Rare Fatal Case of Purpura Fulminans Due to Pneumococcal Sepsis in a Child, Associated with Multiorgan Failure
Abstract
Introduction
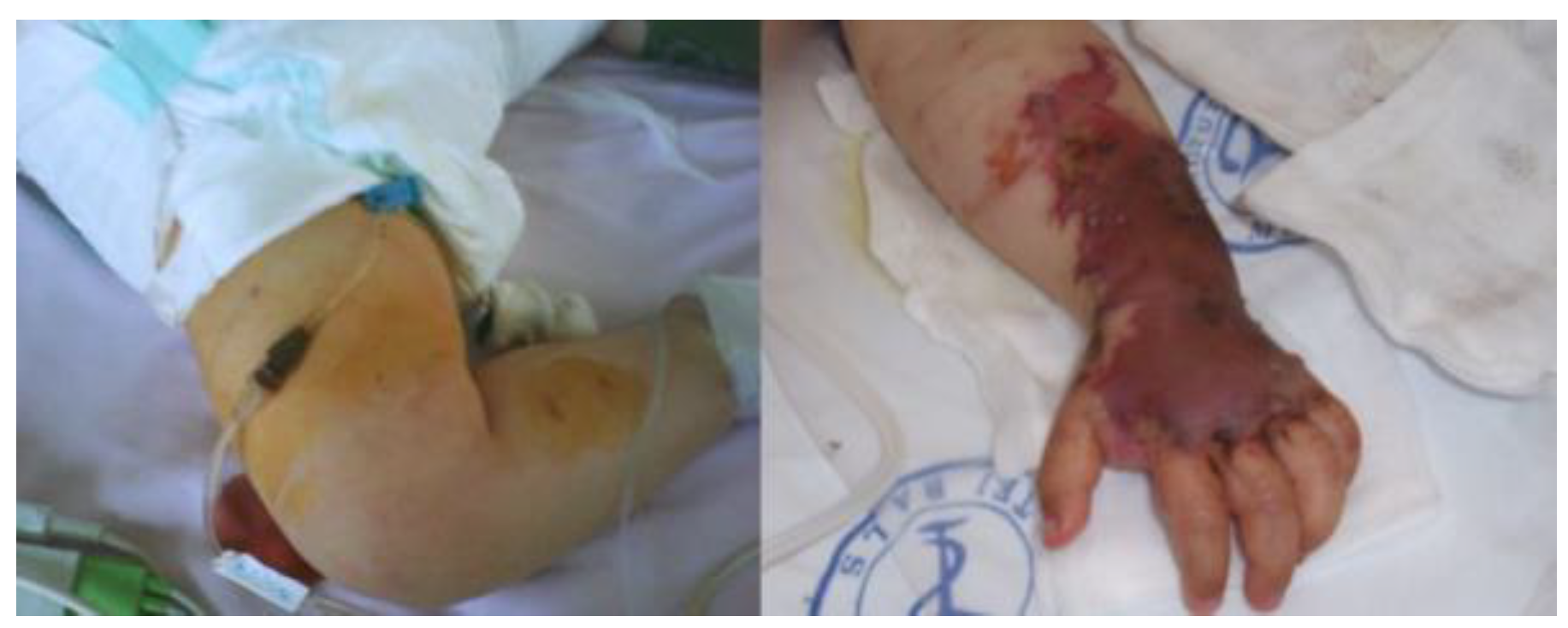
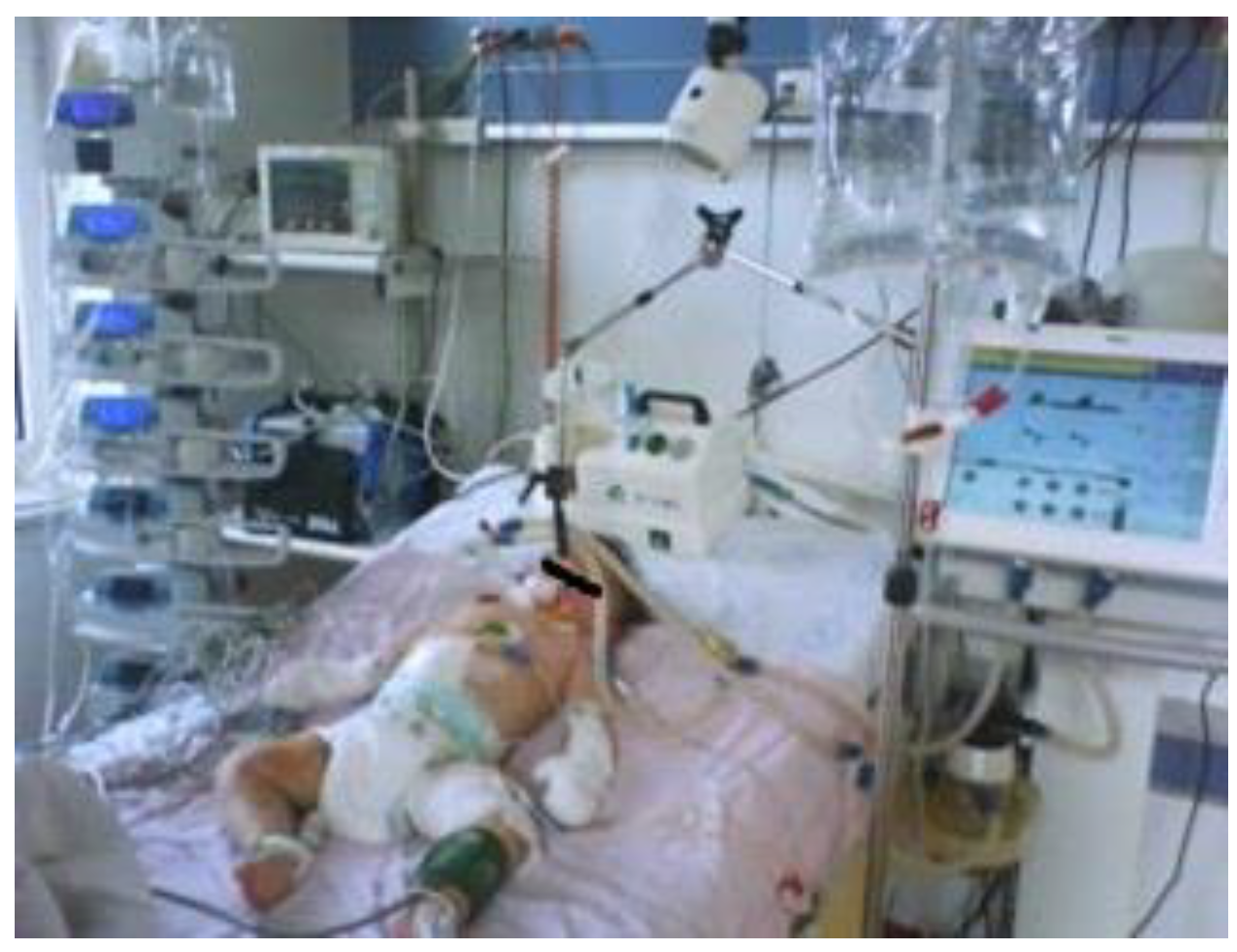
Case report
Discussion
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Irfan Kazi, S.G.; Siddiqui, E.; Habib, I.; Tabassum, S.; Afzal, B.; Khan, I.Q. Neonatal purpura fulminans, a rare genetic disorder due to protein C deficiency: a case report. J Pak Med Assoc. 2018, 68, 463–465. [Google Scholar]
- Chalmers, E.; Cooper, P.; Forman, K.; et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011, 96, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Pombar, R.F.; Tellería, R.L.; Bianco, B.; Centeno, M.D.V.; Cervini, A.B. Postinfectious purpura fulminans: a case report. Arch Argent Pediatr. 2024, 122, e202310137. [Google Scholar] [CrossRef]
- Li, X.L.; Luan, C.Y.; Fan, Y.J.; et al. A rare case of acute infectious purpura fulminans caused by Klebsiella pneumoniae and human herpesvirus type 5. J Inflamm Res. 2022, 15, 4251–4260. [Google Scholar] [CrossRef]
- Findley, T.; Patel, M.; Chapman, J.; Brown, D.; Duncan, A.F. Acquired versus congenital neonatal purpura fulminans: a case report and literature review. J Pediatr Hematol Oncol. 2018, 40, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Djurdjevic, N.; Taweesedt, P.T.; Paulson, M.; et al. Septic shock and purpura fulminans due to Streptococcus pneumoniae bacteremia in an unvaccinated immunocompetent adult: case report and review. Am J Case Rep. 2020, 21, e923266. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Swaminathan Abitbol, V.; Kolhapure, S.; Sathyanarayanan, S. A comprehensive review of meningococcal disease burden in India. Infect Dis Ther. 2020, 9, 537–559. [Google Scholar] [CrossRef]
- Merișescu, M.; Jugulete, G.; Bastian, A.E.; Luminos, M. Evaluation of sepsis mortality in children. Rom J Legal Med. 2018, 26, 37–41. [Google Scholar]
- Jugulete, G.; Merișescu, M.; Bastian, A.E.; Luminos, M. Clinical aspects and medico-legal implications of purpura fulminans in children. Rom J Legal Med. 2017, 25, 364–368. [Google Scholar]
- Lin, C.; Li, D.; Hu, B. Lower limb necrosis secondary to purpura fulminans: a case report. J Burn Care Res. 2023, 44, 477–480. [Google Scholar]
- World Health Organization. 2019. Meningococcal meningitis. Available online: https://www.who.int/en/newsroom/fact-sheets/detail/meningococcal-meningitis (accessed on 30 September 2019).
- Miron, V.D.; Sandulescu, O.; Visan, C.A.; et al. Pneumococcal colonization and pneumococcal disease in children with influenza. Clinical, laboratory and epidemiological features. Rev Chim. 2018, 69, 2749–2753. [Google Scholar] [CrossRef]
- Tekin, R.T.; Dinleyici, E.C.; Ceyhan, M.; et al. The prevalence, serogroup distribution and risk factors of meningococcal carriage in adolescents and young adults in Turkey. Hum Vaccin Immunother. 2017, 13, 1182–1189. [Google Scholar] [CrossRef]
- Wang, B.; Santoreneos, R.; Giles, L.; Afzali, H.H.; Marshall, H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019, 37, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Preziosi, M.P.; Jacob John, T.; Greenwood, B. The epidemiology of meningococcal disease in India. Trop Med Int Health. 2010, 15, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Aye, A.M.M.; Bai, X.; Borrow, R.; et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): the global meningococcal initiative. J Infect. 2020, 81, 698–711. [Google Scholar] [CrossRef]
- Lee, J.; Blackburn, J.; Pham-Huy, A. Uncommon clinical presentation of a common bug: Group A Streptococcus meningitis. Paediatr Child Health. 2020, 26, e129–e131. [Google Scholar] [CrossRef]
- Sernaqué, C.; Ceresetto, J.; Duboscq, C.; et al. Púrpura fulminans asociada a déficit adquirido de proteína S en una paciente con neumonía por Streptococcus pneumoniae. Hematología. 2021, 24, 71–75. [Google Scholar]
- Azzari, C.; Nieddu, F.; Moriondo, M.; et al. Underestimation of invasive meningococcal disease in Italy. Emerg Infect Dis. 2016, 22, 469–475. [Google Scholar] [CrossRef]
- Sharip, A.; Sorvillo, F.; Redelings, M.D.; et al. Population- based analysis of meningococcal disease mortality in the United States: 1990-2002. Pediatr Infect Dis J. 2006, 25, 191–194. [Google Scholar] [CrossRef]
- Ouldali, N.; Levy, C.; Varon, E.; et al. Incidence of paediatric pneumococcal meningitis and emergence of new serotypes: a time-series analysis of a 16-year French national survey. Lancet Infect Dis. 2018, 18, 983–991. [Google Scholar] [CrossRef]
- Green, M.S.; Schwartz, N.; Peer, V. A meta-analytic evaluation of sex differences in meningococcal disease incidence rates in 10 countries. Epidemiol Infect. 2020, 148, e246. [Google Scholar] [CrossRef] [PubMed]
- Stein-Zamir, C.; Shoob, H.; Sokolov, I.; Kunbar, A.; Abramson, N.; Zimmerman, D. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dise J 2014, 33, 777–779. [Google Scholar] [CrossRef]
- Bhatti, U.F.; Williams, A.M.; Raghavendran, K.; Georgoff, P.E. Four-extremity amputation following disseminated intravascular coagulation and purpura fulminans. BMJ Case Rep. 2019, 12, e228028. [Google Scholar] [CrossRef]
- Andreasen, T.J.; Green, S.D.; Childers, B.J. Massive infectious soft-tissue injury: diagnosis and management of necrotizing fasciitis and purpura fulminans. Plast Reconstr Surg. 2001, 107, 1025–1034. [Google Scholar] [CrossRef]
- Tahmasebi, Z.; Asadollahi, P.; Sadeghifard, N.; Ghafourian, S.; Kalani, B.S.; Kalaei, E.G.P.; Pakzad, I. Microbiological and drug resistance patterns of bronchoalveolar lavage samples taken from hospitalized patients in Iran. Germs. 2022, 12, 333–343. [Google Scholar] [CrossRef]
- Chalmers, E.; Cooper, P.; Forman, K.; Grimley, C.; Khair, K.; Minford, A.; et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011, 96, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Plüß, M.; Zeisberg, M.; Müller, G.A.; Vasko, R.; Korsten, P. Therapeutic response to glucocorticoids, anticoagulation and plasma exchange in a patient with primary antiphospholipid syndrome presenting with purpura fulminans. Lupus. 2018, 27, 2170–2173. [Google Scholar] [CrossRef] [PubMed]

| Parameter | 1st week | 2nd week | 3rd week | 4th week | Reference range |
|---|---|---|---|---|---|
| White blood cells (WBC) ×109/L | 34 | 31.5 | 23.3 | 33.8 | 4-11 |
| Platelets ×109/L | 23 | 28 | 29 | 24 | 150-400 |
| Hemoglobin g/dL | 9.7 | 9.6 | 10.8 | 9.4 | 12-15 |
| Fibrinogen mg/dL | 596 | 697 | 331 | 440 | <400 |
| Prothrombin time (PT) seconds | 140 | 81 | 90 | 134 | 11-15 |
| Aspartate aminotransferase (AST) U/L | 112 | 96 | 64 | 102 | 14-50 |
| Alanine aminotransferase (ALT) U/L | 130 | 122 | 120 | 110 | 0-50 |
| Serum creatinine mg/dL | 4.45 | 4.40 | 3.00 | 2.90 | <0.7 |
| D-dimers mg/L | 8.9 | 9 | 9.3 | 10 | 0-0.50 |
© GERMS 2024.
Share and Cite
Jugulete, G.; Merişescu, M.M.; Pavelescu, C.; Luminos, M.L. Rare Fatal Case of Purpura Fulminans Due to Pneumococcal Sepsis in a Child, Associated with Multiorgan Failure. Germs 2024, 14, 204-209. https://doi.org/10.18683/germs.2024.1432
Jugulete G, Merişescu MM, Pavelescu C, Luminos ML. Rare Fatal Case of Purpura Fulminans Due to Pneumococcal Sepsis in a Child, Associated with Multiorgan Failure. Germs. 2024; 14(2):204-209. https://doi.org/10.18683/germs.2024.1432
Chicago/Turabian StyleJugulete, Gheorghiţă, Maria Mădălina Merişescu, Carmen Pavelescu, and Monica Luminiţa Luminos. 2024. "Rare Fatal Case of Purpura Fulminans Due to Pneumococcal Sepsis in a Child, Associated with Multiorgan Failure" Germs 14, no. 2: 204-209. https://doi.org/10.18683/germs.2024.1432
APA StyleJugulete, G., Merişescu, M. M., Pavelescu, C., & Luminos, M. L. (2024). Rare Fatal Case of Purpura Fulminans Due to Pneumococcal Sepsis in a Child, Associated with Multiorgan Failure. Germs, 14(2), 204-209. https://doi.org/10.18683/germs.2024.1432




