Abstract
Introduction: Pseudoglutamicibacter cumminsii (formerly Arthrobacter cumminsii) is a microorganism rarely reported as a cause of infection. It is a Gram-positive, non-motile, and non-spore-forming bacterium belonging to the Micrococcaceae family. It is known for its environmental ubiquity, being frequently found in soil, water, and other ecological niches. Case report: A 39-year-old woman with a history of glucose-6-phosphate dehydrogenase deficiency and multiple surgeries at the left lower extremity for osteomyelitis at the left tibia presented with a relapse of chronic osteomyelitis of the left tibia. She underwent surgical debridement, and cultures grew Enterococcus faecalis, Pseudomonas aeruginosa, Klebsiella oxytoca, Proteus mirabilis, and Pseudoglutamicibacter cumminsii, according to identification with matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Targeted antimicrobial treatment with ciprofloxacin and amoxicillin-clavulanate for six weeks led to patient recovery. Conclusions: Accurate identification by advanced diagnostic techniques is essential for effectively managing rare pathogens. Further research and reporting of cases are needed to understand better the epidemiology, pathogenesis, optimal treatment, and improved clinical outcomes associated with P. cumminsii infections.
Introduction
Pseudoglutamicibacter cumminsii, formerly known as Arthrobacter cumminsii, is a rare and recently reclassified bacterial species within the genus Pseudoglutamicibacter [1]. It is a Gram-positive, non-motile, and non-spore-forming bacterium that belongs to the Micrococcaceae family, and is known for its environmental ubiquity, being frequently found in soil, water, and other ecological niches [1,2,3]. Recently scarce reports implicate this microorganism in human infections, such as in cases of urinary tract infections [3,4].
Despite its previous classification as Arthrobacter cumminsii, recent advances in microbiological taxonomy have necessitated its reclassification, leading to its designation as P. cumminsii. Such taxonomic revisions highlight the evolving nature of microbial identification and underscore the importance of accurate species identification for appropriate diagnosis and treatment [1].
Herein, we present a case of polymicrobial osteomyelitis in a young woman. We discuss the clinical presentation, microbiological identification, treatment strategies, and potential implications for patient management. This case highlights the significance of being vigilant about unusual and emerging pathogens and emphasizes the need for a multidisciplinary approach to manage and control infections caused by such rare bacteria effectively.
Case report
A 39-year-old woman with a history of glucose-6-phosphate dehydrogenase (G6PD) deficiency and multiple surgeries at the left lower extremity for osteomyelitis at the left tibia presented with purulent discharge from a non-healing wound over the left tibia. More specifically, the history of G6PD deficiency was diagnosed based on a positive Guthrie test after birth and had not been further evaluated. The patient had a history of acute osteomyelitis of the left tibia from age 14, and has been hospitalized multiple times ever since. From 2009 to 2021 she was hospitalized 10 times due to acute episodes of infection manifesting as a combination of pain and purulent discharge from a fistula over the tibia, with or without fever on the grounds of chronic osteomyelitis. She had undergone multiple surgeries (more than 20 from 2009 to 2021) for surgical debridement and had received multiple courses of intravenous and oral antimicrobial treatment based on the results of cultures received intraoperatively. The treatment mainly consisted of beta-lactams, quinolones, and tetracyclines. Supplementary Table 1 shows the microbiology of isolated pathogens through surgically retrieved samples.
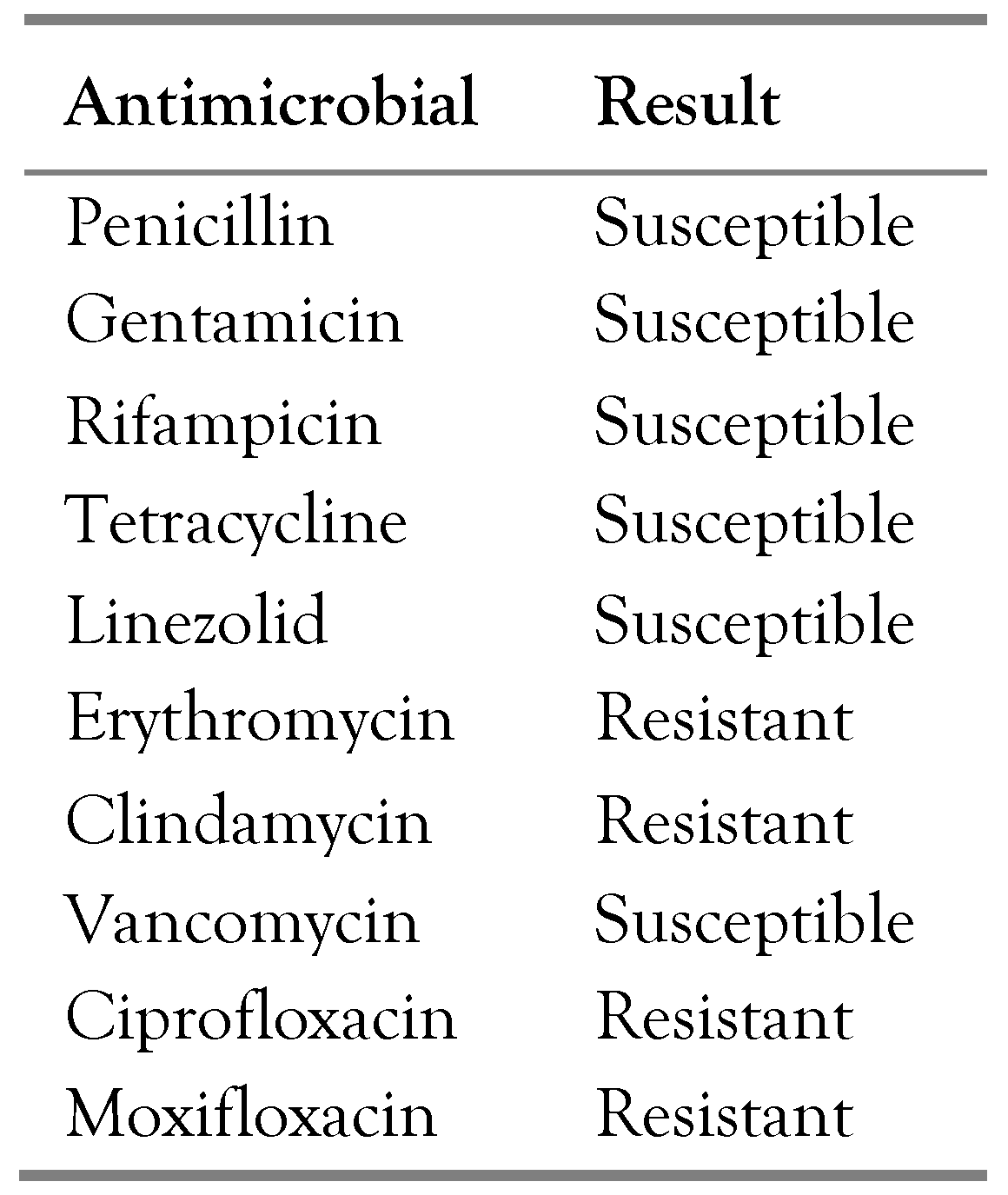
The patient was admitted to the Orthopedics Department of the University Hospital of Heraklion with a working diagnosis of relapse of chronic osteomyelitis of the tibia, and underwent surgical debridement. Necrotic material was removed, and pus cultures were taken. The results of the cultures yielded Enterococcus faecalis (sensitive to ampicillin), Pseudomonas aeruginosa (susceptible to all antipseudomonal antibiotics, including quinolones), Klebsiella oxytoca (sensitive to all antibiotics except for ampicillin), Proteus mirabilis (sensitive to all antibiotics except for ampicillin and quinolones), and Pseudoglutamicibacter cumminsii. The antimicrobial susceptibility of P. cumminsii is shown in Table 1.

Table 1.
Antimicrobial susceptibility of Pseudoglutamicibacter cumminsii from a patient with polymicrobial osteomyelitis of the tibia.
Bacterial strains were identified using the matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) (version 3.2) (BioMérieux, France). Antimicrobial susceptibility was performed by disk diffusion, and the results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2022 guidelines.
When the results of the cultures became available, antimicrobial treatment was given with a combination of ciprofloxacin and amoxicillin-clavulanate for six weeks. The patient improved over the next two months. However, a relapse of the osteomyelitis was noted several months later. Microbiology from surgically retrieved samples yielded Acinetobacter pittii,Enterococcus faecalis, and Klebsiella oxytoca, which were again identified with the use of MALDI-TOF MS. The patient was treated with daptomycin and the combination of piperacillin with tazobactam. She then had a complicated course and eventually had an amputation of the left lower limb at a level lower than the knee that was complicated by surgical site infections, with surgical cultures received during another surgical debridement yielding Escherichia coli, and was again treated with antibiotics.
Discussion
Herein, we present the case of a patient with a very prolonged and complicated history of polymicrobial chronic osteomyelitis, who was treated with surgical debridement combined with antimicrobial treatment based on the results of antimicrobial susceptibility. Five different species were identified during this episode of osteomyelitis, along with P. cumminsii, a bacterium with scarce literature on human infections.
The clinical manifestations observed in this patient do not align with previous reports of P. cumminsii infections. Since this pathogen is rare, most published studies in the literature are case reports. The cases that have been published so far are related to the isolation of the microorganism in the urinary tract [3,4]. Even so, the pathogenic potential of this microorganism is still debatable since it is mainly isolated in the context of contamination [5]. In another report, P. cumminsii was identified as a cause of bacteremia in a patient who also suffered a urinary tract infection from Escherichia coli [6]. An older study reporting clinical data of patients from whom P. cumminsii (A. cumminsii) was isolated shows that in five out of 15 patients, the genitourinary tract was the source of infection, two patients had skin and soft tissue infections, two patients had otitis, two had bacteremia, and one had calcaneus osteomyelitis [7]. Interestingly, in this report, in five out of 15 cases, P. cumminsii was isolated in the context of a polymicrobial infection. With so few cases being published, no solid conclusions can be drawn regarding the risk factors for infections by this pathogen, its pathogenicity, and the exact types of infection that it causes. Future observational studies are needed to clarify risk factors, and whether immunosuppression or other conditions predispose to infections by this rare pathogen. Until then, experience from isolated case reports may be of value to gain insight into this microorganism.
Our patient presented with an open wound over the tibia and had a history of previously treated episodes of osteomyelitis, both with antimicrobials and surgery. Thus, osteomyelitis was very high at the differential diagnosis, making surgery an early option for obtaining adequate microbiology samples and removing devitalized tissues. However, results from microbiology provided unexpected results, showing a polymicrobial infection by five microorganisms, with P. cumminsii being one of them. This study underlines the importance of considering unusual pathogens and also of using advanced diagnostic techniques for accurate identification, such as MALDI-TOF MS or sequencing, since rare pathogens may be misidentified by classical biochemical methods [8,9]. This is especially true in cases where standard empirical treatments fail to achieve the desired clinical response. As P. cumminsii infections are not commonly encountered in clinical settings, misdiagnosis or delayed diagnosis may occur, leading to inappropriate treatment and prolonged hospitalization.
In the present case, one could not be sure whether all the isolated pathogens were truly associated with the infection or if at least some were contaminants. This is particularly true given the polymicrobial result of the culture, as one would probably anticipate fewer pathogens to be the causative ones. Due to the difficulty in differentiating contaminants from pathogenic bacteria, the treatment provided was selected to cover all the isolated pathogens. However, the question of the pathogenic potential of P. cumminsiiremains elusive [5].
Considering the limited evidence on antimicrobial susceptibility profiles, a standardized treatment regimen for P. cumminsii infections has yet to be established. In vitro susceptibility testing should be encouraged to guide therapeutic decisions. In our case, P. cumminsii was sensitive to the antimicrobials tested, except for the quinolones, erythromycin, and clindamycin. Antimicrobial treatment included amoxicillin-clavulanate and quinolone, considering the susceptibility of all the isolated pathogens. The patient was rapidly switched to oral treatment based on the results of the OVIVA trial, which shows that oral therapy for osteomyelitis is non-inferior to intravenous treatment if oral antimicrobials provide adequate coverage [10]. Regarding the duration of treatment, even though there are some studies suggesting that a treatment duration shorter than six weeks may be adequate for low-risk patients with osteomyelitis, due to the complicated history of chronic osteomyelitis in this patient, duration of treatment of six weeks with both ciprofloxacin and the combination of amoxicillin with clavulanate was chosen [10,11].
One factor that could have complicated the choice of antimicrobial treatment for the patient presented herein is the history of the G6PD deficiency. The G6PD gene is located in the X-chromosome, thus, G6PD deficiency is more likely to be diagnosed in male individuals, since female individuals require two defective genes to have significant deficiency that could lead to hemolysis under specific stimuli, such as the use of sulfonamides, primaquine, or specific foods [12]. Even though direct quantification of the G6PD enzyme was not performed during the patient’s hospitalization, the history of a G6PD deficiency diagnosis with the qualitative Guthrie test strongly correlates with a significant deficiency of enzyme activity. Qualitative tests such as the Guthrie test have a threshold to discriminate between deficient and normal individuals at 30-40% of the enzyme activity, andthey have good sensitivity for deficient males, as well as for females with two G6PD deficient alleles [13]. The history of G6PD deficiency could have significantly limited the therapeutic choices in this patient. Medications such as the combination of trimethoprim with sulfamethoxazole and quinolones could lead to hemolysis in patients with this condition [12]. This risk may not be the same with all suspect medications, and in this patient, quinolones had been used safely multiple times in the past. Indeed, the effect of quinolones in patients with G6PD is still disputable, especially in women, where enzyme deficiency may be less severe than in men [14,15].
Conclusions
We report the second case of polymicrobial osteomyelitis, where P. cumminsii was isolated in a patient with a chronic non-healing wound over the left tibia. The patient was treated with combined surgical intervention and antimicrobial treatment based on antibiograms of the isolated microorganisms. Accurate identification by advanced diagnostic techniques is essential for effectively managing rare pathogens. Further research and reporting of cases are needed to understand better the epidemiology, pathogenesis, optimal treatment, and improved clinical outcomes associated with P. cumminsiiinfections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.18683/germs.2024.1421/s1, Table S1: Microbiology of previously identified pathogens through surgically retrieved cultures.
Author Contributions
Conceptualization, PI; methodology, PI; software, PI; validation, PI and SM; formal analysis, PI, and SM; investigation, EV, PI, SM, and DPK; data curation, PI; writing—original draft preparation, PI; writing—review and editing, SM, DPK, and EV; visualization, PI; supervision, DK; project administration, DPK. All authors have read and approved the final version of the manuscript.
Funding
None to declare.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
All authors—none to declare.
References
- Busse, H.J. Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibactergen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibactergen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int J Syst Evol Microbiol. 2016, 66, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dolin, E.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Chaves Blanco, L.; Gómez-Camarasa, C.; Illescas López, M.; Chueca Porcuna, N.; Rojas-García, L.L. Unusual isolation of Pseudoglutamicibacter cumminsii in urine culture. Rev Esp Quimioter. 2022, 35, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Puca, E.; Koci, M.; Puca, E.; Como, N. A case report of urinary tract infection from Pseudoglutamicibacter cumminsii in an immunosuppressed patient. New Microbes New Infect. 2023, 53, 101133. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, G.; Bläckberg, A.; Sunnerhagen, T. Infection or contamination with Rothia, Kocuria, Arthrobacter and Pseudoglutamicibacter- a retrospective observational study of non-micrococcus micrococcaceae in the clinic. J Clin Microbiol. 2023, 61, e0148422. [Google Scholar] [CrossRef] [PubMed]
- Kalluru, D.; Ihle, R. Catch me if you can: An unusual case of bacteremia from Arthrobacter cumminsii. Chest. 2021, 160, A745. [Google Scholar] [CrossRef]
- Funke, G.; Pagano-Niederer, M.; Sjödén, B.; Falsen, E. Characteristics of Arthrobacter cumminsii, the most frequently encountered Arthrobacter species in human clinical specimens. J Clin Microbiol. 1998, 36, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ye, L.; Zhao, Q.; Ma, Y.; Yang, J.; Luo, Y. Comparative study of MALDI-TOF MS and VITEK 2 in bacteria identification. J Thorac Dis. 2014, 6, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.Z.; Matajira, C.E.C.; Poor, A.P.; et al. Identification through MALDI-TOF mass spectrometry and antimicrobial susceptibility profiling of bacterial pathogens isolated from sow urinary tract infection. Vet Q. 2018, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.K.; Rombach, I.; Zambellas, R.; et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Besal, R.; Adamič, P.; Beović, B.; Papst, L. Systemic antimicrobial treatment of chronic osteomyelitis in adults: A narrative review. Antibiotics (Basel). 2023, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood. 2020, 136, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Anderle, A.; Bancone, G.; Domingo, G.; Gerth-Guyette, E.; Pal, S.; Satyagraha, A. Point-of-care testing for G6PD deficiency: Opportunities for screening. Int J Neonatal Screen. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Carmoi, T.; Bordier, L.; Bonnefoy, S.; Callot, D.; Lecoules, S.; Algayres, J.-P. [Ofloxacin is contraindicated in case of G6PD deficiency: Is it evidenced based?]. Rev Med Interne. 2009, 30, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Arcavi, L.; Schechmaster, R.; et al. Medications and glucose-6-phosphate dehydrogenase deficiency: An evidence-based review. Drug Saf. 2010, 33, 713–726. [Google Scholar] [CrossRef]
© GERMS 2024.