Case report
We present a case of a 70-year-old male patient from southern Croatia with an incidentally found liver lesion that was suspicious of a cancer. Previously, he had been diagnosed with rheumatoid arthritis (RA) in 2008 and since then he had been receiving immunosuppressive therapy. Since 2014 the disease had been well controlled with methotrexate (MTX) and anti- TNF agent infliximab (IFX).
In December 2021 he had a regular appointment with his rheumatologist. Routine laboratory tests showed microhematuria, proteinuria and slight kidney function impairment. Until February 2022, his kidney impairment severely progressed and he reported macrohematuria. Therefore, he was admitted to the hospital for further diagnostics and treatment of kidney failure. Finally, the diagnosis of IgA nephropathy (IgAN) was confirmed by histopathological analysis of the kidney-biopsy samples.
Besides IgAN, the diagnostic evaluation of kidney failure revealed a large infiltrative, poorly delineated, inhomogeneous mass of the right liver lobe (16 × 6.8 × 6.7 cm) and a necrotic nodule measuring 7 × 5 cm next to the celiac trunk (
Figure 1a). The described liver lesion was first detected by abdominal ultrasound (US) and then verified by multislice computed tomography (MSCT). The radiographic appearance of the lesion was suspicious of a primary malignant liver tumor. This finding was incidental since our patient had no pain in the right upper abdominal quadrant and he was showing no signs of liver insufficiency, both clinically and according to the laboratory results. Nonetheless, chronic liver disease is often found as an accompanying condition in patients with IgAN.
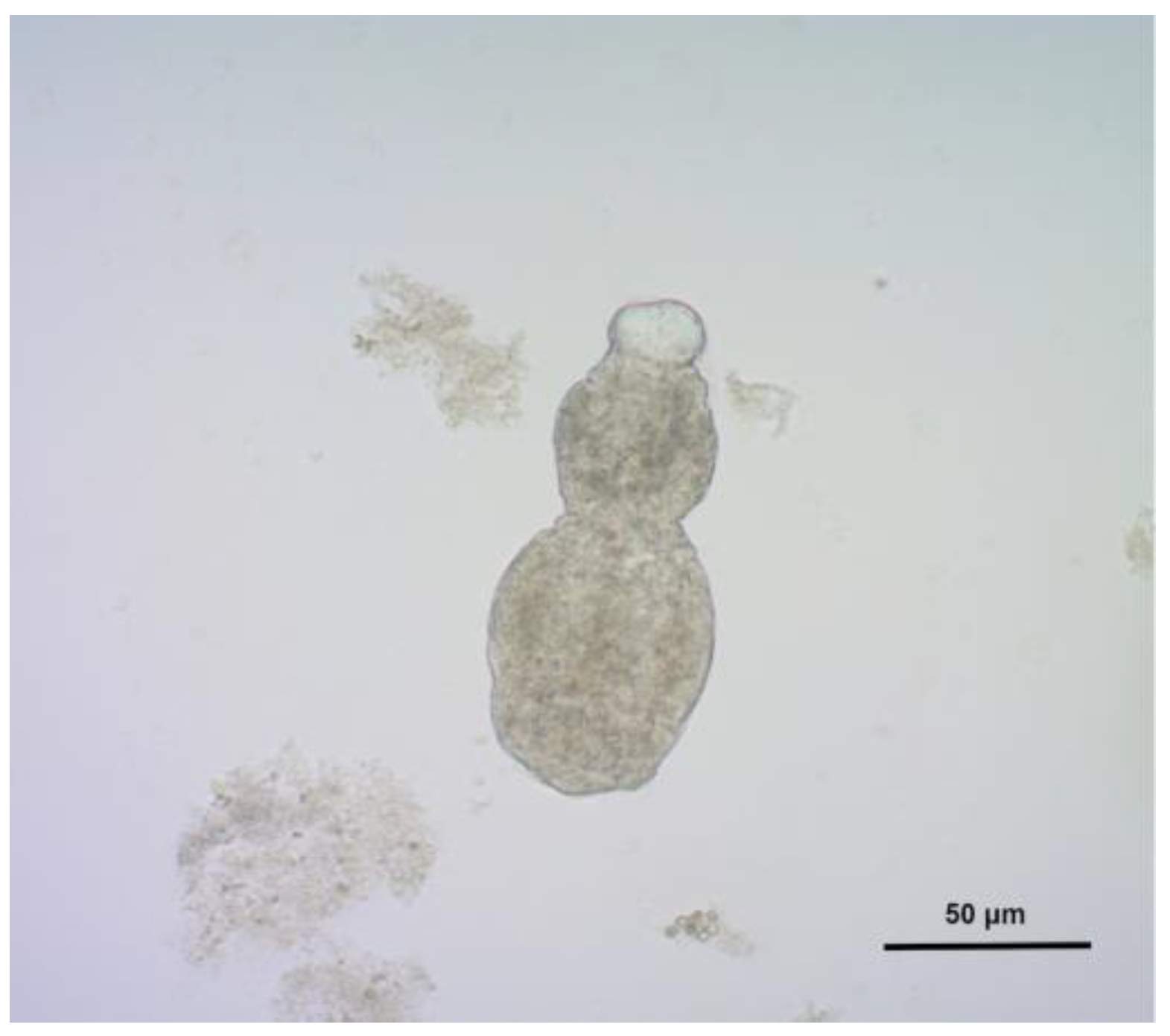
In order to further clarify the described finding a liver core biopsy was performed. Cytological analysis of the liver punctate showed tiny scolex hooks, which raised suspicion of echinococcosis (
Figure 2). The hypothesis was additionally supported by positive serology on echinococcal antigens.
Finally, the diagnosis of AE was confirmed by histopathological examination of the liver-biopsy obtained tissue (
Supplementary Figure S1). and molecular confirmation of
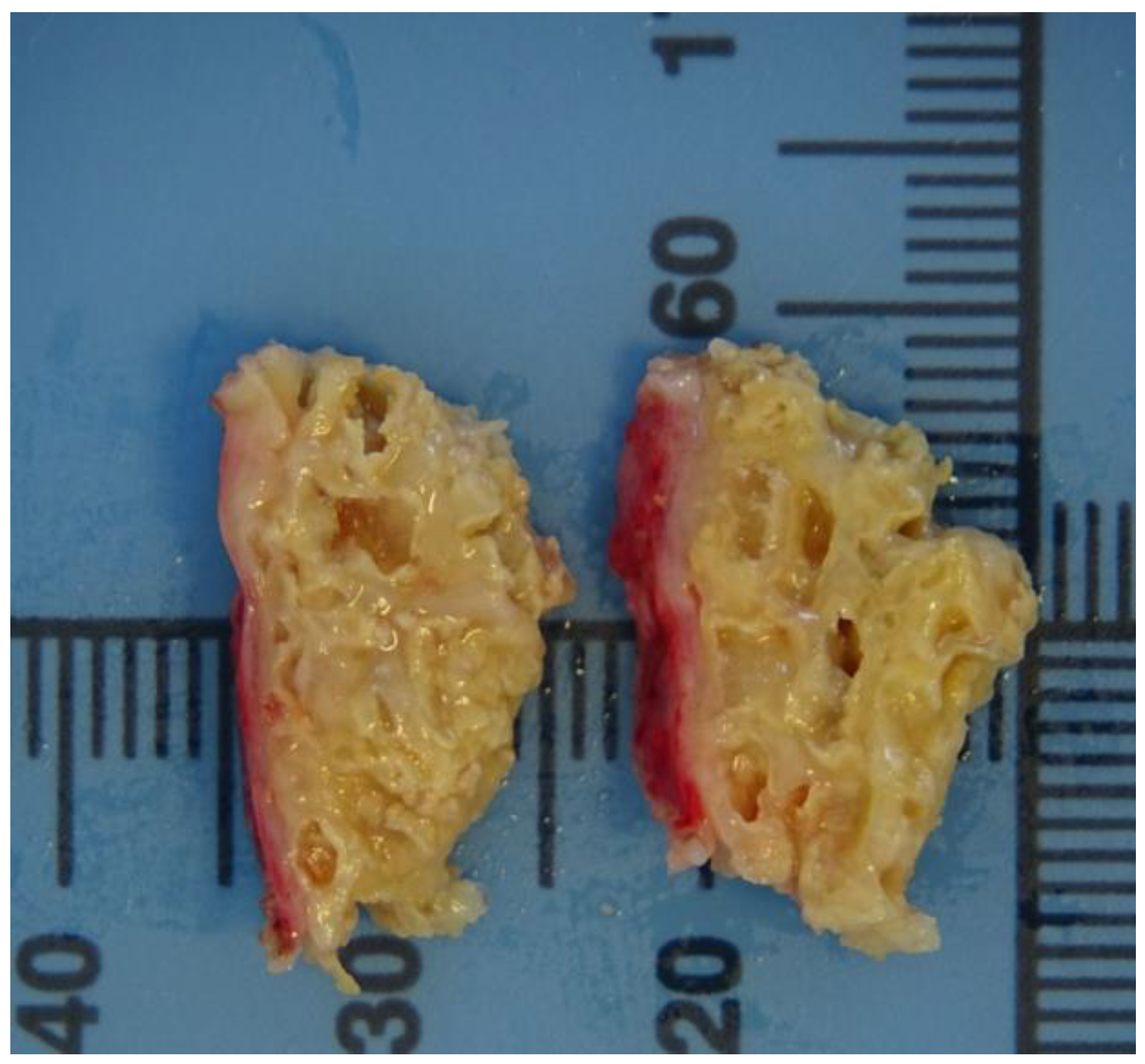
E. multilocularis. At gross pathologic examination, hepatic tissue was multicystically changed with cysts of irregular shape and size (<1 mm to 7 mm) (
Figure 3). Upon histological examination, pseudocysts were focally filled with calcified material, lined with laminated eosinophilic material (laminar membrane) and surrounded by necrotic hepatocytes and dense inflammatory infiltrate of lymphocytes, plasma cells, eosinophils and macrophages. The specific DNA fragments of
E. multilocularis from the biopsy sample were successfully amplified. Following DNA extraction, samples were analyzed in duplicates using conventional PCRs that amplified a 200-bp region in the mitochondrial gene
nad1 and a 395- bp section of the
12S rRNA gene of
E. multilocularis. The obtained sequences were compared using BLAST (
https://blast.ncbi.nlm.nih.gov/Blast.cg) and they were found to be identical to sequences previously described in red foxes and Croatian patients as well as other European isolates of
E. multilocularis.According to the WHO-PNM (P, parasitic mass in the liver; N, involvement of neighboring organs; M, metastasis) staging system of AE, at the moment when diagnosis of AE was reached (April 2022), our patient was in stage P4N0M0. The liver infiltration was irresectable, particularly due to the extension of the lesion along the vessels, but there were no signs of extrahepatic dissemination of the disease. Therefore, the patient presented to the national liver transplantation team at University Hospital Merkur. Considering the complexity of this procedure, it was agreed to proceed with conservative treatment only. Therefore, treatment was continued with albendazole (ABZ) in daily dose of 800 mg, divided in two doses [
6]. Because of the active parasitic infection, in agreement with rheumatologists, immunosuppressive therapy with MTX and IFX was discontinued. However, corticosteroid therapy was initiated (high doses of methylprednisolone with gradual de-escalation) due to IgAN and subsequent kidney impairment.
During a one-year follow-up, our patient attended regular medical assessments with an infectologist and a rheumatologist. No adverse reactions to ABZ have been recorded. The liver and kidney function are satisfactory. He reported no signs of exacerbations regarding RA. He is currently taking 12 mg of methylprednisolone daily and 800 mg of albendazole alongside other chronic therapy. Even though the infection, RA and IgAN were well controlled, he was hospitalized twice during this period due to a high fever accompanied with rigors. Both times it was due to streptococcal bacteremia (S. constellatus). Despite ample diagnostics employed (transesophageal echocardiogram (TOE), abdominal MSCT) there was no definitive clue on the origin of the infection. A prolonged antibiotic treatment (four weeks of intravenously applied ceftriaxone followed by six weeks of co- amoxiclav per os) was performed. To date, there were no relapses of the invasive streptococcal disease.
The control MSCT in January 2023, following 10 weeks of antibiotic therapy and 11 months of ABZ treatment, showed regression of the lesions. The cystoid lesion of the right liver lobe was now better demarcated than initially and smaller in diameter. The necrotic nodule next to the celiac trunk decreased in size too (
Figure 1b).
Discussion
The presented case highlights several worrying aspects regarding AE. It testifies to the increasing incidence of AE among immunocompromised hosts [
2,
3]. Also, since it is the first reported case of AE from southern Croatia, it is another example that demonstrates the spread of
E. multilocularis outside of the historically endemic countries [
7].
It is questionable, however, whether this case was imported from Southern Germany or autochthonously transmitted. Several facts support the hypothesis that the infection was acquired abroad. Our patient had lived in Southern Germany, near the city of Tübingen in Baden-Württemberg for 27 years, from 1987 to 2014, after which he moved back to Croatia. This region is part of the Central European AE belt where
E. multilocularis is highly endemic. In 2020, 77% of all reported cases in EU/EEA were from Germany and France. Furthermore, it is the south of Germany that is known as an epicenter of the disease within the country with 80% of all cases reported in the years 2012 to 2016 [
8]. In addition, he had a dog while living in Germany and the “dog ownership” per se has been identified as a risk factor associated with AE with 2.5-fold increased odds ratio for acquiring the disease [
9]. The epidemiological situation regarding AE in Croatia has recently dramatically changed. There were no confirmed cases of AE in Croatia until 2017, but since then six new cases were reported (all of them from continental Croatia) which was the highest regional incidence ever reported in Europe. Nevertheless, to this day there were no reports of AE from southern Croatia [
5]. What is more, a recent surveillance program examining
E. multilocularis prevalence in red foxes in different parts of Croatia detected no positive foxes in southern parts of the country [
10]. This is not surprising because these two regions have very different climate and geography. Our patient lives in southern Croatia, in the coastal city of Split, his lifestyle was not associated with a higher risk of acquiring
E. multilocularis and he did not have significant contact with continental parts of the country. Taken together, it seems plausible that this case was imported rather than autochthonous.
However, several facts make it impossible to completely rule out an autochthonous infection. First, the disease has been diagnosed 8 years after he had left Germany. Despite the long latency of the disease, immunocompromised patients often experience faster progression of the disease which, on average, becomes clinically apparent within 4 years. Exceptionally fast-progression of the disease has been described in severely immunocompromised patients, namely in solid organ transplantation recipients [
11]. Second, our patient had had an abdominal US in 2018 (4 years after he had left Germany), which was described as normal. Possibly, the lesions were too small and hard to detect with ultrasonography at that time even if the infection had occurred well before. Third, the emergence of AE in continental Croatia makes the exclusion of autochthonous infection even more difficult. Additional studies are required to rule out the presence of
E. multilocularis in wild animals from the region.
Whether it was imported or autochthonous
, it is evident that the incidence of human AE cases is rising. From 2001 through 2018, 14 countries reported their first cases of AE, mostly in Europe and central Asia. The incidence in endemic countries is rising too [
4,
7]. It is partly due to the increased prevalence and geographical extension of the tapeworm-infected red foxes which significantly augmented the
E. multilocularis-infection pressure. Besides that, another reason for the observed rise in human AE cases is the growing number of immunocompromised patients who are more susceptible to the infection. Humans are considered generally resistant
to E. multilocularis infection. Estimates are that only 1-10% of infected individuals will develop the disease [
1]. Accordingly, various immunosuppressive agents could significantly alter this balance in favor of
E. multilocularis. A review of 4 cohort studies found that among 870 confirmed AE cases, 115 of them were diagnosed in immunocompromised patients which makes 13.2% of all cases. These numbers show that immunosuppression associated condition (IAC) are significantly overexpressed among AE patients compared to the general population [
2,
3].
AE is always a challenge from both diagnostic and therapeutic point of view. The treatment of choice is a radical surgical resection of the lesions, followed by a 2-year ABZ treatment. However, due to its rareness and malignant nature of the disease, it is often misdiagnosed or diagnosed late when radical surgical treatment is impossible to perform (the rate of resectability ranges from 20% to 50%). Therefore, the majority of AE patients present with an advanced disease that requires long-term treatment with ABZ. Unfortunately, 20-30% of patients do not tolerate ABZ and yet there is no satisfactory data on reliable alternatives [
1]. All aspects of the disease are far more challenging in immunocompromised patients as they often present with atypical imaging, negative serology, rapid progression and unusually extensive lesions. Moreover, a higher rate of ABZ intolerability (up to 40%) was documented among these patients. Finally, they require careful management of pre- existing immunosuppressive therapy and yet there are no official guidelines and recommendations on optimal immunosuppressive modalities to follow. Theoretically, and based on clinical data, it seems that long-term administration of steroids and TNF-
α inhibitors could accelerate AE progression [
2,
3]. Our patient is steroid-dependent due to IgAN and RA. He had normal abdominal ultrasonography in 2018 and less than 4 years later he was diagnosed with an inoperable AE, which necessitates long-term ABZ treatment. Even though he lives in an
E. multilocularis non- endemic area, due to a high clinical suspicion, the diagnosis of AE was reached in less than 2 months since the first finding of the liver infiltration and ABZ treatment had been started even before. Diagnostic delay and treatment initiation are usually much longer. A cohort study from Hungary reported mean diagnostic delay of 33 months [
12].