Introduction
Actinotignum schaalii is a small Gram-positive facultative anaerobic coccoid rod that was reclassified in 1997 from the genus
Actinomyces [
1]. It is considered to be a frequent commensal microorganism that colonizes the skin and mucosa of the urinary and genital tract, but not the colon [
2]. Although
A. schaalii can be detected on Gram stain as a coccoid rod, it is difficult to culture with usual microbiological techniques and slow-growing. To isolate this microorganism, cultures should be incubated for 48 to 72 hours on a blood agar plate in anaerobic conditions or in CO
2-enriched conditions [
3]. Furthermore, reliable identification of
A. schaalii requires the use of 16S rRNA sequencing or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) [
4,
5,
6,
7].
A. schaalii is an emerging human pathogen that is most commonly implicated in urinary tract infections (UTIs) [
8,
9,
10], but has also been isolated less frequently from abscesses of various sites (such as the skin, the genitourinary tract, surgical sites or intraabdominal) [
4,
8,
9,
11,
12,
13,
14,
15]. However,
A. schaalii is probably under-reported considering the difficulties in isolation and identification discussed above.
Herein, we report two cases of diabetic foot osteomyelitis involving
A. schaalii (isolated from bone cultures) with this study being the second report of
A. schaalii osteomyelitis [
16]. Furthermore, all
A. schaalii strains cultured from patient samples in the Microbiology Laboratory of the University Hospital of Heraklion (Crete, Greece) were retrospectively reviewed.
Methods
All patients’ data from whom A. schaalii strains were isolated in the University Hospital of Heraklion were retrospectively reviewed. The following data were collected: site of isolation, co-isolates in polymicrobial cultures, and patient characteristics (age, gender, site of infection).
Gram staining was performed in all samples.
A. schaalii strains grew on Columbia sheep blood agar and chocolate agar with polyvitex incubated in 5% CO
2 at 36°C and on Schaedler agar with 5% sheep blood at 36°C after 48 h of incubation in an anaerobic atmosphere (all products of bioMérieux, France). Identification of the isolates was performed by MALDI–TOF-MS (bioMérieux, database version 3.2) (confidence level of 99.9%). The antimicrobial susceptibility was determined with the gradient diffusion method using E-test strips (bioMérieux), according to the manufacturer’s recommendations. Although there are not yet established guidelines regarding the antimicrobial susceptibility testing for
A. schaalii, the E-test method appears to be reliable [
8,
17]. The following antimicrobials were tested: penicillin, ampicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam, cefoxitin, imipenem, meropenem, erythromycin, clindamycin, rifampicin, moxifloxacin, metronidazole, chloramphenicol, tetracycline, tigecycline, and vancomycin. Isolates were suspended in Brucella broth (Becton Dickinson, USA), to match a turbidity equivalent to that of a 1.0 McFarland standard and then inoculated onto Brucella agar supplemented with 5% sheep blood, 5 mg/L of hemin and 1 mg/L of vitamin K1 (bioMérieux). Plates were incubated at 37°C for 48 hours in anaerobiosis.
Bacteroides fragilis ATCC 25285, and
Bacteroides thetaiotaomicron ATCC 29741 were used as quality control strains. The minimum inhibitory concentration (MIC) values were interpreted according to the CLSI guidelines [
18]. For tigecycline the U.S. Food and Drug Administration (FDA)-recommended MIC breakpoints were applied (susceptible ≤4 mcg/mL, resistant ≥16 mcg/mL) [
19].
Results
During a 6-year period (January 2016 – January 2022),
A. schaalii was isolated in 11 cultures from 10 patients (
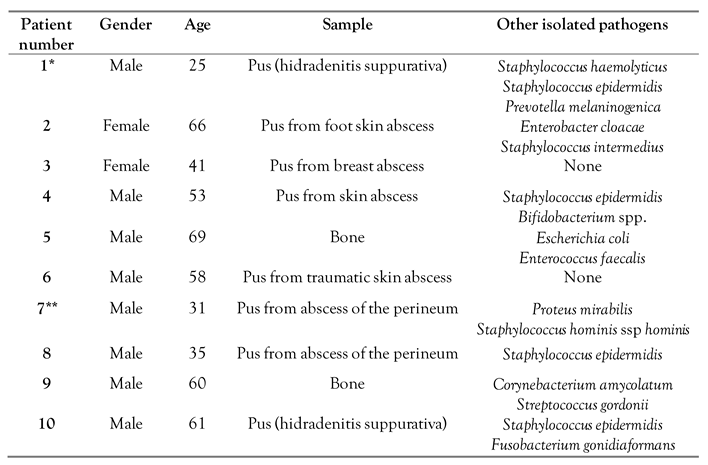
Table 1). The site of infection was skin and soft tissue in nine out of eleven cultures (81.8%) and bone was the site of infection in two patients (18.2%). Most (72.7%, n=8) cultures were polymicrobial, the most common co-isolate being coagulase-negative staphylococci (6 of 8 polymicrobial cultures).
A. schaalii was isolated from pus culture in most cases (n=8) and from bone culture in two patients. The median age of affected patients was 55.5 years (interquartile range 34-63 years), and 80% (n=8) were male.
The two cases of diabetic foot osteomyelitis are presented in more detail below. Case 1 (patient 5), a 69-year-old man with a past medical history of coronary artery disease with a coronary artery bypass graft, hypertension, dyslipidemia, type 2 diabetes mellitus (T2DM), active smoking and peripheral artery disease for which he had undergone an angioplasty of the right common and the external iliac artery and the common femoral artery, was admitted due to ischemia of the left lower limb. He underwent an angioplasty at several levels of the superficial femoral artery and the left popliteal artery. However, two transmetatarsal amputations were required and a negative pressure drainage system was placed in order to facilitate recovery. A Gram stain from material of the bones from the removed digits that were the source of infection in this patient revealed a Gram-positive coccus, a Gram-positive rod and a Gram-negative rod, while the culture yielded an extended spectrum beta-lactamase producing Escherichia coli, an ampicillin susceptible Enterococcus faecalis and A. schaalii. Antimicrobial treatment was changed from ciprofloxacin and clindamycin that had been administered empirically, to tigecycline, as E. coli and E. faecalis were resistant to the empirical antimicrobial regimen, while all microorganisms were susceptible to tigecycline and the patient improved slowly. Repeated surgical debridement was performed and the surgical site slowly showed signs of adequate healing. He was discharged in an improved condition after four months of hospital stay.
Case 2 (patient 9), a 60-year-old man with a medical history of T2DM and peripheral artery disease that had required digital amputation at the left foot, was admitted due to acute digital ischemia at the 4th digit of the right foot. He underwent open digital amputation of the 4th and 5th digit of the right foot and was treated with a negative pressure drainage system; however, he required a transmetatarsal amputation of the 4th and 5th digits of the right foot. A Gram-stain from material of the bones of the excised digits, which were the source of infection in this patient, revealed Gram-positive cocci and Gram-positive rods, while the culture yielded Corynebacterium amycolatum, Streptococcus gordonii and A. schaalii. The patient was treated with daptomycin and piperacillin/tazobactam, since all microorganisms were susceptible to this antimicrobial combination, and the patient showed significant improvement. After the surgical site healed adequately, he was discharged after 20 days of hospital stay on amoxicillin/clavulanate with a plan to perform a magnetic imaging resonance exam in order to rule out osteomyelitis at the remaining foot.
Table 1 shows the characteristics of the patients with infection by
A. schaalii and
Table 2 shows the results of the antimicrobial susceptibility testing.
Discussion
Herein, we report ten cases where A. schaalii was identified, and describe two cases of diabetic patients diagnosed with polymicrobial diabetic foot osteomyelitis from this organism. Both patients were successfully managed with targeted antimicrobial treatment and surgical management.
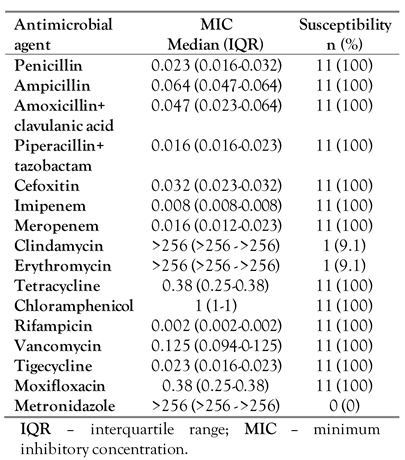
All isolates were uniformly susceptible to all beta-lactams tested, rifampicin, moxifloxacin, chloramphenicol, tetracycline, tigecycline, and vancomycin, and resistant to clindamycin, metronidazole and erythromycin. These findings are in agreement with prior reports according to which
A. schaalii appears to be susceptible to almost all beta-lactams, tetracyclines, linezolid and rifampicin [
11,
12,
14,
20]. Resistance has been described to erythromycin, sulfamethoxazole/trimethoprim, clindamycin, norfloxacin and ciprofloxacin, while most strains seem to be susceptible to levofloxacin and moxifloxacin [
11,
12,
14,
15,
20,
21]. More specifically, herein, we found resistance of
A. schaalii to clindamycin and erythromycin to be around 91%, which is in contrast to the literature, thus implying that treatment of this microorganism with these agents should be avoided, unless previous confirmation of its susceptibility has been performed [
21]. Resistance to colistin and metronidazole is almost universal among
A. schaalii strains [
20]. In our cases, tigecycline was used in one patient, while the other patient was treated with beta-lactam agents and they both improved slowly but required surgical intervention as well.
A. schaalii has more commonly been described as a cause of UTIs [
8,
9,
10]. There are reports, though, where it has been isolated in various other sites (such as the skin, surgical sites, bone or intraabdominal) [
4,
8,
9,
12,
13,
14,
15,
16]. A recently published study described seven patients that developed bacteremia by
A. schaalii, most commonly in the context of a urinary tract infection in patients who had been diagnosed with a urological condition, however, two patients had developed Fournier’s gangrene and pyometra [
22]. Interestingly,
A. schaalii has been identified again in cases of Fournier’s gangrene [
23,
24], while other reports also implicate it as a skin pathogen [
9]. In the present study,
A. schaalii was isolated from pus drained from skin and soft tissues in eight out of ten patients, thus confirming its potential as a putative pathogen of skin and soft tissues. However,
Actinotignum spp. was not isolated from the urinary tract of any patient herein. This could be associated with differences in culturing technique among different hospitals, leading to underrepresentation of
Actinotignum in urinary samples in this study. On the other hand, the rarity of this pathogen could imply the possibility of underrepresentation of the types of infections identified in the present study in the published literature so far.
There are scarce data regarding virulence factors of
A. schaalii in the literature, however, a recent genomic study identified that
A. schaalii expresses several virulence factors that allow this pathogen to colonize and evade host tissues, such as fimbrial and adhesion proteins, sialidase and NlpC-P60 protein and heat-shock proteins [
25].
Conclusions
In conclusion, A. schaalii is an emerging pathogen that is likely under-reported due to difficulties in isolation and identification. A. schaalii is susceptible to a variety of antibiotic classes (including beta-lactams, tetracyclines, vancomycin, linezolid, rifampicin and selected fluoroquinolones) but highly resistant to metronidazole and resistant to erythromycin and clindamycin. Herein, two cases of diabetic foot osteomyelitis are reported, successfully managed with targeted antimicrobial therapy and surgical debridement.
Author Contributions
Conceptualization: PI, SM. Data curation: PI, SK, SM. Formal analysis: PI, SM. Investigation: PI, SK, SM, ET, NK. Methodology: PI, SM. Project administration: DPK. Resources: GC, DPK. Software: PI. Supervision: DPK. Validation: DPK. Visualization: PI. Roles/Writing - original draft: PI, SM. Writing - review & editing: SK, ET, NK, GC, DPK.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of University Hospital of Heraklion (protocol code 08/24-03-2021).
Informed Consent Statement
Written informed consent was obtained from the patients for publication of their case report.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors – none to declare.
References
- Lawson, P.A.; Falsen, E.; Akervall, E.; Vandamme, P.; Collins, M.D. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997, 47, 899–903. [Google Scholar] [CrossRef]
- Könönen, E.; Wade, W.G. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015, 28, 419–442. [Google Scholar] [CrossRef]
- Bank, S.; Cattoir, V.; Lienhard, R.; et al. Recommendations for optimal detection and identification of Actinobaculum schaalii in urine. APMIS. 2014, 122, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Bing, A.U.; Loh, S.F.; Morris, T.; Hughes, H.; Dixon, J.M.; Helgason, K.O. Actinomyces species isolated from breast infections. J Clin Microbiol. 2015, 53, 3247–3255. [Google Scholar] [CrossRef]
- Tuuminen, T.; Suomala, P.; Harju, I. Actinobaculum schaalii: identification with MALDI-TOF. New Microbes New Infect. 2014, 2, 38–41. [Google Scholar] [CrossRef]
- Prigent, G.; Perillaud, C.; Amara, M.; Coutard, A.; Blanc, C.; Pangon, B. Actinobaculum schaalii: a truly emerging pathogen?: Actinobaculum schaalii: un pathogène réellement émergent? New Microbes New Infect. 2016, 11, 8–16. [Google Scholar] [CrossRef]
- Lotte, L.; Lotte, R.; Durand, M.; et al. Infections related to Actinotignum schaalii (formerly Actinobaculum schaalii): a 3-year prospective observational study on 50 cases. Clin Microbiol Infect. 2016, 22, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Lotte, R.; Lotte, L.; Ruimy, R. Actinotignum schaalii (formerly Actinobaculum schaalii): a newly recognized pathogen-review of the literature. Clin Microbiol Infect. 2016, 22, 28–36. [Google Scholar] [CrossRef]
- Maraki, S.; Evangelou, G.; Stafylaki, D.; Scoulica, E. Actinotignum schaalii subcutaneous abscesses in a patient with hidradenitis suppurativa: Case report and literature review. Anaerobe. 2017, 43, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, C.M.; Gupta, S. Actinotignum schaalii abscess in a patient with common variable immunodeficiency. Pathogens. 2020, 9, 494. [Google Scholar] [CrossRef]
- Nielsen, H.L.; Søby, K.M.; Christensen, J.J.; Prag, J. Actinobaculum schaalii: a common cause of urinary tract infection in the elderly population. Bacteriological and clinical characteristics. Scand J Infect Dis. 2010, 42, 43–47. [Google Scholar] [CrossRef]
- Beguelin, C.; Genne, D.; Varca, A.; et al. Actinobaculum schaalii: clinical observation of 20 cases. Clin Microbiol Infect. 2011, 17, 1027–1031. [Google Scholar] [CrossRef]
- Tschudin-Sutter, S.; Frei, R.; Weisser, M.; Goldenberger, D.; Widmer, A.F. Actinobaculum schaalii - invasive pathogen or innocent bystander? A retrospective observational study. BMC Infect Dis. 2011, 11, 289. [Google Scholar] [CrossRef]
- Reinhard, M.; Prag, J.; Kemp, M.; et al. Ten cases of Actinobaculum schaalii infection: clinical relevance, bacterial identification, and antibiotic susceptibility. J Clin Microbiol. 2005, 43, 5305–5308. [Google Scholar] [CrossRef]
- Tena, D.; Fernández, C.; Lago, M.R.; Arias, M.; Medina, M.J.; Sáez-Nieto, J.A. Skin and soft-tissue infections caused by Actinobaculum schaalii: report of two cases and literature review. Anaerobe. 2014, 28, 95–97. [Google Scholar] [CrossRef]
- Haller, P.; Bruderer, T.; Schaeren, S.; et al. Vertebral osteomyelitis caused by Actinobaculum schaalii: a difficult-to-diagnose and potentially invasive uropathogen. Eur J Clin Microbiol Infect Dis. 2007, 26, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. Actinobaculum schaalii: review of an emerging uropathogen. J Infect. 2012, 64, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Food and Drug Administration (FDA). 2010. Prescribing information for Tygacil (tigecycline). 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021821s021lbl.pdf (accessed on 9 February 2022).
- Cattoir, V.; Varca, A.; Greub, G.; Prod'hom, G.; Legrand, P.; Lienhard, R. In vitro susceptibility of Actinobaculum schaalii to 12 antimicrobial agents and molecular analysis of fluoroquinolone resistance. J Antimicrob Chemother. 2010, 65, 2514–2517. [Google Scholar] [CrossRef]
- Barberis, C.; Budia, M.; Palombarani, S.; et al. Antimicrobial susceptibility of clinical isolates of Actinomyces and related genera reveals an unusual clindamycin resistance among Actinomyces urogenitalis strains. J Glob Antimicrob Resist. 2017, 8, 115–120. [Google Scholar] [CrossRef]
- Nakaoka, Y.; Kitagawa, H.; Kitano, H.; et al. Clinical characteristics of Actinotignum schaalii bacteremia in a Japanese tertiary hospital. Anaerobe. 2022, 102513. [Google Scholar] [CrossRef] [PubMed]
- Sahra, S.; Jahangir, A.; Kandlakunta, H.; Glaser, A. Actinotignum schaalii caught for the second time in Fournier's gangrene! Cureus. 2021, 13, e13288. [Google Scholar] [CrossRef] [PubMed]
- Kus, N.J.; Kim, B.J.; Ross, H.M. A case report of necrotizing fasciitis with growth of Actinomyces europaeus and Actinotignum schaalii. J Surg Case Rep. 2019, 2019, rjz286. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.F.; Langenberg, S.; Huntemann, M.; et al. Draft genome sequence of Actinotignum schaalii DSM 15541T: genetic insights into the lifestyle, cell fitness and virulence. PLoS One. 2017, 12, e0188914. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Characteristics of patients and cultures where Actinotignum spp. were isolated.
Table 1.
Characteristics of patients and cultures where Actinotignum spp. were isolated.
Table 2.
Antimicrobial susceptibility of 11 A. schaalii strains isolated from 10 patients.
Table 2.
Antimicrobial susceptibility of 11 A. schaalii strains isolated from 10 patients.