Systematic Review on Epidemiology of Escherichia coli in Bloodstream Infection of Patients Undergoing Hematopoietic Stem Cell Transplantation
Abstract
Introduction
Methods
Search strategy
Eligibility criteria
Evaluation of the quality
Extraction of data
Results
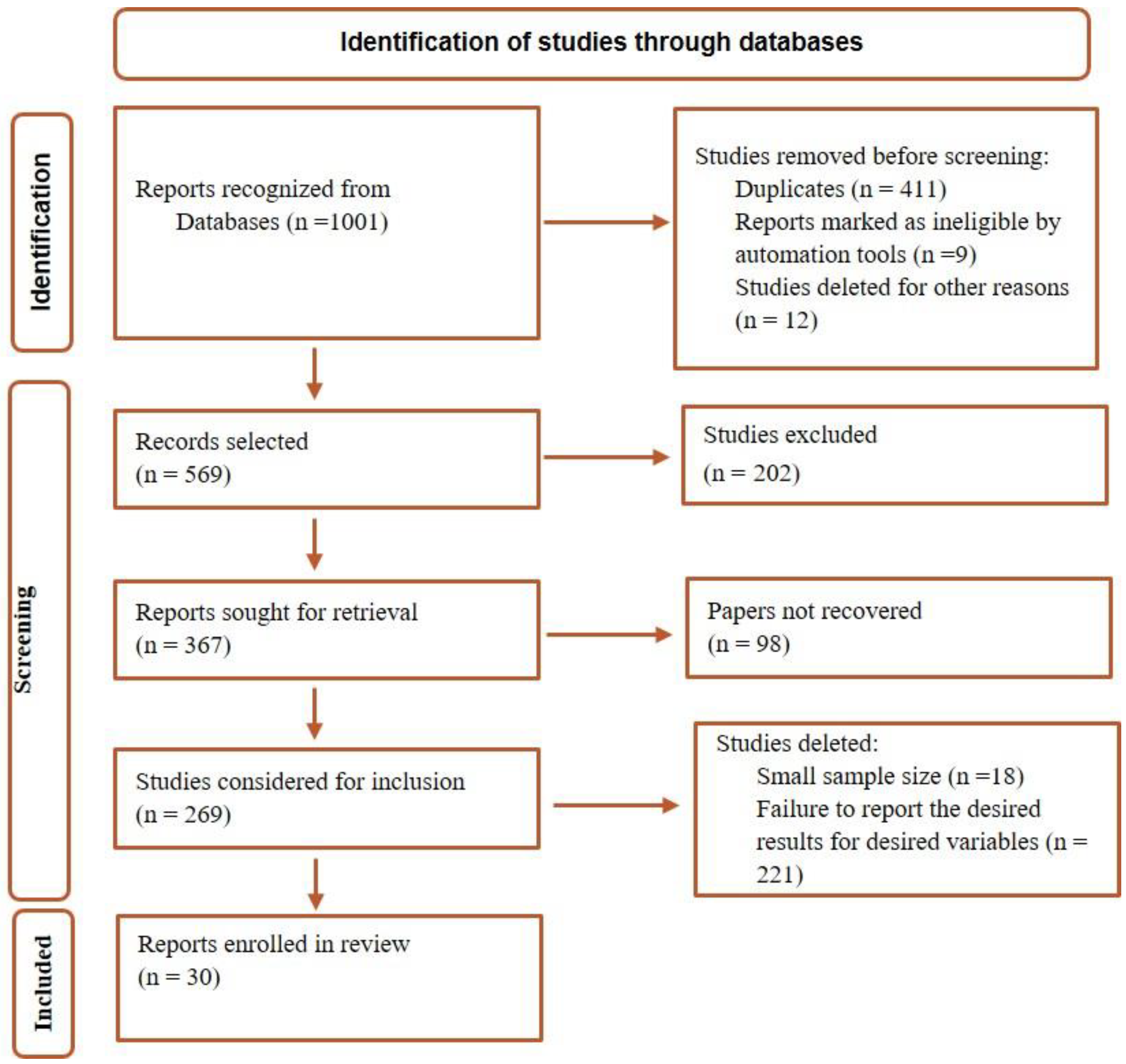
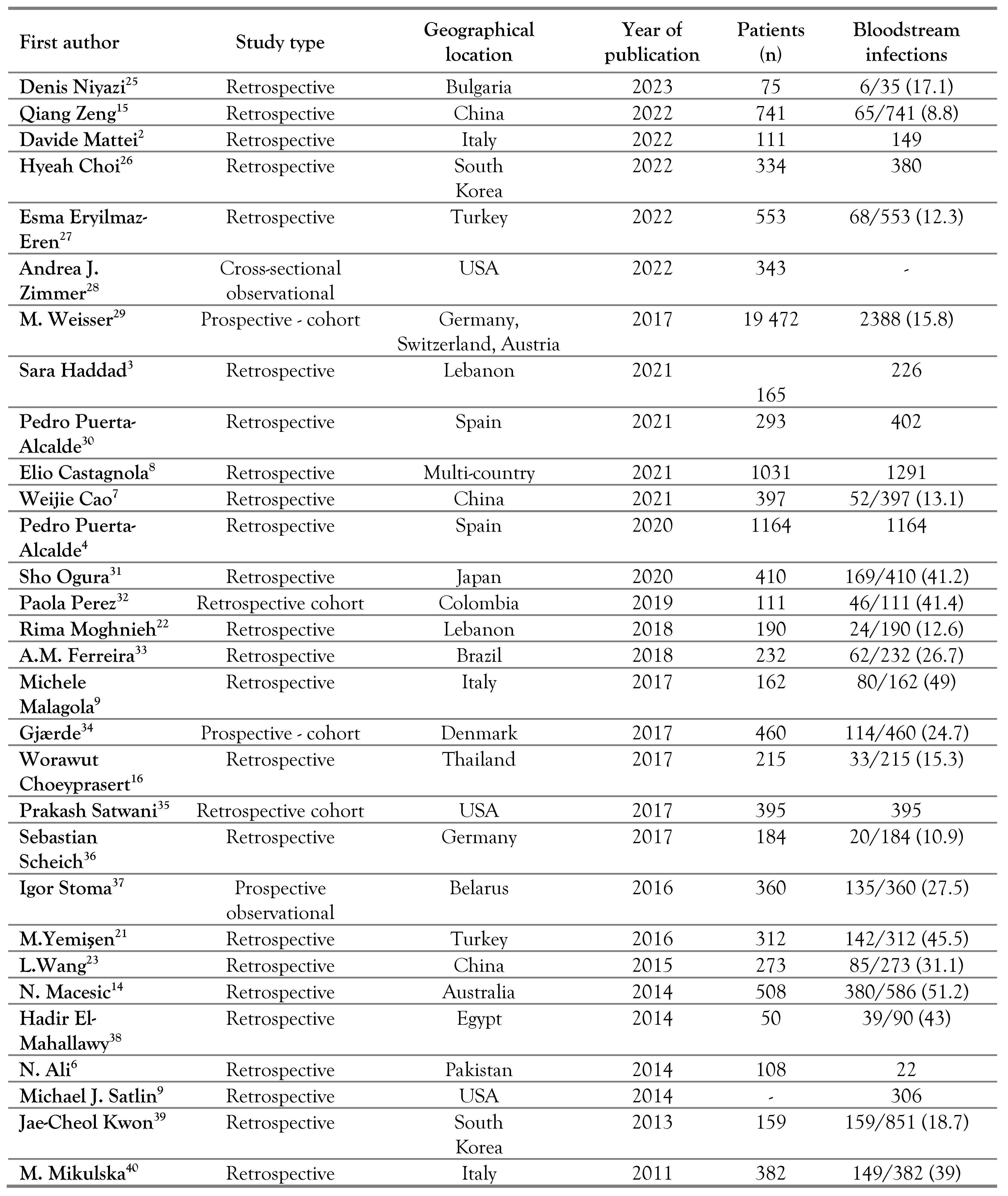
Study selection and characteristics
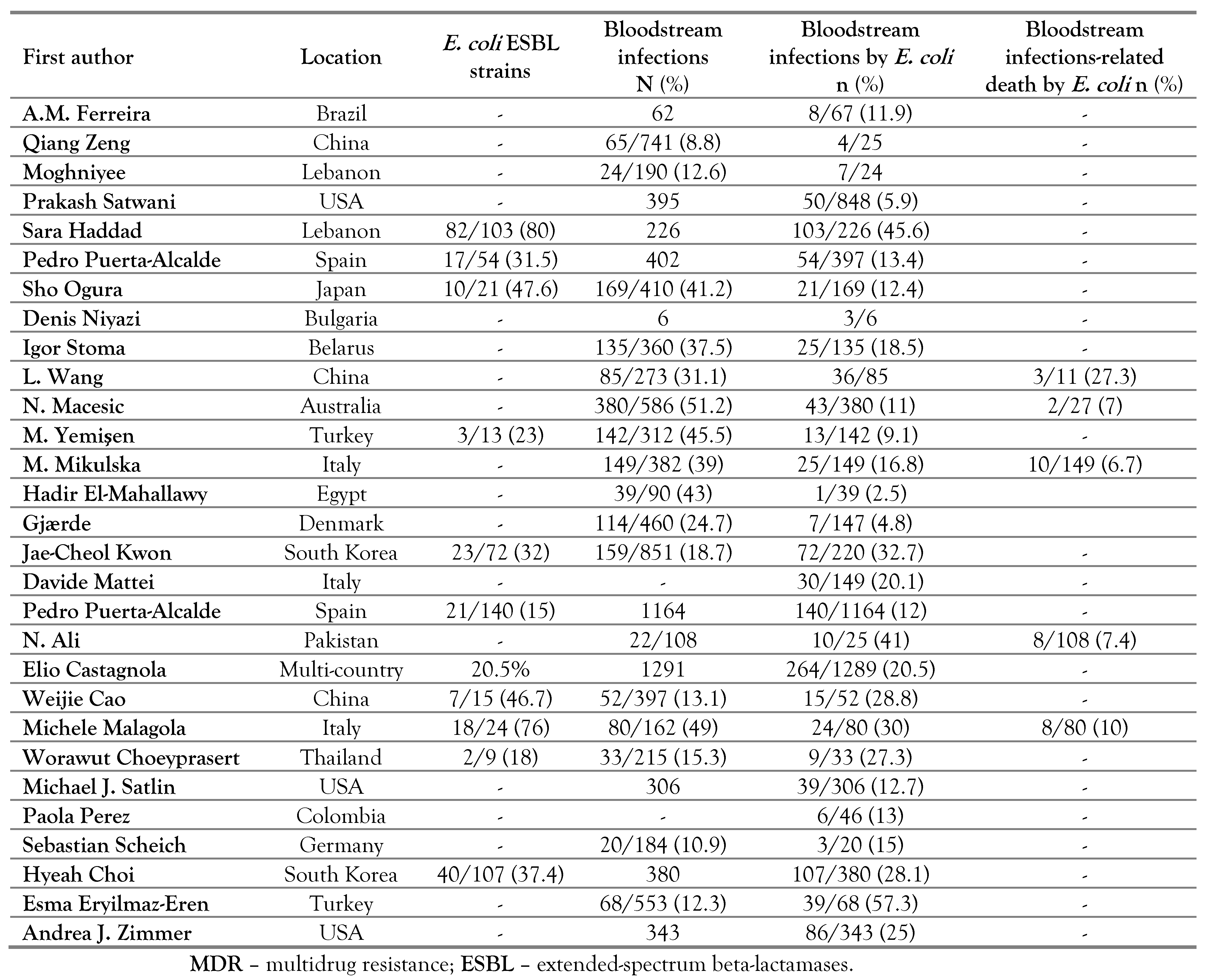
Prevalence of ESBL strains and mortality caused by E. coli strains
Antibiotic susceptibility in E. coli strains
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mert, D.; Ceken, S.; Iskender, G.; et al. Epidemiology and mortality in bacterial bloodstream infections in patients with hematologic malignancies. J Infect Dev Ctries. 2019, 13, 727–735. [Google Scholar] [CrossRef]
- Mattei, D.; Baretta, V.; Mazzariol, A.; et al. Characteristics and outcomes of bloodstream infections in a tertiary-care pediatric hematology-oncology unit: A 10-year study. J Clin Med. 2022, 11, 880. [Google Scholar] [CrossRef]
- Haddad, S.; Jabbour, J.F.; Hindy, J.R.; et al. Bacterial bloodstream infections and patterns of resistance in patients with haematological malignancies at a tertiary centre in Lebanon over 10 years. J Glob Antimicrob Resist. 2021, 27, 228–235. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Cardozo, C.; Marco, F.; et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: Current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transplant. 2020, 55, 603–612. [Google Scholar] [CrossRef]
- Bow, E.J. Fluoroquinolones, antimicrobial resistance and neutropenic cancer patients. Curr Opin Infect Dis. 2011, 24, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Adil, S.; Shaikh, M.U. Bloodstream and central line isolates from hematopoietic stem cell transplant recipients: Data from a developing country. Transpl Infect Dis. 2014, 16, 98–105. [Google Scholar] [CrossRef]
- Cao, W.; Guan, L.; Li, X.; et al. Clinical analysis of bloodstream infections during agranulocytosis after allogeneic hematopoietic stem cell transplantation. Infect Drug Resist. 2021, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Bagnasco, F.; Mesini, A.; et al. Antibiotic resistant bloodstream infections in pediatric patients receiving chemotherapy or hematopoietic stem cell transplant: Factors associated with development of resistance, intensive care admission and mortality. Antibiotics. 2021, 10, 266. [Google Scholar] [CrossRef]
- Malagola, M.; Rambaldi, B.; Ravizzola, G.; et al. Bacterial blood stream infections negatively impact on outcome of patients treated with allogeneic stem cell transplantation: 6 years single-centre experience. Mediterr J Hematol Infect Dis. 2017, 9, e2017036. [Google Scholar] [CrossRef]
- Hosseini, S.M.J.; Naeini, N.S.; Khaledi, A.; Daymad, S.F.; Esmaeili, D. Evaluate the relationship between class 1 integrons and drug resistance genes in clinical isolates of Pseudomonas aeruginosa. Open Microbiol J. 2016, 10, 188–196. [Google Scholar] [CrossRef]
- Jafarabadi, M.; Khaledi, A. Escherichia coli bloodstream infections and associated antibiotic resistance pattern in hematological malignancy populations, a global systematic review. EJMO. 2024, 8, 15–23. [Google Scholar] [CrossRef]
- Mikulska, M.; Del Bono, V.; Raiola, A.M.; et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: Reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009, 15, 47–53. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Morrissey, C.; Cheng, A.; Spencer, A.; Peleg, A.Y. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: Increasing resistance over a 9-year period. Transpl Infect Dis. 2014, 16, 887–896. [Google Scholar] [CrossRef]
- Zeng, Q.; Xiang, B.; Liu, Z. Profile and antibiotic pattern of blood stream infections of patients receiving hematopoietic stem cell transplants in Southwest China. Infect Drug Resist. 2022, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Choeyprasert, W.; Hongeng, S.; Anurathapan, U.; Pakakasama, S. Bacteremia during neutropenic episodes in children undergoing hematopoietic stem cell transplantation with ciprofloxacin and penicillin prophylaxis. Int J Hematol. 2017, 105, 213–220. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Tumbarello, M.; Spanu, T.; et al. Incidence and clinical impact of extended-spectrum-β-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli in patients with hematological malignancies. J Infect. 2009, 58, 299–307. [Google Scholar] [CrossRef]
- Liang, T.; Xu, C.; Cheng, Q.; Tang, Y.; Zeng, H.; Li, X. Epidemiology, risk factors, and clinical outcomes of bloodstream infection due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniaein hematologic malignancy: A retrospective study from Central South China. Microb Drug Resist. 2021, 27, 800–808. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.; Abtahi-Eivary, S.H.; Pirouzi, A.; Khaledi, A.; Rahimi, M. A systematic review and meta-analysis of urinary tract infection, frequency of IS elements and MDR isolates retrieved from adult patients. Gene Rep. 2020, 20, 100707. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]
- Yemişen, M.; Balkan, İ.İ.; Salihoğlu, A.; et al. The changing epidemiology of bloodstream infections and resistance in hematopoietic stem cell transplantation recipients. Turk J Hematol. 2016, 33, 216–222. [Google Scholar] [CrossRef]
- Moghnieh, R.; Abdallah, D.; Awad, L.; et al. Bacteraemia post-autologous haematopoietic stem cell transplantation in the absence of antibacterial prophylaxis: A decade’s experience from Lebanon. Infection. 2018, 46, 823–835. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Fan, X.; Tang, W.; Hu, J. Prevalence of resistant Gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: A single center retrospective cohort study. Medicine. 2015, 94, e1931. [Google Scholar] [CrossRef]
- Gudiol, C.; Garcia-Vidal, C.; Arnan, M.; et al. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014, 49, 824–830. [Google Scholar] [CrossRef]
- Niyazi, D.; Stoeva, T. In vitro activity of ceftazidime-avibactam against 3rdgeneration cephalosporin and carbapenem resistant Gram-negative bacteria obtained from blood and fecal samples of hematopoietic stem cell transplanted patients. Acta Microbiol Bulg. 2023, 39, 78–82. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, H.; Lee, R.; Cho, S.-Y.; Lee, D.G. Bloodstream infections in patients with hematologic diseases: Causative organisms and factors associated with resistance. Infect Chemother. 2022, 54, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz-Eren, E.; Izci, F.; Ture, Z.; Sagiroglu, P.; Kaynar, L.; Ulu-Kilic, A. Bacteremia in hematopoietic stem cell recipients receiving fluoroquinolone prophylaxis: Incidence, resistance, and risk factors. Infect Chemother. 2022, 54, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.J.; Stohs, E.; Meza, J.; et al. Bloodstream infections in hematologic malignancy patients with fever and neutropenia: Are empirical antibiotic therapies in the United States still effective? Open Forum Infect Dis. 2022, 9, ofac240. [Google Scholar] [CrossRef]
- Sava, M.; Bättig, V.; Gerull, S. Bloodstream infections in allogeneic haematopoietic cell recipients from the Swiss Transplant Cohort Study: Trends of causative pathogens and resistance rates. Bone Marrow Transplant. 2023, 58, 115–118. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Chumbita, M.; Charry, P.; et al. Risk factors for mortality in hematopoietic stem cell transplantation recipients with bloodstream infection: Points to be addressed by future guidelines. Transplant Cell Ther. 2021, 27, 501.e1–6. [Google Scholar] [CrossRef]
- Ogura, S.; Kimura, M.; Takagi, S.; et al. Characteristics of gram-negative bacteremia during febrile neutropenia among allogeneic hematopoietic stem cell transplant recipients on levofloxacin prophylaxis. Eur J Clin Microbiol Infect Dis. 2021, 40, 941–948. [Google Scholar] [CrossRef]
- Perez, P.; Patiño, J.; Estacio, M.; Pino, J.; Manzi, E.; Medina, D. Bacteremia in pediatric patients with hematopoietic stem cell transplantation. Hematol Transfus Cell Ther. 2020, 42, 5–11. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Moreira, F.; Guimaraes, T.; et al. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: Importance of previous gut colonization. J Hosp Infect. 2018, 100, 83–91. [Google Scholar] [CrossRef]
- Gjaerde, L.I.; Moser, C.; Sengeløv, H. Epidemiology of bloodstream infections after myeloablative and non-myeloablative allogeneic hematopoietic stem cell transplantation: A single-center cohort study. Transpl Infect Dis. 2017, 19, e12730. [Google Scholar] [CrossRef]
- Satwani, P.; Freedman, J.L.; Chaudhury, S.; et al. A multicenter study of bacterial blood stream infections in pediatric allogeneic hematopoietic cell transplantation recipients: The role of acute gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2017, 23, 642–647. [Google Scholar] [CrossRef]
- Scheich, S.; Reinheimer, C.; Brandt, C.; et al. Clinical impact of colonization with multidrug-resistant organisms on outcome after autologous stem cell transplantation: A retrospective single-center study. Biol Blood Marrow Transplant. 2017, 23, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Stoma, I.; Karpov, I.; Milanovich, N.; Uss, A.; Iskrov, I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016, 51, 102–106. [Google Scholar] [CrossRef]
- El-Mahallawy, H.; Samir, I.; Fattah, R.A.; Kadry, D.; El-Kholy, A. Source, pattern and antibiotic resistance of blood stream infections in hematopoietic stem cell transplant recipients. J Egyp Natl Canc Inst. 2014, 26, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.C.; Kim, S.H.; Choi, J.K.; et al. Epidemiology and clinical features of bloodstream infections in hematology wards: One year experience at the catholic blood and marrow transplantation center. Infect Chemother. 2013, 45, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Del Bono, V.; Bruzzi, P.; et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012, 40, 271–278. [Google Scholar] [CrossRef]
 |
 |
© GERMS 2024.
Share and Cite
Janani, F.; Azami, P.; Sanani, M.G.; Bamneshin, K. Systematic Review on Epidemiology of Escherichia coli in Bloodstream Infection of Patients Undergoing Hematopoietic Stem Cell Transplantation. Germs 2024, 14, 85-94. https://doi.org/10.18683/germs.2024.1420
Janani F, Azami P, Sanani MG, Bamneshin K. Systematic Review on Epidemiology of Escherichia coli in Bloodstream Infection of Patients Undergoing Hematopoietic Stem Cell Transplantation. Germs. 2024; 14(1):85-94. https://doi.org/10.18683/germs.2024.1420
Chicago/Turabian StyleJanani, Fatemeh, Pouria Azami, Mohammad Ghenaatpisheh Sanani, and Khadijeh Bamneshin. 2024. "Systematic Review on Epidemiology of Escherichia coli in Bloodstream Infection of Patients Undergoing Hematopoietic Stem Cell Transplantation" Germs 14, no. 1: 85-94. https://doi.org/10.18683/germs.2024.1420
APA StyleJanani, F., Azami, P., Sanani, M. G., & Bamneshin, K. (2024). Systematic Review on Epidemiology of Escherichia coli in Bloodstream Infection of Patients Undergoing Hematopoietic Stem Cell Transplantation. Germs, 14(1), 85-94. https://doi.org/10.18683/germs.2024.1420




