Abstract
Introduction: This paper examines the use of local antibiotic therapy in one-stage septic revision surgery for late periprosthetic joint infections (PJIs). This case study suggests that morselized bone allografts impregnated with antibiotics in powder form are a preferable alternative to polymethyl methacrylate (PMMA) because they can generate higher local antibiotic concentrations. Current research also recommends using vancomycin and aminoglycosides as the preferred choice of antibiotics, as they may have low diffusion in tissues when administered intravenously, but are effective when administered locally. The article emphasizes the importance of achieving high local antibiotic concentrations to eradicate bacterial biofilms and provides guidelines for the preparation of bone allografts. Case report: The paper assesses the case study of a 68-year-old male patient who underwent two-stage total revision surgery for a late septic failure of the endoprosthesis (approximatively one year after implantation). The first stage involved removing the implant, debridement, lavage, and setting a fixed spacer manually made from polymethyl methacrylate impregnated with 4 g of vancomycin. The second stage of revision surgery utilized a morselized bone allograft impregnated with 4 g of vancomycin as a means of local antibiotic therapy and bone defect coverage. Systemic levels of vancomycin were measured at 4, 8, 12 and 24 hours respectively after surgery. During this period, no systemic vancomycin was administered. After the second stage was carried out and the testing was carried out, systemic vancomycin therapy was initiated and the plasma levels of the drug were assessed and subsequently therapeutic doses were adjusted. Conclusions: Vancomycin loaded bone allograft achieved higher systemic concentrations that its polymethyl methacrylate counterpart, when being manually loaded with the same amount of antibiotic. Nonetheless, the levels were well below nephrotoxic levels, indicating that this may be a valuable tool for local means of antibiotic therapy in selected patients that could not endure such a systemic therapeutic regiment. For septic revisions, antibiotic loaded bone allograft holds a valuable place in the surgical arsenal of local antimicrobial treatment, by far exceeding that of the polymethyl methacrylate.
Introduction
The management of late periprosthetic joint infections (PJI) in the arthroplasty field has been primarily focused on two-stage revision surgery. However, the development of novel methods for diagnosis and local antibiotic therapy may shift the treatment paradigm towards one-stage revision, which would significantly improve patient outcomes and reduce healthcare costs. To consider this approach, certain criteria must be met, such as the absence of a fistula, a positive microbiological diagnosis, and the ability to achieve high local antibiotic concentrations. This can be challenging with parenteral or oral administration, as the necessary concentrations to destroy bacterial biofilms can require up to ten times the typical dose, highlighting the need for a local antimicrobial agent [1]. Polymethyl methacrylate (PMMA) has been proposed as a method for delivering antibiotics to infected sites, but it has limitations due to its polymerization and structural strength properties. Furthermore, its effectiveness against mature bacterial biofilms is uncertain, as antibiotic-loaded formulas available do not typically achieve the required local concentrations [2]. Therefore, additional antibiotics may be necessary to achieve the required bactericidal concentrations. If the required local antibiotic concentrations are not reached, PMMA may be colonized by bacteria forming a biofilm [3]. Given these considerations, using cemented implants in one-stage septic revision surgery carries a high risk of failure [4].
The aim of the current case study is to assess the use of the morselized bone allograft embedded with vancomycin as a mean of obtaining higher local antibiotic levels, able to eradicate mature biofilm.
The use of a morselized bone allograft impregnated with antibiotics in powder form presents a more effective alternative to local antimicrobial therapy for the management of periprosthetic joint infections (PJIs). Effective management of PJIs requires careful preparation, including the morselization, washing, and drying of the graft to enhance the incorporation of higher antibiotic quantities and minimize local inflammatory responses [5]. Studies have shown that bone marrow, specifically lipids, can produce immune-mediated local inflammatory responses that hinder antibiotic incorporation. On the other hand, processed bone allografts promote the incorporation of host tissue [6]. To ensure the success of the treatment, it is crucial to achieve high local antibiotic concentrations to eradicate the remaining bacterial biofilm. Vancomycin and aminoglycosides are the preferred antibiotics for this purpose because they may have low diffusion in tissues when administered intravenously. This property is beneficial when administered locally because of their lower resorption rate and lower vascular diffusion, reducing the likelihood of systemic adverse reactions [5].
Local antibiotic therapy must achieve high local concentrations, estimated to be between 200 and 500 mg/mL for vancomycin, for at least 72 hours in order to eradicate mature bacterial biofilms [5]. When combined with bone graft, the local antibiotic concentration significantly surpasses those observed with PMMA. Moreover, all of the antibiotic is available, whereas up to 95% of the antibiotic can become trapped in the structure of PMMA [2,7]. The European Tissue Bank recommends impregnating 1 g of vancomycin and 0.4 grams of tobramycin for every 10 ccs of bone graft [5,8].
Case report
The objective of this case report is to describe the surgical management of a 68-year-old male patient who had undergone total knee replacement and subsequently developed a late local periprosthetic infection. The patient presented at approximatively one year postoperatively with symptoms indicative of a late periprosthetic septic complication, including, pain, local edema, elevated temperature, and loss of range of motion. Laboratory tests revealed an inflammatory response, as evidenced by elevated levels of C reactive protein (CRP) (52 mg/L), fibrinogen (440 mg/dL), and erythrocyte sedimentation rate (ESR, 27 mm/h) and no other significant modifications in blood cell count other than mild anemia, with a hemoglobin of 11.2 g/dL. Radiographs showed alterations in bone structure suggestive of implant loosening and osteolysis at the tibial level (Figure 1). The patient had no significant comorbidities. Procalcitonin testing was negative, thus the patient was not in a septic condition; blood cultures were not collected.
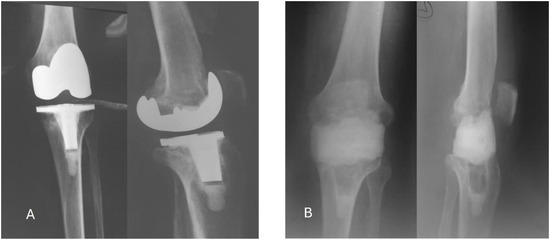
Figure 1.
Radiological studies of the case. A. Before surgery – frontal and lateral; B. After the first surgery – frontal and lateral.
Based on these findings, a two-stage total revision surgery was deemed necessary. The first stage of the procedure, started at one day after admission, involved the removal of the implant, debridement, antiseptic lavage, and the placement of a fixed PMMA spacer manually impregnated with 4 g of vancomycin.
After explantation, the prosthetic implant was sent to the microbiology laboratory and subjected to a sonication analysis.
Intraoperatively, in order to create the PMMA spacer, we utilized two doses of commercially available orthopedic cement (Stryker Simplex 40 cc, Stryker, USA). The first dose served as the core of the spacer, and hence, no antibiotics were mixed with the formula. The second dose, which formed the outer layer, was impregnated with 3 g of vancomycin (Kabi, Germany), and an additional fourth gram of vancomycin was added when the formula reached a doughy consistency. This was done to optimize the local release of vancomycin and achieve higher concentrations of the antibiotic at the site of infection. Upon polymerization, the compound appeared normal on macroscopic examination. The spacer was then fixed in place on the explanted knee without the use of drainage tubes.
Blood samples were collected at 4, 8, 12, and 24 h after surgery and tested for plasma levels of vancomycin, representing antibiotic diffusion from the spacer. After the last sample was taken, systemic intravenous vancomycin therapy was initiated at a dose of 1 g every 12 h. Subsequent blood tests were performed 36 and 48 h after surgery to determine if the antibiotic had reached therapeutic plasma levels at the proposed dose.
The microbiological results from the implant sonication identified a Staphylococcus epidermidis strain that was susceptible to methicillin; we continued the antibiotic regimen proposed, vancomycin i.v. 1 g per 12 h and ceftriaxone 2 g per 24 h.
Antibiotic treatment was continued for 4 weeks during hospitalization. Postoperatively, the patient developed mild leukopenia, neutropenia, and mild renal insufficiency following the administration of vancomycin (serum creatinine levels 1.5 mg/dL and serum urea 81 mg/dL).
In the second stage of the revision surgery, approximately 8 weeks after the first surgery, a morselized bone allograft was used as a means of negotiating the remaining bone defects and as a means of local antibiotic therapy.
During surgery and before the removal of the PMMA spacer, samples of the intra-articular fluid were collected and analyzed for vancomycin concentration. In addition, the local effects of the spacer were noted, as it created a pseudo-membrane in the joint.
A freshly frozen bone allograft was used to reconstruct bone defects. The management of this case required a femoral head obtained from our hospitals’ Tissue Bank, totaling approximately 40 cc of morselized graft. After deicing, the graft was manually morselized utilizing rongeurs, resulting in bone fragments measuring between 3 and 5 mm in diameter. Subsequently, a manual washing method was imposed and the bone marrow and fat were cleared using pulsatile lavage. Washing was repeated three times, after which the resulting liquid was discarded. After the initial lavage, 20 mL of peroxide was added to the bone graft and subsequently washed to remove lipids on the surface. Approximately 500 mL of saline solution were used for lavage.
The quantity of bone allograft was then measured utilizing a 50 milliliters syringe (1 mL equals 1 cc) to estimate the quantity of vancomycin to be added (1 g of vancomycin for each 10 cc of bone allograft). Fifteen cc of antibiotic-impregnated bone allografts were inserted intramedullary in the femur and 5 cc intramedullary in the tibia. A total of 20 cc were impacted at the bone-implant interface.
Blood samples were taken at 4, 8, 12, and 24 hours after surgery to determine the plasma vancomycin concentration that diffused from the allograft.
The liquid chromatography-mass spectrometry system used for analysis comprised an Eksigent MicroLC 200 Plus pump, a CTC PAL model QTRAP 5500 autosampler, and a SCIEX 5500 QTrap mass spectrometer, equipped with an electrospray source operated in positive ionization. Analyst 1.6.3 software was used to control the instrument and acquire the data.
After the last sample was collected, systemic therapy with vancomycin and rifampicin was initiated in order to obtain both an antimicrobial and an anti-biofilm barrier surrounding the newly implanted prosthesis. The patient remained hospitalized for a reminder of two weeks, and then received oral antimicrobial treatment for another four weeks.
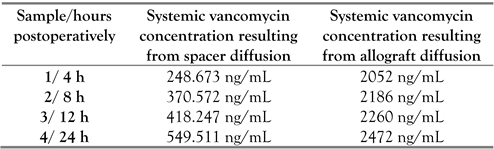
The results of the plasma vancomycin levels analysis are presented in Table 1.

Table 1.
Results of the plasma vancomycin levels analysis after each step of the two-stage revision was carried out.
The local joint levels of vancomycin after 8 weeks, resulting from the spacer diffusion, were determined and the result was 7623 ng/mL.
The plasma concentration of vancomycin following systemic administration was 12052 ng/mL after 36 and 48 h at intravenous administration of 1 gram per 12 hours. Thus, in order to achieve efficient antimicrobial levels of the antibiotic, the administered dose of vancomycin was increased to 1.5 grams per 12 h intravenously.
IBM SPSS Statistics 23 was utilized for conducting statistical analyses. The plasma levels of vancomycin were evaluated and the outcomes were categorized into two groups: PMMA and allograft. The mean discrepancies were determined with a 95% confidence interval. The threshold for statistical significance was set at α=0.05% (p<0.05). The results indicated that there was a significant disparity between the two groups in terms of the mean plasma concentration of vancomycin during the initial 24 hours after the administration of the antibiotic-loaded medium. Consequently, it can be deduced that antibiotic-loaded morselized bone allograft serves as a reliable mode of local antibiotic therapy. We suggest that this compound can attain adequate levels of local antibiotic to eliminate bacteria and may be able to prevent biofilm formation.
Discussion
The key findings of the study on this case demonstrate significant potential for a bone allograft impregnated with vancomycin for local antibiotic delivery in managing and preventing infections in an orthopedic procedure.
Although the testing period for the bone allograft was limited to 24 hours, the results are encouraging in terms of avoiding systemic antimicrobial treatment; one of the most notable outcomes is the achievement of five-fold higher plasma concentrations of vancomycin generated while remaining below systemic toxic levels. This is a crucial balance in anti-microbial therapy, as it allows for effective local treatment without risking adverse effects associated with high systemic concentrations. The ability to maintain high local vancomycin concentrations (7623 ng/mL) in joint fluid for up to eight weeks (values recorded at revision time with spacer removal) is particularly promising. This sustained release could potentially provide extended protection against infection, which is especially valuable in the context of orthopedic reconstruction – revision surgeries where the risk of infection can persist for weeks after the procedure.
The controlled release of antibiotics from bone allografts offers several advantages over traditional PMMA spacers. PMMA spacers are known to release their antibiotic load rapidly, which can lead to suboptimal antibiotic concentrations over time. In contrast, the bone allograft system appears to provide a more sustained and controlled release, potentially offering more consistent protection against infection over an extended period [9]. A complementary research regarding this matter is underway, and this study group is keen on analyzing its results.
The potential applications of antibiotic-loaded bone allografts are diverse and significant. They may prove useful not only for preventing infections in primary surgeries but also for managing existing infections in revision procedures. This dual functionality could make them a versatile tool in the orthopedic surgeon's arsenal. The higher local antibiotic concentrations achieved with this method may also improve success rates in managing septic complications, which are often challenging to treat effectively [10].
However, we rightfully acknowledge several limitations of our study. The testing period was limited to 24 hours, which may not fully capture the long-term in-vivo release profile of the antibiotic from the allograft. This limitation underscores the need for extended studies to evaluate the sustained release characteristics over weeks or months. Additionally, further research is needed to determine the antimicrobial and antibiofilm properties of these antibiotic-loaded allografts. Understanding these properties is crucial for assessing their effectiveness against various pathogens and their ability to prevent or disrupt biofilm formation, which is a common challenge in orthopedic implant-related infections.
The discussion also addresses conflicting evidence in the field. Some studies have discouraged the use of antibiotic-impregnated joint spacers, citing concerns about potential biofilm formation and interference with systemic antibiotics [11,12]. These concerns highlight the complexity of local antibiotic delivery and the need for careful consideration of potential drawbacks. However, recent studies have suggested that rates of infection clearance with joint spacers are similar to those achieved with two-stage revision procedures, with higher complication rates observed only in long-term use [13]. This conflicting evidence accentuates the need for further research to clarify the optimal approaches for local antibiotic delivery in orthopedic infections.
To further strengthen this discussion, several aspects could be elaborated upon. First, the potential clinical implications of these findings could be explored in more depth. For instance, how might the use of antibiotic-loaded bone allografts impact current treatment protocols for orthopedic infections? Could they potentially reduce the need for systemic antibiotics or shorten treatment durations?
Second, the limitations of the study could be addressed more comprehensively. In addition to the short testing period, other potential limitations such as sample size, in vitro versus in vivo efficacy, and the specific properties of the bone allograft used could be discussed.
Third, a more detailed comparison of these results to other local antibiotic delivery methods would provide valuable context. How do the release profiles and local concentrations achieved with this method compare to other approaches such as antibiotic-loaded cement, collagen sponges, or hydrogels?
Lastly, specific directions for future research could be suggested. These might include long-term in vivo studies, investigations into the efficacy against different bacterial strains, or clinical trials comparing this approach to current standard treatments for orthopedic infections.
Conclusions
In conclusion, while the results of this case study are promising, they also highlight the need for continued research in this area. The potential benefits of antibiotic-loaded bone allografts in orthopedic procedures are important, but careful evaluation of their long-term efficacy and safety is essential before widespread clinical adoption can be recommended.
The article discusses the use of local antibiotic therapy in one-stage septic revision surgery for late periprosthetic joint infections (PJIs). It proposes that a bone allograft impregnated with antibiotic powder offered a superior alternative to PMMA for PJI management. This approach aims to deliver high local antibiotic concentrations while minimizing the risk of systemic toxicity. The article presents a case study of a 68-year-old male patient who underwent a two-stage total revision surgery: first stage: removal of the implant - debridement - lavage - insertion of a fixed spacer impregnated with vancomycin; second stage: use of morselized bone allograft to address remaining bone defects - utilization of the allograft as a local antibiotic delivery system.
The study emphasizes the potential of a vancomycin-loaded bone allograft in eluting high local antibiotic concentrations without the risk of systemic toxicity. However, it is noted that further research is needed to fully explore the efficacy and safety of this approach in managing periprosthetic joint infections.
Author Contributions
AB and IT contributed to the design and implementation of the research. DL contributed to the analysis of the results and to the writing of the manuscript. DO supervised the sampling and testing procedure. AB conceived the original and supervised the project. RM and GP edited the final manuscript. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Institutional Review Board Statement
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Institutional review board approval was obtained (04/12.02.2024).
Informed Consent Statement
Written informed consent was obtained by the author for the publication of this case report and any accompanying images.
Conflicts of interest
All authors – none to declare.
References
- Bunea, A. Antibiotic elution from vancomycin embedded polymethyl methacrylate cement used in orthopedic surgery. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Bertazzoni Minelli, E.; Della Bora, T.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe. 2011, 17, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Slane, J.; Gietman, B.; Squire, M. Antibiotic elution from acrylic bone cement loaded with high doses of tobramycin and vancomycin. J Orthop Res. 2018, 36, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Sanz-Ruiz, P.; Abenojar, J.; Vaquero-Martín, J.; Forriol, F.; Del Real, J.C. Evaluation of elution and mechanical properties of high-dose antibiotic-loaded bone cement: comparative "in vitro" study of the influence of vancomycin and cefazolin. J Arthroplasty. 2015, 30, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Haiden, P. Allograft bone as antibiotic carrier. J Bone Jt Infect. 2017, 2, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Thoren, K. Lipid extraction enhances bank bone incorporation. An experiment in rabbits. Acta Orthop Scand 1990, 61, 546–548. [Google Scholar] [CrossRef]
- Sultan, A.M.; Mahmoud, N.M. Detection of resistance integrons among biofilm and non-biofilm producing clinical isolates of Pseudomonas aeruginosa. Germs. 2024, 14, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Witso, E.; Persen, L.; Loseth, K.; Bergh, K. Adsorption and release of antibiotics from morselized cancellous bone. In vitro studies of 8 antibiotics. Acta Orthop Scand 1999, 70, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Boelch, S.P.; Rueckl, K.; Fuchs, C.; et al. Comparison of elution characteristics and compressive strength of biantibiotic-loaded PMMA bone cement for spacers: Copal® spacem with gentamicin and vancomycin versus Palacos® R+G with vancomycin. Biomed Res Int. 2018, 2018, 4323518. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, Y.; An, H.; et al. Recent advances in prevention, detection and treatment in prosthetic joint infections of bioactive materials. Front Bioeng Biotechnol. 2022, 10, 1053399. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Q.; Fang, X.Y.; Huang, C.Y.; et al. Destination joint spacers: a similar infection-relief rate but higher complication rate compared with two-stage revision. Orthop Surg. 2021, 13, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Citak, M.; Masri, B.A.; Springer, B.; Argenson, J.N.; Kendoff, D.O. Are preformed articulating spacers superior to surgeon-made articulating spacers in the treatment of PJI in THA? A literature review. Open Orthop J. 2015, 9, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Frew, N.M.; Cannon, T.; Nichol, T.; Smith, T.J.; Stockley, I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with 'home-made' preparations. Bone Joint J. 2017, 99-B, 73–77. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2024.