Fosfomycin: A Potential Oral Option for Treatment of Urinary Tract Infections in Sri Lanka in the Context of High Antibiotic Resistance
Abstract
Introduction
Methods
Antimicrobial susceptibility testing (AST)
Laboratory data
Statistical analysis
Ethical considerations
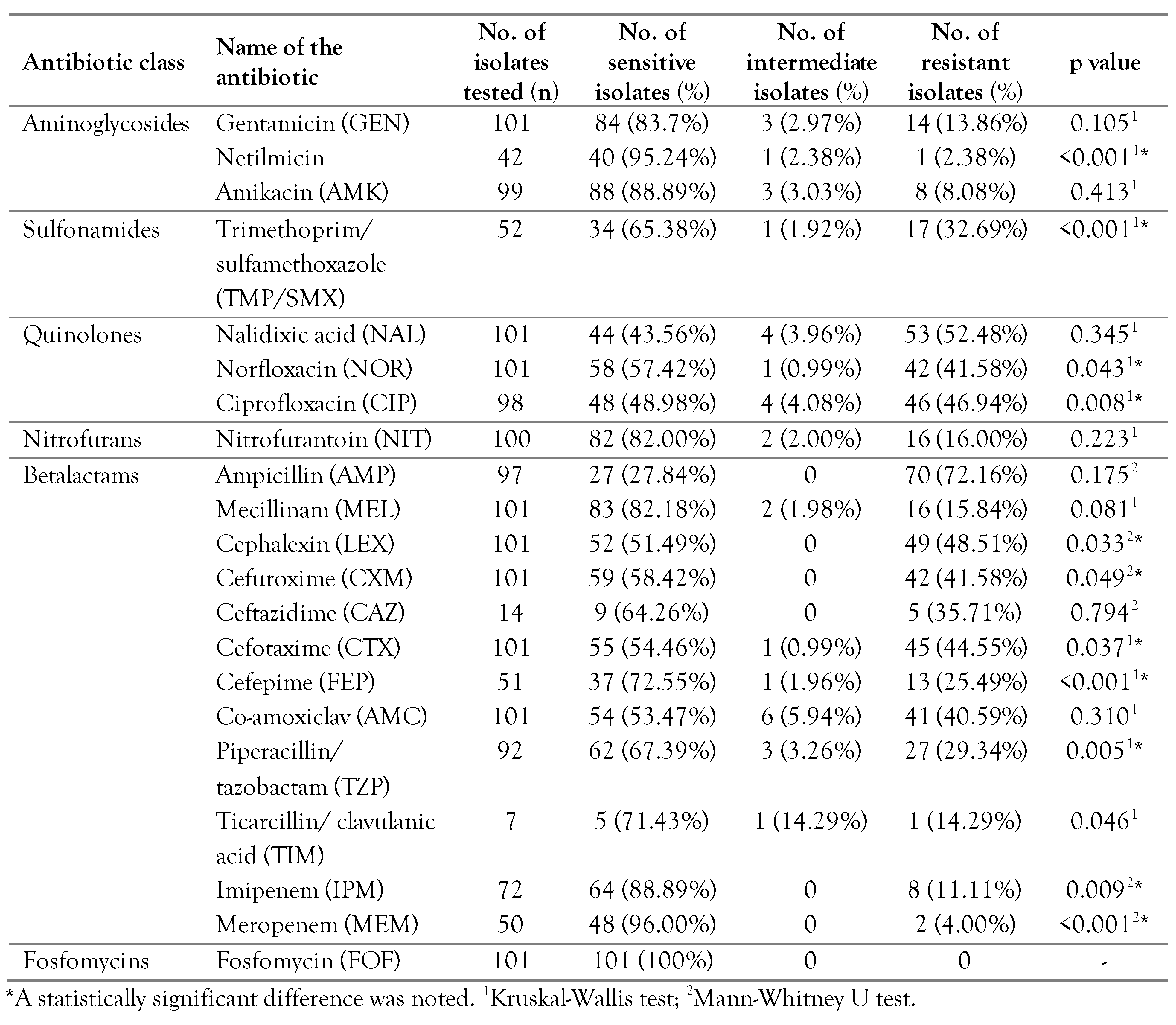
Results
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ani, O.C.; Mgbechi, E.K. Prevalence of urinary tract infections (UTI) in sexually active women of Abakaliki, Ebonyi State, Nigeria. Anim. Res. Int. 2008, 5, 876–879. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Odoki, M.; Almustapha Aliero, A.; Tibyangye, J.; et al. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi District, Uganda. Int. J. Microbiol. 2019, 2019, 4246780. [Google Scholar] [CrossRef] [PubMed]
- Sime, W.T.; Biazin, H.; Zeleke, T.A.; Desalegn, Z. Urinary tract infection in cancer patients and antimicrobial susceptibility of isolates in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS ONE. 2020, 15, e0243474. [Google Scholar] [CrossRef] [PubMed]
- Opatowski, M.; Brun-Buisson, C.; Touat, M.; et al. Antibiotic prescriptions and risk factors for antimicrobial resistance in patients hospitalized with urinary tract infection: A matched case-control study using the French health insurance database (SNDS). BMC Infect. Dis. 2021, 21, 571. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshana, U.; Piyasiri, L.B.; Wijesinghe, C. Prevalence, antibiotic sensitivity pattern and genetic analysis of extended spectrum beta lactamase producing Escherichia coli and Klebsiella spp among patients with community acquired urinary tract infection in Galle district, Sri Lanka. Ceylon Med. J. 2019, 64, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.M.B.T.; Fernando, S.S.N.; Chandrasiri, N.S. The distribution and characteristics of extendedspectrum β-Lactamase producing Escherichia coli and Klebsiella species among urinary isolates in a tertiary care hospital. Sri Lankan J. Infect. Dis. 2012, 2, 30–36. [Google Scholar] [CrossRef]
- Gopichand, P.; Agarwal, G.; Natarajan, M.; et al. In vitro effect of fosfomycin on multi-drug resistant gramnegative bacteria causing urinary tract infections. Infect. Drug Resist. 2019, 12, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.C.; Barrett, L.K.; Warren, S.; et al. Oral fosfomycin for treatment of urinary tract infection: A retrospective cohort study. BMC Infect. Dis. 2016, 16, 556. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Scalia, G.; Stefani, S.; Mezzatesta, M.L. In vitro fosfomycin study on concordance of susceptibility testing methods against ESBL and carbapenem-resistant Enterobacteriaceae. J. Glob. Antimicrob. Resist. 2020, 23, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Aris, P.; Boroumand, M.A.; Rahbar, M.; Douraghi, M. The activity of fosfomycin against extended-spectrum betalactamase-producing isolates of Enterobacteriaceae recovered from urinary tract infections: A single-center study over a period of 12 years. Microb. Drug Resist. 2018, 24, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Centers for Disease Control and Prevention. 2022. Glossary of terms related to antibiotic resistance. Available online: https://www.cdc.gov/narms/resources/glossary.html (accessed on day month year).
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, C.N.; Dassanayake, K.M.M.P.; Pathmeswaran, A. Antimicrobial susceptibility patterns and empirical prescribing practices in adult in patients with urinary tract infection: Is there a need for changing clinical practices? Sri Lankan J. Infect. Dis. 2014, 4, 9–21. [Google Scholar] [CrossRef]
- Kanda, N.; Hashimoto, H.; Sonoo, T.; et al. Gram-negative organisms from patients with community-acquired urinary tract infections and associated risk factors for antimicrobial resistance: A single-center retrospective observational study in Japan. Antibiotics. 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2017, 129, 24258. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.H.; Saleh, S.E.; Hamed, S.M.; Aboshanab, K.M. Febrile illness of bacterial etiology in a public fever hospital in Egypt: High burden of multidrug resistance and WHO priority Gram negative pathogens. Germs 2022, 12, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Norafika Arbianti, N.; Prihatiningsih, S.; Indriani, D.W.; Indriati, D.W. A retrospective cross-sectional study of urinary tract infections and prevalence of antibiotic resistant pathogens in patients with diabetes mellitus from a public hospital in Surabaya, Indonesia. Germs. 2020, 10, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rosso-Fernández, C.; Sojo-Dorado, J.; Barriga, A.; et al. Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum βlactamase-producing Escherichia coli (FOREST): Study protocol for an investigator-driven randomised controlled trial. BMJ Open. 2015, 5, e007363. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens. 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
 |
© GERMS 2023.
Share and Cite
Jayathilaka, N.; Pathirana, T.; Kumari, C.; Navaratne, V.; Gunasekara, S.; Nakkawita, D.; Senaratne, T. Fosfomycin: A Potential Oral Option for Treatment of Urinary Tract Infections in Sri Lanka in the Context of High Antibiotic Resistance. GERMS 2023, 13, 314-320. https://doi.org/10.18683/germs.2023.1400
Jayathilaka N, Pathirana T, Kumari C, Navaratne V, Gunasekara S, Nakkawita D, Senaratne T. Fosfomycin: A Potential Oral Option for Treatment of Urinary Tract Infections in Sri Lanka in the Context of High Antibiotic Resistance. GERMS. 2023; 13(4):314-320. https://doi.org/10.18683/germs.2023.1400
Chicago/Turabian StyleJayathilaka, Nishadi, Tharushi Pathirana, Chathurika Kumari, Varuna Navaratne, Samanmalee Gunasekara, Dilini Nakkawita, and Thamarasi Senaratne. 2023. "Fosfomycin: A Potential Oral Option for Treatment of Urinary Tract Infections in Sri Lanka in the Context of High Antibiotic Resistance" GERMS 13, no. 4: 314-320. https://doi.org/10.18683/germs.2023.1400
APA StyleJayathilaka, N., Pathirana, T., Kumari, C., Navaratne, V., Gunasekara, S., Nakkawita, D., & Senaratne, T. (2023). Fosfomycin: A Potential Oral Option for Treatment of Urinary Tract Infections in Sri Lanka in the Context of High Antibiotic Resistance. GERMS, 13(4), 314-320. https://doi.org/10.18683/germs.2023.1400




