Abstract
Pseudomonas aeruginosa is one of the most common causes of diabetic foot infection globally. This study aimed to determine the global distribution of P. aeruginosa isolated from diabetic foot ulcer infection. PRISMA procedure was used to perform the current systematic review and meta-analysis. The Web of Science, MEDLINE/PubMed, Scopus, and other databases were searched for studies published in English from 2000 to 2022. Data was analyzed using the Comprehensive Meta-Analysis software (CMA). Keywords and MESH phrases included Pseudomonas aeruginosa, diabetic foot ulcer, P. aeruginosa, and diabetic foot infection. As a result of this review, 16.6% of diabetic foot wound infections were caused by P. aeruginosa. About 37.9% of strains were multidrug resistant (MDR). P. aeruginosa infection rates in diabetic foot ulcers ranged from 0.5 to 100% globally. In total, the prevalence rates of P. aeruginosa in diabetic foot ulcer infection from Asia, Africa, and Western countries were reported at 18.5%, 16.3%, and 11.1%, respectively. Data have shown that the prevalence of P. aeruginosa, particularly MDR strains, isolated from diabetic foot ulcer infection was relatively high; inherent resistance to antibiotics is also high; the wound either does not heal or if it does, it will be delayed. Therefore, timely treatment is essential.
Introduction
Diabetes is a major public health concern worldwide . The number of patients suffering from diabetes has increased from 108 million in 1980 to 442 million in 2014, and the disease's prevalence is growing year after year [1]. Diabetes already affects 7% of the global population, and is expected to rise to 8.3% by 2030 [2]. Diabetes can cause many complications such as renal failure, heart attack, blindness, stroke, and amputations of the lower limbs. Diabetic foot ulcers are a major health concern worldwide, and they are one of the most prevalent diseases among diabetic people. A diabetic foot ulcer is one of the main causes of hospitalization in patients with diabetes. If the wound becomes infected, the length of hospitalization rises, causing more issues for the patient and increasing treatment expenses. About 25% of all wounds do not heal, and this can result in the amputation of the lower leg [2].
In Europe, predominantly Gram-positive bacteria such as Staphylococcus aureus are prevalent, but in Asia and Africa, mostly Gramnegative bacteria, particularly P. aeruginosa are prominent [3]. In general, Gram-negative bacteria are the most common cause of diabetic foot infection [2,4]. P. aeruginosa causes serious injury in patients with diabetic foot ulcers and should never be considered contamination or natural flora, since this microorganism is always a pathogen in this patient population, and the leading cause of diabetes-associated sepsis and amputation [5]. P. aeruginosa is susceptible to few antibiotics due to the intrinsic poor permeability of its cell wall, making it a particularly deadly infection [6,7]. Furthermore, effective antibiotics against P. aeruginosa such as ceftazidime, ciprofloxacin, levofloxacin, amikacin, and piperacillin have only been found to be effective in 64 to 80% of cases [8].
Diabetic foot wound infection is more difficult to cure and is treated later than in nondiabetic patients [5]. Numerous factors contribute to the enhancement of diabetic foot ulcer infection, including vascular system abnormalities, peripheral neuropathy, a high glycemic index, and immune system suppression, which all lead to infection by microorganisms [9].
Poor blood sugar management is linked to biofilm development in diabetic foot ulcer infections . P. aeruginosa can create a biofilm in the wound, making it stronger and more difficult to heal [10]. Biofilm production increases the expression of inflammatory factors, which eventually leads to vascular system damage and delayed wound healing. On the other hand, P. aeruginosa is found in chronic wound infections more frequently than other microorganisms, and inhibits wound healing [11,12]. Antibiotic resistance is becoming a major global issue due to the high inherent resistance in P. aeruginosa, and produced infections are typically difficult to cure [13]. Antibiotic resistance in P. aeruginosa is currently spreading [5]. Despite several studies to investigate the prevalence and resistance of P. aeruginosa in various countries, a global study of the prevalence and antibiotic resistance of this microorganism in diabetic foot infection has seldom been conducted. Thus, the aim of this study is to provide an overview of the prevalence of P. aeruginosa and its antibiotic resistance in people with diabetic foot ulcers worldwide.
Methods
Literature Search
The search was conducted in Scopus, PubMed, Web of Science (ISI), and other databases for studies giving the prevalence of Pseudomonas aeruginosa retrieved from diabetic foot infections (DFI) all over the world between 2000 and end of 2022. MESH terms and Entry words included Pseudomonas, Pseudomonas spp., Pseudomonas aeruginosa, P. aeruginosa, diabetic ulcer, diabetic foot, foot ulcer, diabetic feet, foot diseases, prevalence, frequency, and occurrence.
Eligibility Criteria
Studies that presented the prevalence of P. aeruginosa in DFI patients, which used the correct methods of sampling, processing, detection and isolation of bacteria were included in the present review. For sampling, needle aspiration under aseptic conditions is recommended by the protocol. For ulcers and other open wounds, biopsy was essential. Microbiological and biochemical methods such as culture on McConkey agar, chocolate agar, appearance of pyocyanin pigment on cetrimide-based media, growth at 42°C, oxidase and catalase test, nitrate reduction, and motility were used to determine this microorganism [14]. Studies before 2000, studies with missed/unclear information, meeting abstracts, case reports, and reviews were omitted.
Quality Assessment
Quality assessment was evaluated through Critical Appraisal Skills Programme (CASP) checklist for cross-sectional studies (www.caspuk.net). Based on the criteria included in this protocol, the studies were classified into three categories: weak, strong and moderate. Finally, poor studies were excluded from the current review.
Data Extraction
As shown in Supplementary Table S1, two authors independently considered the selected studies and extracted the appropriate data such as: first author, location (country), published year, sample size (all bacteria), event (Pseudomonas aeruginosa), multidrug resistant (MDR) strains, age, sex (male, female), and stage of disease.
Meta-Analysis
Analysis was done with the Comprehensive Meta-Analysis software (Version 3.3.070, Biostat, USA). The prevalence was calculated by 95% confidence intervals (CIs). The possible statistical heterogeneity between studies was evaluated by Cochran’s Q-test, and I2. Due to significant statistical heterogeneity, the random-effect model was applied. Moreover, Egger’s regression asymmetry test, and Funnel plot were used for judging publication bias in the included studies.
Results
Study Selection and Characteristics of Included Studies
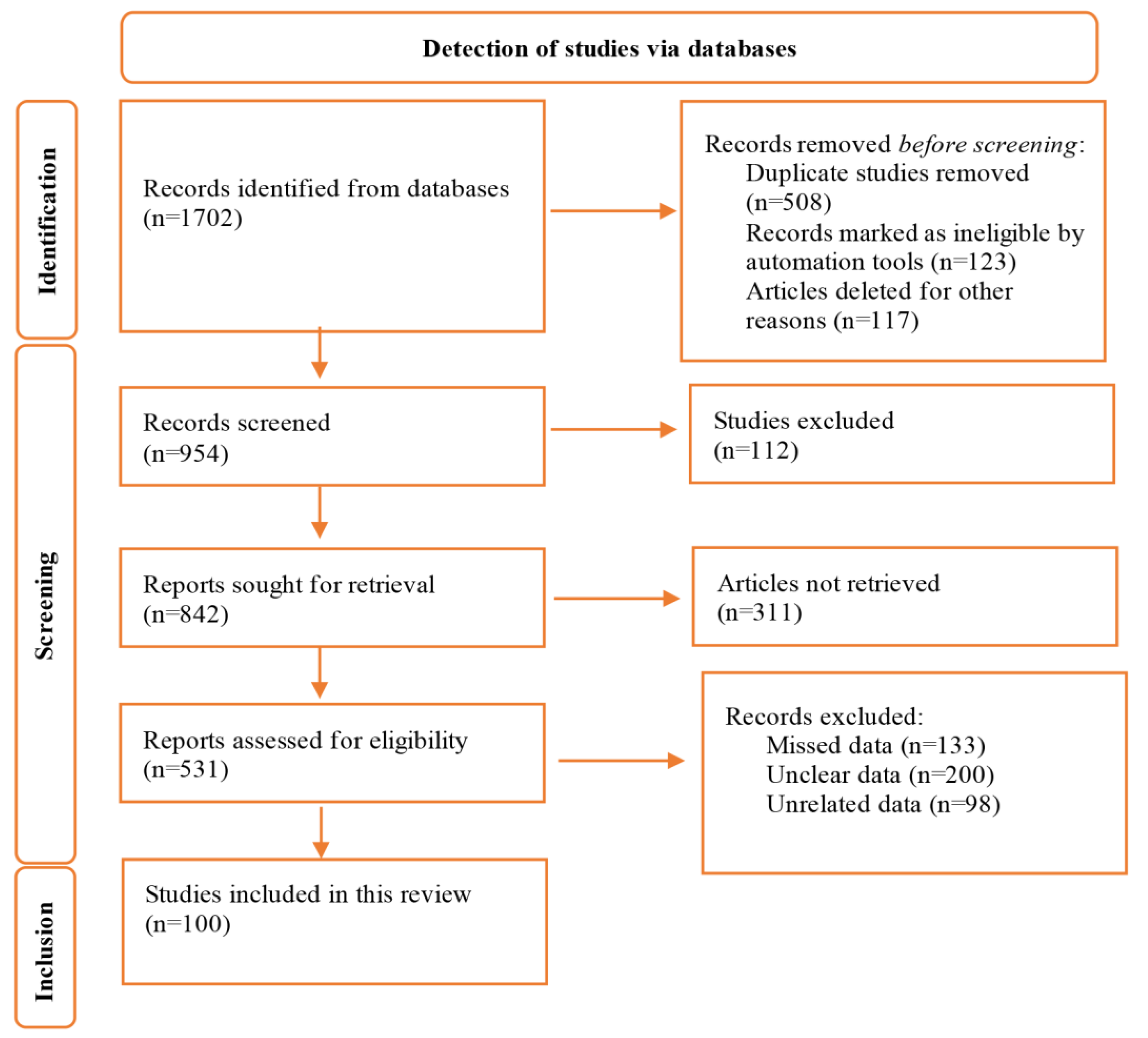
A total of 1702 studies were retrieved in the initial search. After all assessments for titles, abstracts, and full-texts, finally, 100 studies met eligibility criteria, were approved and included the present review (Figure 1). Of these 100 studies, 69, 25, and 5 were from Asian, European, and African countries, respectively.
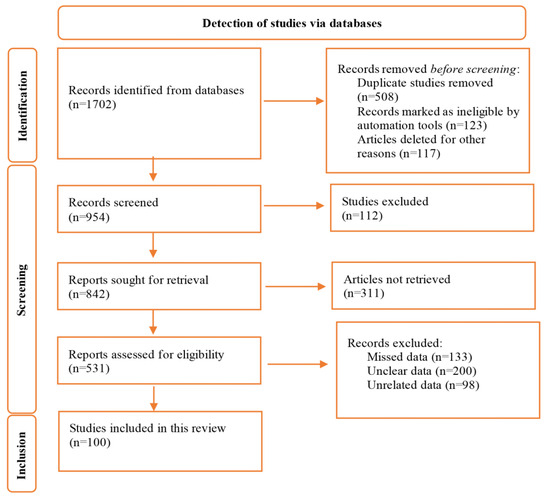
Figure 1.
Flow diagram of the study selection process.
Overall Effects
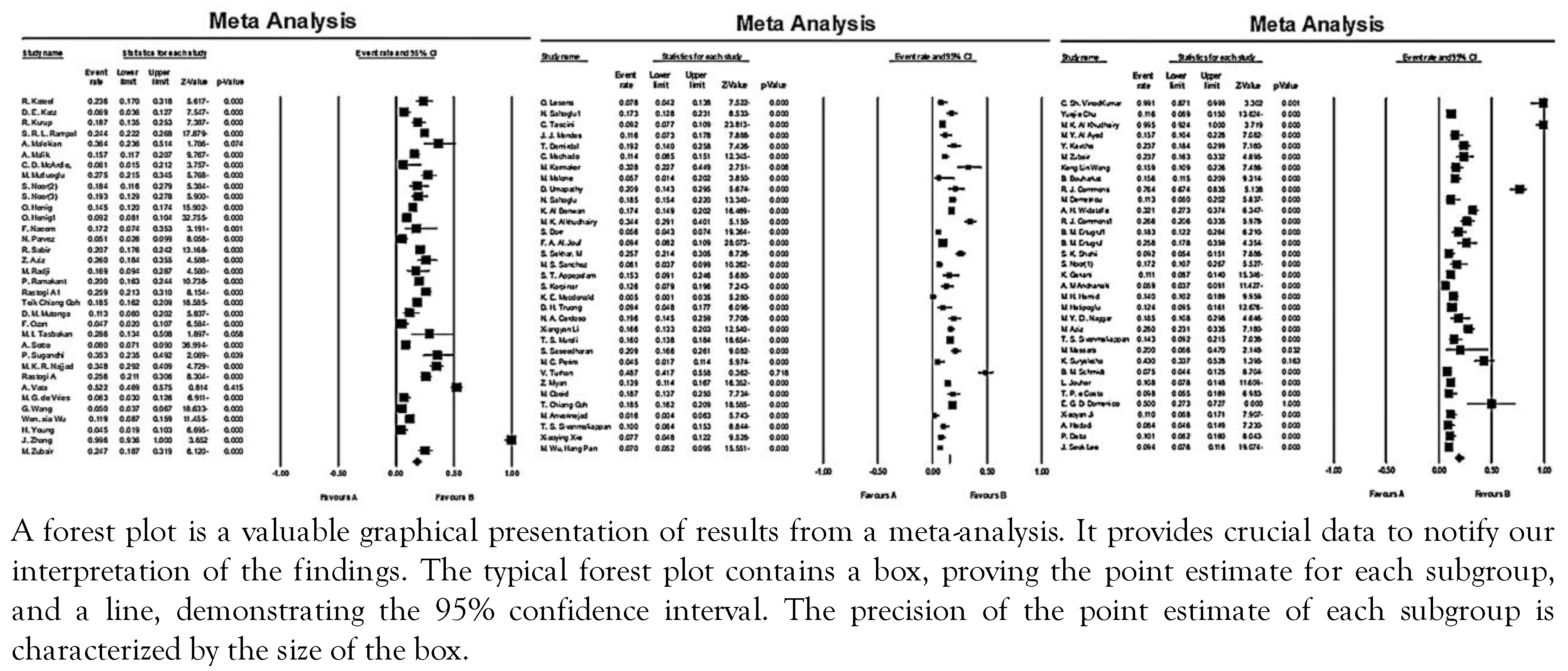
As shown in Figure 2, the combined prevalence of P. aeruginosa in diabetic ulcer infections varied between 0.5-100% all over the world. In general, the pooled prevalence of P. aeruginosa in the world from 2000 to 2022 was reported at 16.6% (95%CI: 14.7-18.8), of which 37.9% (95%CI: 25.3-16.5) were MDR strains. The prevalence of P. aeruginosa in Asian, African and Western countries was 18.5% (95%CI: 16.220.9), 16.3% (95%CI: 9.4-26.8), and 11.1% (95% CI: 8.7-14.2), respectively. In Asian countries, the highest and lowest prevalence of P. aeruginosa isolated from diabetic ulcer infection were 100% and 1.63%, respectively. Also, the highest and lowest prevalence of this microorganism from African countries were 32.2% and 9.4%, respectively. Similarly, values of 0.5% and 50% were reported from Western countries.
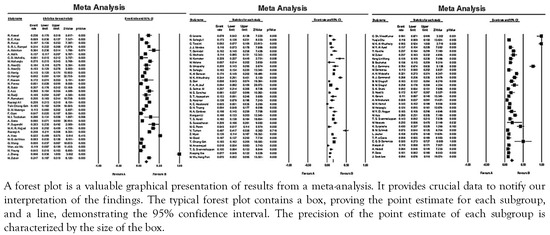
Figure 2.
Forest plot of the meta-analysis of the prevalence of P. aeruginosa isolated from diabetic foot infections.
Heterogeneity and Publication Bias
Data showed the heterogeneity between studies included (Q=1919.7 and Z=21.5, and I2=94.8). Funnel plot showed the bias in the studies. Egger’s linear regression test was performed to further investigate this subject. According to Egger’s linear regression, publication bias was not confirmed between studies (p=0.43).
Discussion
Diabetic foot is a chronic complication in patients with diabetes around the world [15]. Every 30 seconds in the world, a lower limb is amputated due to diabetes [16], and the risk of amputation in patients with diabetic foot infection is 154.5 times higher than in people without infection [17]. One of the common causes of diabetic foot infection is P. aeruginosa, and now, the prevalence of this bacterium in diabetic foot wound infection worldwide is 16.6%.
As mentioned in the results, the prevalence of P. aeruginosa in diabetic foot ulcer infection in Asia varied from 1.63% to 100%. This rate is related to 69 studies included in this review from the Asian continent and in Western countries it varied from 0.5 to 50%, with most of the mentioned studies having reported less than 20%. Also, according to the review, the prevalence of P. aeruginosa in diabetic foot ulcers in Africa varied from 9.4% to 32.2%, which is related to 6 studies, but in Africa, most of the data reported was above 10%, which indicated a higher prevalence of infection in Africa, although the numbers of studies in Africa are lower than in Asia and Western countries, which needs further investigation. In total, the prevalence of P. aeruginosa in diabetic foot ulcer infection in Asia, Africa, and Western countries is 18.5%, 16.3%, and 11.1%, respectively.
The bacteria causing diabetic foot ulcer infection in the studies included may be different, and the reasons for this difference are:
1. Differences in the bacteria causing the infection, 2. Geographical changes, 3. The type and intensity of the infection, and 4. Methods used in the isolation of bacteria [18]. In general, the highest rate of prevalence of P. aeruginosa in diabetic foot ulcer infection was found in Asia, Africa, and, lastly, Western countries.
The most common cause of diabetic foot ulcer infection in European and North American countries is S. aureus, but in Asian, African, and South American countries, Gram-negative bacteria such as P. aeruginosa were common, for example, the prevalence of diabetic foot ulcer infection with S. aureus in Asian countries such as China, India, Thailand, Iran, Turkey, Saudi Arabia, Kuwait, and Malaysia varied from 11.4% to 44% [19], but in studies such as the one conducted by Amanda Thurler Palomo et al. in Brazil, the prevalence of Gram-negative bacilli (88%) was more than that of Gram-positive cocci, with a prevalence of 68% [20]. Also, in other studies included in the current review, such as the studies carried out by Esaki Muthu et al. [21] and Sivanmaliappan et al., [5] both from India,
Alkhudhairy et al. [22] in Iraq, Saltoglu et al. [23] in Turkey, Xiangyan et al. [24] in China, and Agwuet et al. [25] in Nigeria, the predominant microorganism isolated from diabetic foot ulcer infection was P. aeruginosa.
In general, no relationship has been observed between the prevalence of P. aeruginosa in diabetic foot ulcer infection and age. Among patients who have recently been treated with antibiotics or patients whose foot wound infection has become chronic, the infection usually occurs in the form of polymicrobial infection, and so, the simultaneous infection with aerobic Gramnegative bacilli and obligate anaerobic bacteria also occurs [18].
Among Gram-negative bacteria, P. aeruginosa causes important inflammatory responses and mediators that delay wound healing due to the assembly of many virulence contributors like hemolysins, collagenase, protease, and resistance to phagocytosis. Also, P. aeruginosa is potentially dangerous due to its quorum sensing systems, induction of passive resistance, and sharing of metabolic substances and DNA in the wound [18]. One of the other reasons for the lack of healing seen in diabetic foot ulcers is the formation of biofilm by microorganisms. Biofilm reduces local immunity, hinders angiogenesis, creates a barrier between antibiotics and the bacterial environment, causes the colonization with other bacteria in the wound, and postpones wound healing [26].
P. aeruginosa has the ability to form a biofilm in the wound, which makes it stronger and harder to cure; the formation of biofilm in the wound causes the wound to become chronic [27]. The biofilm created by P. aeruginosa creates an inflammatory condition in the wound, subsequently, the host cannot eradicate the biofilm and the healing of the wound is delayed [28].
P. aeruginosa can lead to gangrene in chronic conditions of DFU such as osteomyelitis [29]. New treatment methods include debridement, antibiotic therapy, and hyperbaric oxygen therapy, which have provided hope for treating chronic cases of impaired repair infections [29]. Acyl-homoserine lactones, as a group of quorum sensing molecules, regulate biofilm formation by P. aeruginosa strains. These factors also provide colonization grounds for opportunistic pathogens [29].
In disease conditions, biofilms hinder the process of angiogenesis and delay wound healing, as mentioned above. Multiple drug-resistant microorganisms use these mechanisms to resist different antibiotic classes, including Gramnegative microorganisms such as P. aeruginosa and Klebsiella spp., which have a high tendency to create a strong biofilm [30].
According to the Infectious Diseases Society of America (IDSA), the risk factors considered for P. aeruginosa infection in DFI include a high local prevalence of P. aeruginosa, hot and humid weather, excessive exposure of the foot to water [31], immunocompromised status, failed outpatient DFI antibiotic therapy [32]. Also, Lebowitz et al. [33] and Michael P. Veve et al. [32] reported that patients who had recurrent diabetic foot infections had a higher rate of P. aeruginosa compared to people who did not. Another problem in the treatment of diabetic foot wound infection is the prevalence of multidrug-resistant strains in wounds, including Gram-negative extended spectrum betalactamase-producing bacilli and, especially, multidrug-resistant P. aeruginosa, which makes their treatment more difficult [34].
Repeated exposure to common antibiotics against Pseudomonas causes the emergence of drugresistant strains, so that highly efficient antibiotics against P. aeruginosa are difficult to find today [35]. In this review, 37.9% of P. aeruginosa strains isolated from diabetic foot ulcers were MDR, this rate varied in different parts of the world, and was around 28% in European countries [34], but it varied between 47% and 62% in African and Latin American countries [36].
Investigating the prevalence of MDR P. aeruginosa strains is very important, because these bacteria can be able to transfer resistance genes to other bacteria through mobile genetic elements such as transposons, integrons, and plasmids. The level of resistance in different countries has been reported to be related to the pattern of prescribing antibiotics, the number of foreign devices used in the patient's body, and the care facilities in each country [14]. The occurrence of resistance in P. aeruginosa, especially the formation of resistance to carbapenems, occurs for three main reasons: 1. a defect in the oprD porin, 2. production of the carbapenemase enzyme, 3. overexpression of the efflux pumps [37].
Amputation of the patient's limb is the last option in patients with diabetic foot ulcers when the injured tissue becomes necrotic [38]. The presence of infection caused by multidrug resistant bacteria worsens the situation, and response to single-drug treatments is usually suboptimal. In these cases, combined therapy with proteins, nanoparticles, herbal extracts, or other nutritional substances can be useful to some extent [39]. For more than a decade, there has been no completely efficient treatment for multidrug-resistant strains of P. aeruginosa. Moreover, the gene transfer that takes place during the phenomenon of biofilm formation makes Pseudomonas resistant to different combinations of antibiotics [40].
Improving diabetic foot ulcers through different strategies as well as studying the microbiome in different stages of the disease, both acute and chronic, may provide better knowledge regarding the prescription of antibiotics.
Conclusions
Data have shown that the prevalence of P. aeruginosa, particularly MDR strains, isolated from diabetic foot ulcer infection was relatively high; inherent resistance to antibiotics is also high; the wound either does not heal or if it does, it will be delayed. Therefore, timely treatment is essential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.18683/germs.2023.1406/s1, Table S1: Characteristics of selected studies.
Author Contributions
MG, and SMT conceptualized the study; HM and ZF carried out the search process; AK interpreted the findings; AS edited and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Availability of data
The data supporting the findings are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors would like to acknowledge Research Center on Global Emerging and Re-emerging Infectious Diseases, Institute of Tropical Disease – Universitas Airlangga to facilitate this study.
Conflicts of interest
All authors – none to declare.
References
- Jneid, J.; Lavigne, J.P.; La Scola, B.; Cassir, N. The diabetic foot microbiota: a review. Human Microbiome. Human Microbiome J 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Iraj, B.; Khorvash, F.; Ebneshahidi, A.; Askari, G. Prevention of diabetic foot ulcer. Int J Prev Med. 2013, 4, 373–376. [Google Scholar]
- Son, S.T.; Han, S.-K.; Lee, T.Y.; Namgoong, S.; Dhong, E.-S. The microbiology of diabetic foot infections in Korea. J Wound Manage Res. 2017, 13, 8–12. [Google Scholar] [CrossRef]
- Bush, K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Sivanmaliappan, T.S.; Sevanan, M. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcers. Int J Microbiol. 2011, 2011, 605195. [Google Scholar] [CrossRef]
- Slama, T.G. Gram-negative antibiotic resistance: there is a price to pay. Crit Care. 2008, 12 (Suppl 4), S4. [Google Scholar] [CrossRef]
- Esmaeili, D.; Daymad, S.F.; Neshani, A.; Rashki, S.; Marzhoseyni, Z.; Khaledi, A. Alerting prevalence of MBLs producing Pseudomonas aeruginosa isolates. 2019. Gene Reports. 2019, 16, 100460. [Google Scholar] [CrossRef]
- Hsueh, P.R. Study for monitoring antimicrobial resistance trends (SMART) in the Asia-Pacific region, 2002-2010. Int J Antimicrob Agents. 2012, 40 (Suppl), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, S.; Dorairaj, A.P. Appraisal of biofilm formation in diabetic foot infections by comparing phenotypic methods with the ultrastructural analysis. J Foot Ankle Surg. 2018, 57, 309–315. [Google Scholar] [CrossRef]
- Barrigah-Benissan, K.; Ory, J.; Dunyach-Remy, C.; Pouget, C.; Lavigne, J.P.; Sotto, A. Antibiofilm properties of antiseptic agents used on Pseudomonas aeruginosa isolated from diabetic foot ulcers. Int J Mol Sci. 2022, 23, 11270. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Hoffstad, O. Response to Comment on Hoffstad et al. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015;38:1852–1857. Diabetes Care. 2016, 39, e8. [Google Scholar] [CrossRef][Green Version]
- Karami, P.; Khaledi, A.; Mashoof, R.Y.; et al. The correlation between biofilm formation capability and antibiotic resistance pattern in Pseudomonas aeruginosa. Gene Reports. 2020, 18, 100561. [Google Scholar] [CrossRef]
- Bonfiglio, G.; Carciotto, V.; Russo, G.; et al. Antibiotic resistance in Pseudomonas aeruginosa: an Italian survey. J Antimicrob Chemother. 1998, 41, 307–310. [Google Scholar] [CrossRef][Green Version]
- Hosseini, S.M.; Naeini, N.S.; Khaledi, A.; Daymad, S.F.; Esmaeili, D. Evaluate the relationship between class 1 integrons and drug resistance genes in clinical isolates of Pseudomonas aeruginosa. Open Microbiol J. 2016, 10, 188–196. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet. 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.J.; Lavery, L.A.; Armstrong, D.G. Diabetic lower extremity infection: influence of physical, psychological, and social factors. J Diabetes Complications. 2005, 19, 107–112. [Google Scholar] [CrossRef]
- Roberts, A.D.; Simon, G.L. Diabetic foot infections: the role of microbiology and antibiotic treatment. Semin Vasc Surg; 2012, 25, 75–81. [Google Scholar] [CrossRef]
- Dunyach-Remy, C.; Ngba Essebe, C.; Sotto, A.; Lavigne, J.P. Staphylococcus aureus toxins and diabetic foot ulcers: role in pathogenesis and interest in diagnosis. Toxins 2016, 8, 209. [Google Scholar] [CrossRef]
- Palomo, A.T.; Pires, A.P.M.; Matielo, M.F.; et al. Microbiology of diabetic foot infections in a tertiary care hospital in São Paulo, Brazil. Antibiotics 2022, 11, 1125. [Google Scholar] [CrossRef]
- Muthu, S.E.; Aberna, R.A.; Mohan, V.; et al. Phenotypes of isolates of Pseudomonas aeruginosa in a diabetes care center. Arch Med Res. 2006, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Khudhairy, M.K.; Al-Shammari, M.M.M. Prevalence of metallo-β-lactamase–producing Pseudomonas aeruginosa isolated from diabetic foot infections in Iraq. New Microbes New Infect. 2020, 35, 100661. [Google Scholar] [CrossRef] [PubMed]
- Saltoglu, N.; Ergonul, O.; Tulek, N.; et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int J Infect Dis. 2018, 70, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, X.; Yuan, G.; et al. Microbiological profile and clinical characteristics of diabetic foot infection in northern China: a retrospective multicentre survey in the Beijing area. J Med Microbiol. 2018, 67, 160–168. [Google Scholar] [CrossRef]
- Agwu, E.; Ihongbe, J.; Inyang, N. Prevalence of quinolonesusceptible Pseudomonas aeruginosa and Staphylococcus aureus in delayed-healing diabetic foot ulcers in Ekpoma, Nigeria. Wounds 2010, 22, 100–105. [Google Scholar]
- Ul Hassan, F.; Qudus, M.S.; Sehgal, S.A.; et al. Prevalence of extended-spectrum β-lactamases in multi-drug resistant Pseudomonas aeruginosa from diabetic foot patients. Endocr Metab Immune Disord Drug Targets. 2019, 19, 443–448. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Moser, C.; Jensen, P.Ø.; Thomsen, K.; et al. Immune responses to Pseudomonas aeruginosa biofilm infections. Front Immunol. 2021, 12, 625597. [Google Scholar] [CrossRef]
- Srivastava, P.; Sivashanmugam, K. Combinatorial drug therapy for controlling Pseudomonas aeruginosa and its association with chronic condition of diabetic foot ulcer. Int J Low Extrem Wounds. 2020, 19, 7–20. [Google Scholar] [CrossRef]
- Hwang, I.S.; Hwang, J.H.; Choi, H.; Kim, K.J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Veve, M.P.; Mercuro, N.J.; Sangiovanni, R.J.; Santarossa, M.; Patel, N. Prevalence and predictors of Pseudomonas aeruginosa among hospitalized patients with diabetic foot infections. Open Forum Infect Dis. 2022, 9, ofac297. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, D.; Gariani, K.; Kressmann, B.; et al. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int J Infect Dis. 2017, 59, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and characteristics of metallo-β-lactamaseproducing Pseudomonas aeruginosa. Infect Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef]
- Gasink, L.B.; Fishman, N.O.; Weiner, M.G.; Nachamkin, I.; Bilker, W.B.; Lautenbach, E. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med. 2006, 119, 526. [Google Scholar] [CrossRef]
- Akya, A.; Salimi, A.; Nomanpour, B.; Ahmadi, K. Prevalence and clonal dissemination of metallo-beta-lactamaseproducing Pseudomonas aeruginosa in Kermanshah. Jundishapur J Microbiol. 2015, 8, e20980. [Google Scholar] [CrossRef]
- Quale, J.; Bratu, S.; Gupta, J.; Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006, 50, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Baby, S.; George, V. Essential oils and new antimicrobial strategies. In New strategies. In New strategies combating bacterial infection; Ahmad, I., Aqil, F., Eds.; Wiley-VCH: Weinheim, 2008; pp. 165–203. [Google Scholar] [CrossRef]
- Rahim, K.; Qasim, M.; Rahman, H.; et al. Antimicrobial resistance among aerobic biofilm producing bacteria isolated from chronic wounds in the tertiary care hospitals of Peshawar, Pakistan. J Wound Care. 2016, 25, 480–486. [Google Scholar] [CrossRef]
© GERMS 2023.