 vs. 2006 (p=0.002), 2004 vs. 2007 (p=0.0302), 2004 vs. 2008 (p=0.0013) and 2004 vs. 2009 (p=0.0018).
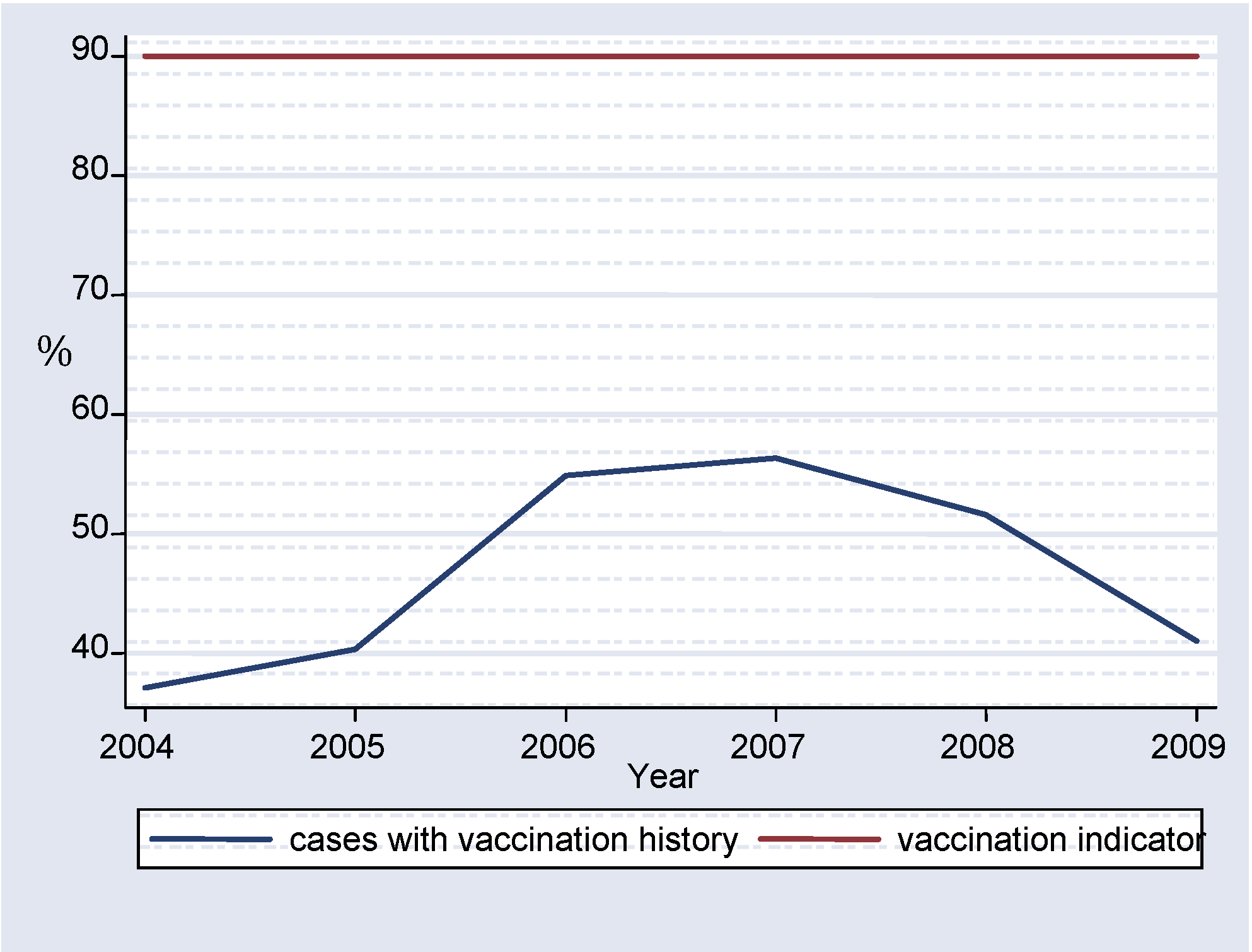
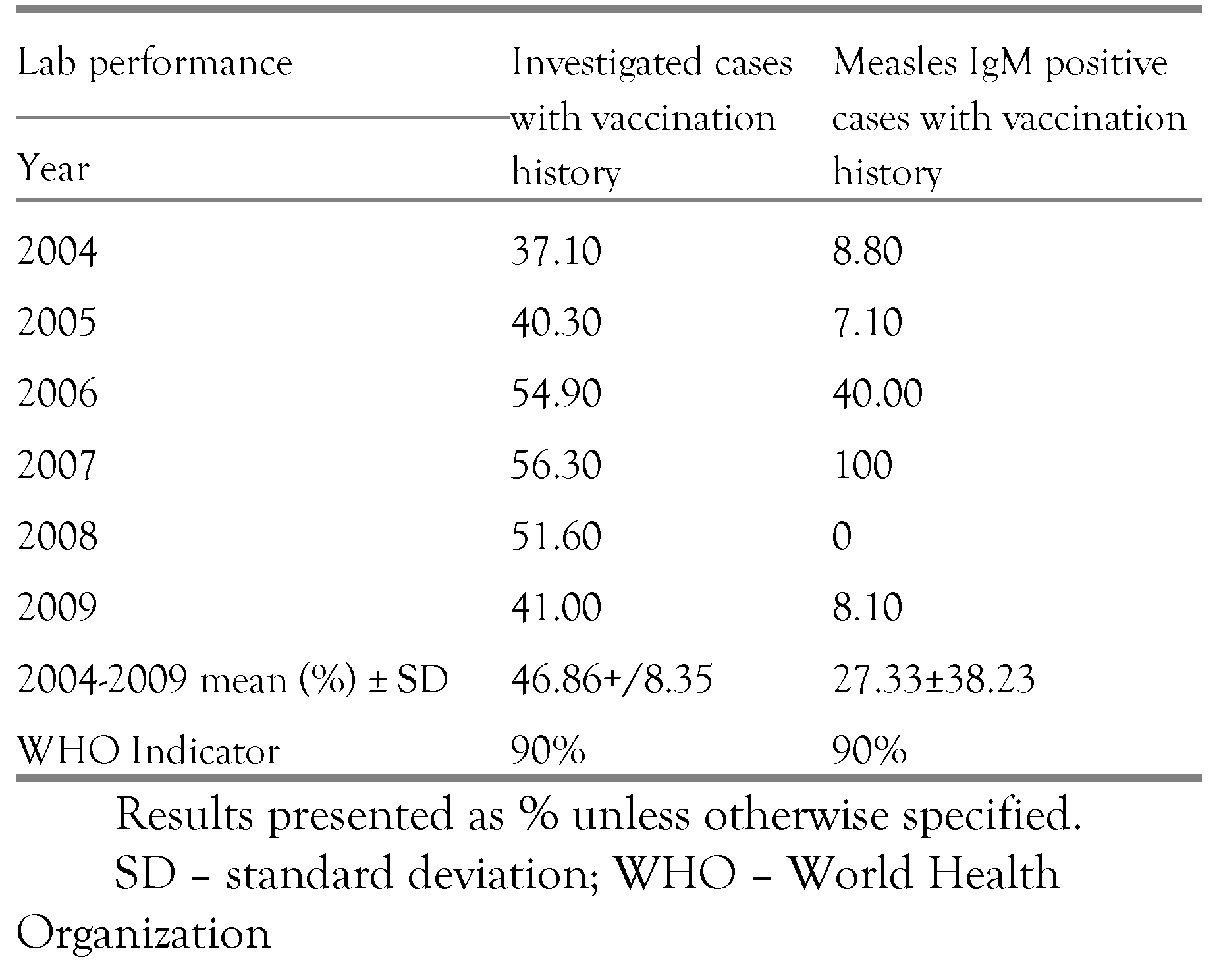
Half of the suspected measles cases brought to the laboratory for confirmation (53.14%) had no vaccination history and only an average 27.33% of the measles IgM confirmed cases had vaccination history (
table II).
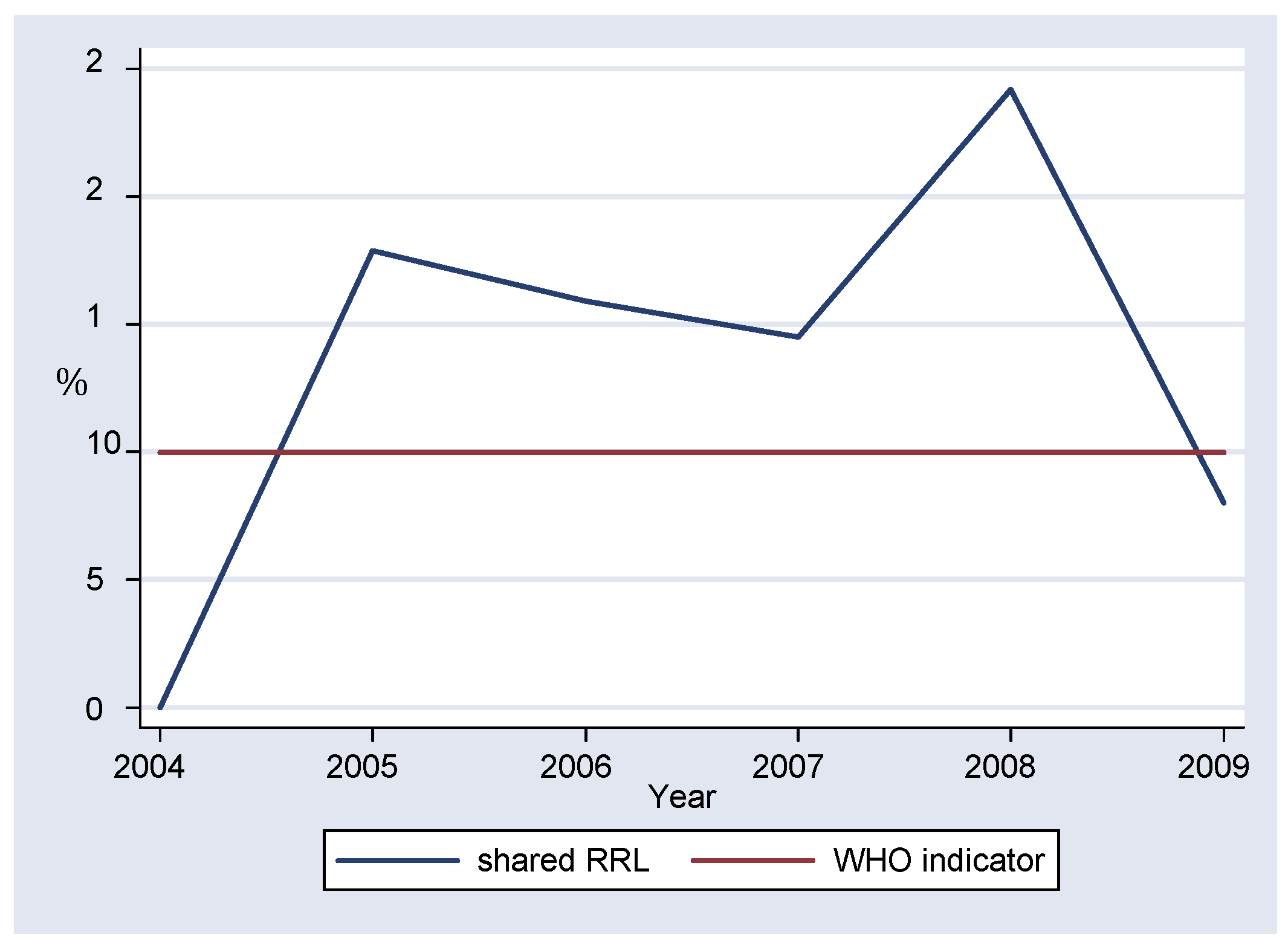
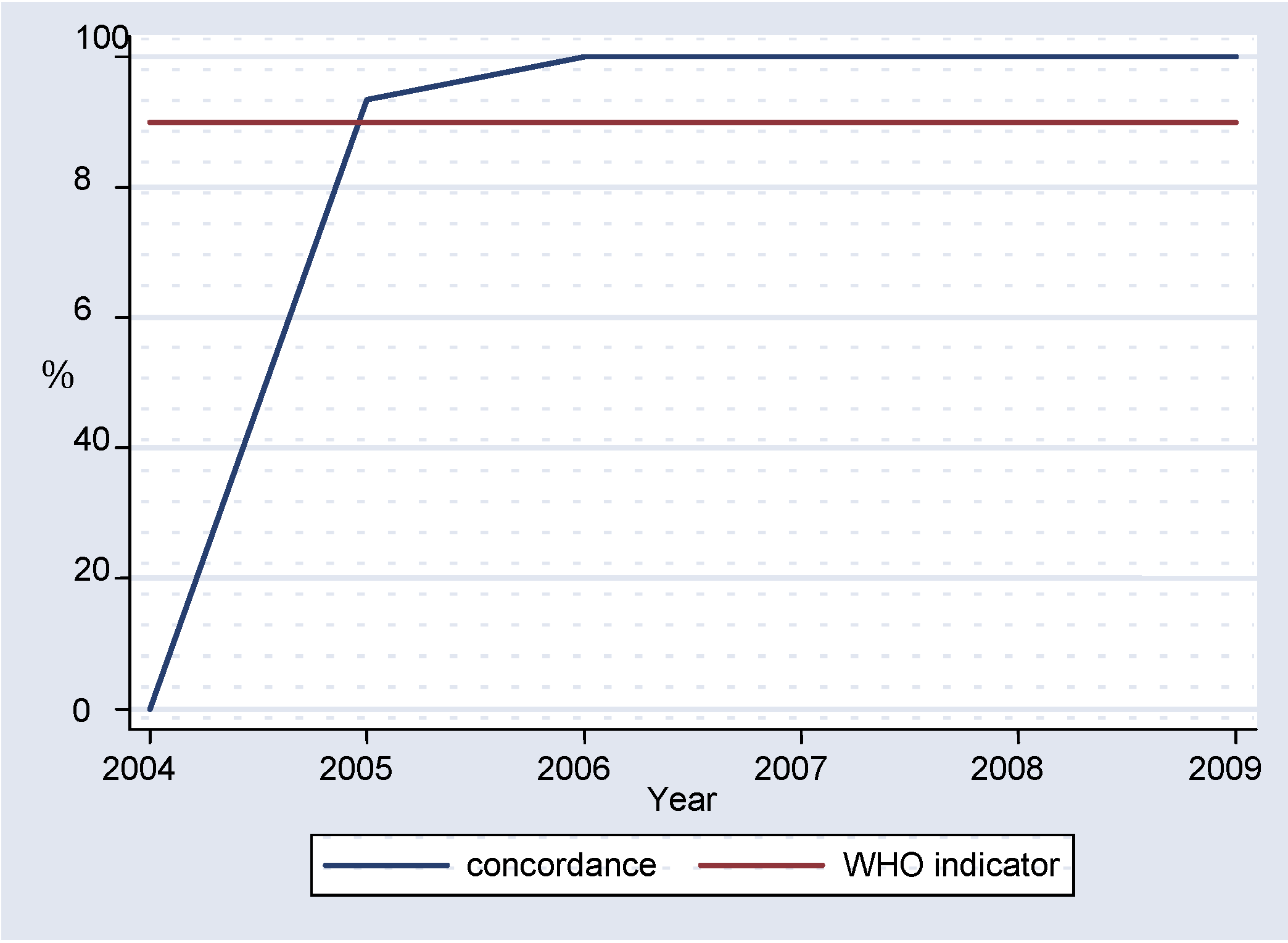
Overall, more than 10% of all specimens tested in the laboratory were sent to the RRL for confirmation, with the lowest value of 8.00% recorded in 2009 (
figure 4). The available results showed an average concordance of 82.25% (
figure 5), with 100% concordance between 2006-2009.
Discussion
During 2004-2009, an average of 92.20% of specimens reached the laboratory in good condition in the period under review and met the WHO target [
12] of over 90%. The condition of the specimen correlates with the quality of the test, having a direct impact on measles enzyme-linked immunosorbent assay (ELISA) IgM results. Although the proportion of specimens received in good condition in 2005 was lower than the WHO standard and differed significantly from the rest of the years (p<.0001), all the other years met the WHO indicator, without statistically significant differences between the proportions. Therefore, it appears that the laboratory has been able to maintain the required target of specimen quality as recommended by WHO surveillance standards.
An average 62.81% (±9.54) of specimens (
table I) reached the laboratory within the recommended three days from the date of collection, which is below the WHO target of 80%. There were no statistically significant differences in the percentage of specimens received within the recommended time, showing that the laboratory consistently failed to meet this important target. Timeliness of serum specimens arriving in the laboratory is very important as it ensures that there is maintenance of the cold chain system, without major temperature fluctuations during transportation and it also has an impact on how prompt the clinical diagnosis can be confirmed.
As indicated in the results section, during 2004 and 2005 less than 80% of the serology results were sent to the national centers within seven days, failing to meet the WHO criterion. The following years recorded values above the recommended target, without statistically significant differences, indicating consistency in meeting the required standard.
WHO recommends that there is at least 90% concordance of measles IgM results between the RRL and the national laboratory. More than 95% of the compared results displayed such a concordance, pointing to the validity of the results of the national laboratory.
The laboratory passed with 100% on all the serum specimens sent from WHO for proficiency testing. However data for the year 2004 was missing. The national laboratory also managed to send the recommended representative proportion of specimens to the regional reference laboratory for co-testing, as quality control measure.
Almost 50% of all investigated cases had no last date of vaccination (
figure 6). It is difficult to tell whether this was a failure in immunization, or one in reporting. This does show however that the cases were not adequately investigated. Thorough investigation of cases is very important in elimination of measles as it ensures that transmission is interrupted. [
14]
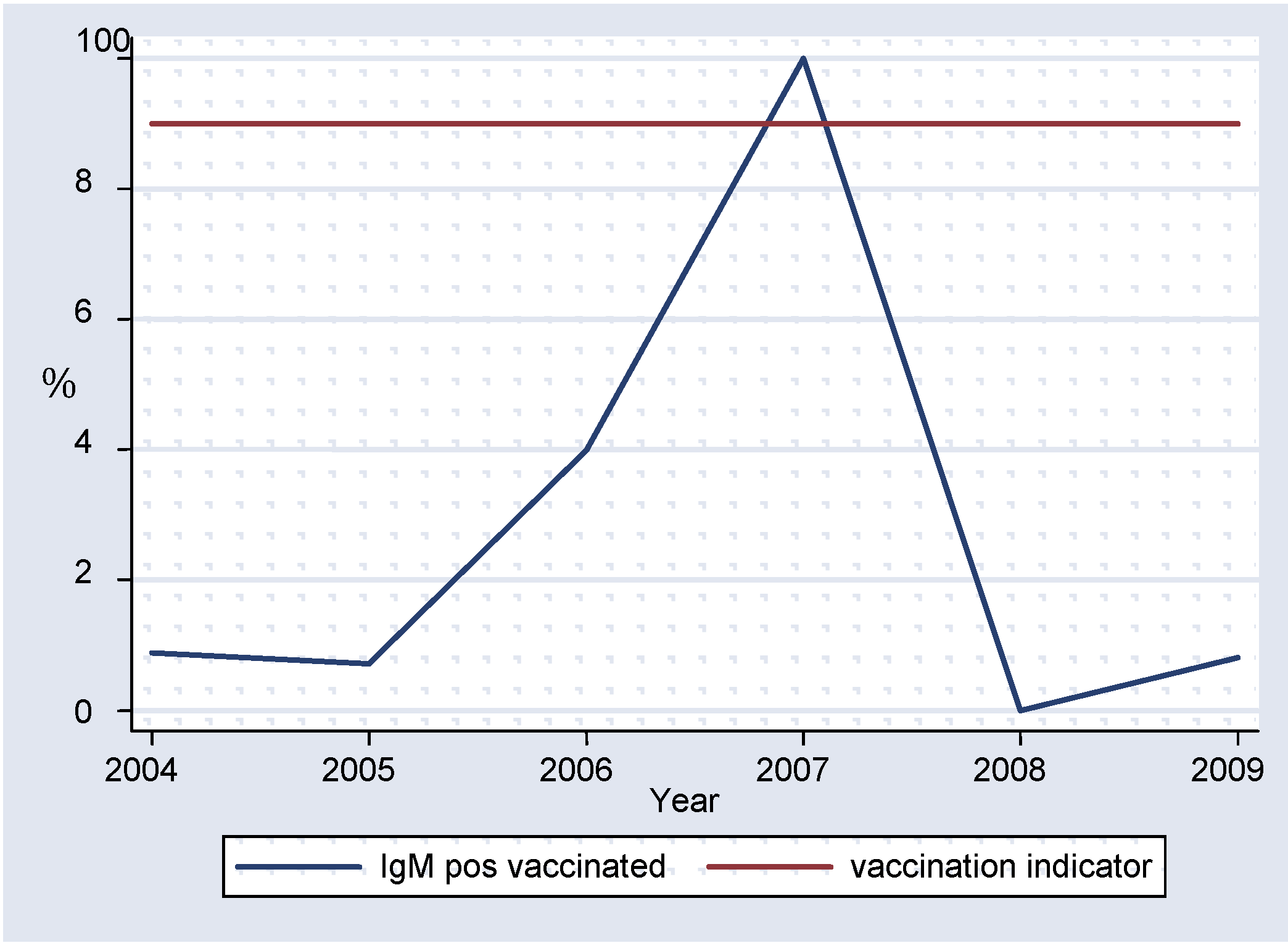
Over 70% of measles IgM-positive results had no vaccination history (
figure 7). Lack of investigation or poorly investigated unvaccinated cases, if allowed to accumulate over time, may result in a substantial number of susceptible individuals which can lead to future measles outbreaks [
13]. These indicators are very important as they guide surveillance officers in planning supplementary immunization activities.
In the elimination and ultimate eradication of infectious diseases [
15], the laboratory becomes critical in determining pathogen levels, the presence or absence of a pathogen. It is important that the laboratory produces valid and reliable results, which can be used by health authorities and other decision-makers. As a result, the laboratory should meet the standards set by disease control programs. It is through the process of monitoring and evaluation that we are able to tell whether the NML in Zimbabwe is able to play its role in the elimination of measles by measuring its performance against predefined WHO indicators. This enables us to identify areas where there is need for training, funding, and development of new approaches and it also provides a comparison with other laboratories in the network.
One of the limitations of this study was that not all forms were filled out completely and another one resides in the retrospective nature of the study, which relies on previous observations and records. The strength of the study is that it is derived from a large number of cases (3614), which allows a pertinent interpretation of measles data. Also, given the fact that measles is a notifiable disease in Zimbabwe, we have the benefit that all cases are recorded within the same database, using the same set of criteria for each case investigation form. This ensures that our study included cases from all over Zimbabwe and selected districts and cities.