Case report
A 42-year-old mentally ill woman was admitted to University Hospital of Heraklion, Crete, Greece due to low grade fever, weight loss and chronic diarrhea.
The patient had been generally well until approximately 6 years before this admission, when diarrhea with up to 4–5 stools/day without mucus or blood, arthralgia, weakness, anorexia with significant weight loss of almost 20 kg developed. The patient was repeatedly evaluated by several specialists in the past 6 years, the first of whom was a rheumatologist who misdiagnosed a seronegative arthritis and commenced the patient on p.o. prednisolone 20 mg once daily for 20 days resulting in a remarkable deterioration of the arthralgia and subsequent discontinuation.
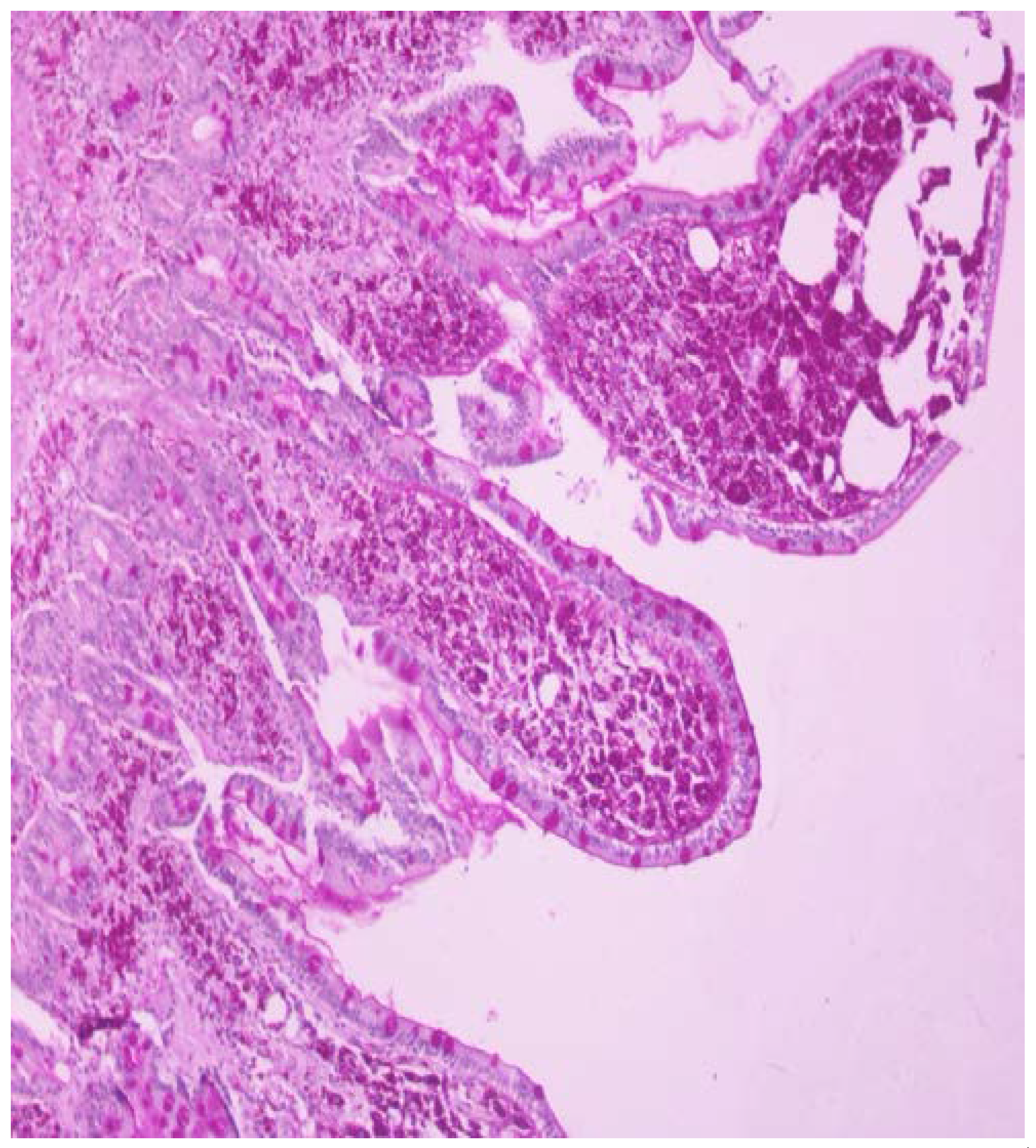
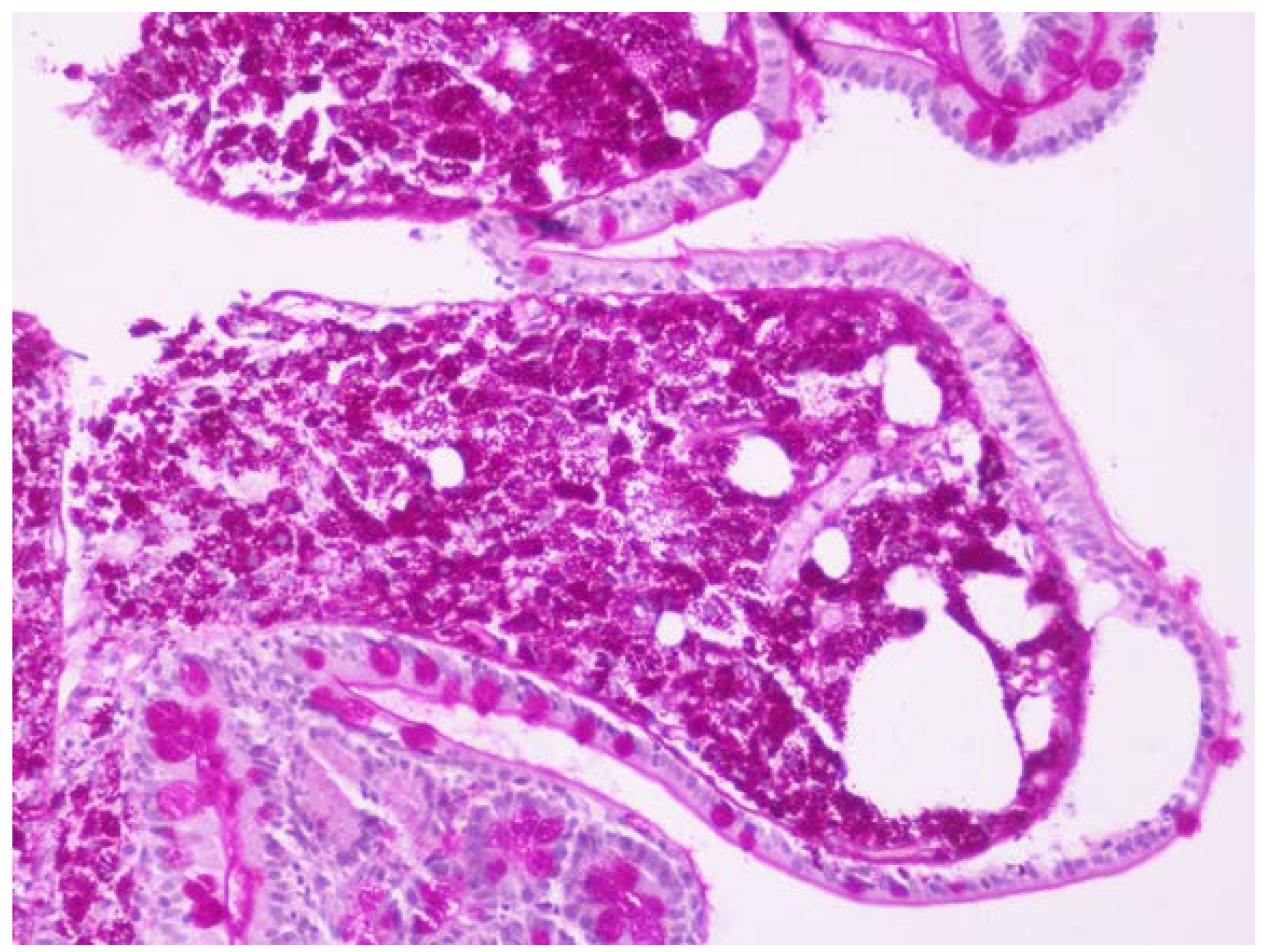
One month before admission the patient was referred to our endoscopy department due to chronic diarrhea and weight loss as an outpatient and she underwent gastroscopy with 2nd portion of the duodenum biopsies. One week before admission she was seen by her physician in the local hospital for low grade fever, weight loss and chronic diarrhea and she was referred to this hospital. Based on the initial history and findings a malabsorption syndrome was suspected. The slides were stained with hematoxylin and eosin (HE) and PAS and the biopsies revealed flattened villi, dense infiltrate of foamy macrophages with frequent PAS-positive bacilli within them, so Whipple’s disease diagnosis was suspected (
Figure 1 and
Figure 2).
Her previous medical history included epilepsy and total hysterectomy due to uterine fibroids. The patient was mentally ill, something already known since her childhood. There were no syndromic facial features but her cognitive function was not normal. Her mental status had been attributed to a specific mutation detected in the karyotype, DEL 13q32.3q53kb. No drinking alcohol and smoking was referred and she did not have any significant family medical history.
Upon admission she appeared underweighted, acutely ill, confused, with tachypnea. The temperature was 39 °C, the blood pressure 80/50 mmHg, the pulse 105 beats per minute, the respiratory rate was 20 breaths per minute and oxygen saturation was 96% while the patient was breathing ambient air.
Physical examination revealed lymphadenopathy and ascites. Lung auscultation revealed rales mainly in the right lung. The neurological examination was normal.
The chest X-ray showed linear atelectasis while the abdominal X-ray showed remarkable intestinal distention. Upon admission the laboratory tests showed anemia (Hb 8.5 g/dL, HCT 27.3%, MCV 69.3 fL, ferritin 874.52 ng/mL, folic acid 10.3 ng/mL, B12 280 pg/mL), thrombocytopenia (PLT 133 K/μL), and low white blood cell count (WBCs 2.5 K/μL), along with low sodium (Na 131 mEq/L), low albumin 2.6 g/dL and increased level of C-reactive protein (CRP 9.18 mg/dL).
Blood, urine, and sputum specimens were taken for culture and she was started on antimicrobials empirically (i.v. meropenem 1g TID. and i.v. vancomycin 1 g BID) and appropriate fluids for hydration. Stool culture for common bacteria, Clostridioides difficile and parasites was negative. Furthermore, blood and urine cultures were negative as well.
A lumbar puncture was performed and the neurologic involvement was excluded since PCR and PAS staining of cerebrospinal fluid was negative. The transthoracic echocardiogram showed ejection fraction (EF) 60%, dilation of right atrium, pulmonary artery systolic pressure (PASP) 55 mmHg and small pericardial effusion. Mantoux test was negative.
The computed tomography (CT) scan of the cervical spine and neck showed lymphadenopathy, whereas the CT of the thorax showed lymphadenopathy, bilateral pleural effusion of small volume and a small pericardial effusion. The abdominal CT showed large volume ascites, lymphadenopathy, edema of the 3rd and 4th portion of the duodenum and the proximal part of the jejunum whereas there were multiple hypodense lesions in the liver. The MRI of the abdomen clarified that these focal lesions of the liver were accumulations of fat. The central nervous system MRI revealed no abnormalities. A pill cam-endoscopy was performed revealing villous atrophy and lymphangiectasia of the duodenum and jejunum.
In the next 7 days after starting treatment the patient showed remarkable clinical improvement. However, on day 8 of i.v. antimicrobial treatment (meropenem 1 g TID and vancomycin 1 g BID) the patient developed dry cough and dyspnea and became febrile again. Meropenem and vancomycin were discontinued and the patient was commenced empirically on i.v. tigecycline 100 mg BID based on the fact that tigecycline is a tetracycline derivative antibiotic, not only effective on intracellular pathogens like Tropheryma whipplei but with a broad spectrum as well. Clinical improvement accompanied by CRP and WBC normalization was observed on day 5 of i.v. tigecycline treatment. However, a worsening of ascitic fluid with a serum ascites albumin gradient (SAAG) 2.6 and negative culture was observed. Inguinal lymph node biopsy and biopsies from the second portion of the duodenum were both positive for PAS stain, while PCR confirmed the Tropheryma whipplei infection. Establishing the diagnosis and after two weeks of tigecycline treatment, the parenteral antibiotic was discontinued.
The patient was commenced then on maintenance therapy with trimethoprim sulfamethoxazole (TMP-SMX) one double strength tablet every 12 h orally. Three days later fever and inflammatory syndrome relapsed and i.v. corticosteroids were added in the patient’s treatment as an immune reconstitution inflammatory syndrome (IRIS) was suspected. Nevertheless, the patient did not improve, after 2 days on corticosteroids and 5 days in total on p.o. TMP-SMX, until when she was commenced again on parenteral tigecycline 100 mg BID. Furthermore, tissue biopsies from the 2nd portion of the duodenum were obtained and resistance to TMP-SMX was established by detecting a mutation in the position N4S of the gene of dihydropteroate synthase (DHPS), the target of SMX.
After 21 days of parenteral tigecycline treatment and after clinical and laboratory improvement was obtained, the patient was commenced on maintenance treatment with doxycycline 100 mg BID orally. However, 2 days later a second relapse of the fever and inflammatory syndrome occurred and the patient was commenced once again on parenteral tigecycline 100 mg BID.
After 45 days of parenteral tigecycline and a total of almost 3 months of parenteral treatment with tigecycline 100 mg BID, the patient was commenced on maintenance therapy with doxycycline 100 mg BID, along with hydroxychloroquine 200 mg TID, and finally remission of the disease was maintained at this time. In the follow up visit six months later the patient underwent a new transthoracic echocardiogram that showed ejection fraction (EF) 60%, normal right atrium, pulmonary artery systolic pressure (PASP) 35 mmHg and absence of pericardial effusion. One year after the initiation of the oral doxycycline 100 mg BID along with hydroxychloroquine 200 mg TID the patient is free of symptoms, her present weight is 70 kg but still the 2nd duodenum portion biopsies reveal infiltrate of foamy macrophages with frequent PAS-positive bacilli in the cytoplasm.
Discussion
Whipple’s disease is a rare, systemic illness and its most prominent symptoms are weight loss, diarrhea, and arthralgia. It is caused by the actinomycete
Tropheryma whipplei, a Gram-positive bacillus. The initial name
Tropheryma whippelii is derived from the Greek words “trophe” for nourishment and “eryma” for barrier, and “whippelii” in honor of George Whipple [
1].
Classic Whipple’s disease is rare and is found in middle-aged individuals, approximately three times more often in men than in women [
2].
Actinobacteria can be found everywhere in the environment, in soil, freshwater, or seawater sediments. The prevalence ranges from 1.1 to 3 per 1 million and the incidence is one to six per 10 million population.
T. whipplei can be detected in the stools and saliva of patients with Whipple’s disease and in asymptomatic carriers (roughly 2–11%) [
1,
3].
The clinical spectrum of
T. whipplei infection varies, depending on the form of disease: the classic form (mainly affecting the intestinal tract causing diarrhea and weight loss and arthropathies), the localized forms (endocarditis, central nervous system disease etc., affecting extra-intestinal organs without gastrointestinal symptoms)
, the transient and acute form (self-limiting acute gastroenteritis or pneumonia) and the asymptomatic carriers [
2,
4]. In recent years Whipple’s disease in association with immunosuppression (after anti-TNF or immunosuppressive treatment) has been reported as well [
5,
6].
The majority of patients with classic Whipple’s disease experience arthralgia in the prodromal stage which can evolve several months or years before the onset of symptoms of the gastrointestinal tract. Other symptoms are low-grade fever, anemia, and lymphadenopathy as in the present case. Furthermore, in some patients, central nervous system involvement, known as a serious complication of classic Whipple’s disease, predicts a poor disease outcome. Diagnosis is established usually by duodenal biopsy showing typical foamy macrophages in the lamina propria with PAS-positive particles within them and should be confirmed by PCR as in the present case or immunohistochemistry, which can be performed from various organ samples or bodily fluids [
1].
Treatment of Whipple’s disease is long lasting and current recommendations are only based on retrospective analysis of small patient cohorts. Antibiotics induce clinical improvement in a short period of time in most patients with Whipple’s disease. Diarrhea and fever usually disappear within 1 week of therapy, whereas other symptoms often require more, 2 to 4 weeks. The laboratory findings normalize often over several weeks. Symptoms improvement usually is followed by a gradual reconstitution of the villi of the small intestine and by elimination of
Tropheryma whipplei in the following weeks [
2].
Although the suggested therapy includes induction treatment for 2 weeks with IV antibiotics either ceftriaxone 2 g QD or meropenem 1 g TID for 14 days, followed by oral TMP-SMX 960 mg twice daily for one year (with an alternative option of doxycycline 100 mg BID along with hydroxychloroquine 200 mg TID for 12 months) [
2,
4], it is rather preferred to check duodenal biopsy every 6 months and treat the patient for as long these remain positive. However, macrophages can be present in the lamina propria for years after successful treatment. On the other hand, PCR and fluorescence in situ hybridization (FISH) may be false-negative because of the biofilm that hinders the detection of a low bacterial load after treatment. Moreover, there is evidence supporting genetic predisposition which renders the patients to lifelong risk of reinfection. Thirty percent of cases with Whipple’s disease relapse and because of this, recent therapeutic recommendations propose that doxycycline (200 mg/day) and hydroxychloroquine (600 mg/day) for 12 months should be followed by lifetime doxycycline [
10]. Hydroxychloroquine causes alkalinization of the vacuoles and this results in enhancement of the bactericidal activity of doxycycline against several intracellular pathogens including
Tropheryma whipplei.
Although most patients recover completely after antibiotic therapy, on occasion, inflammation reappears (mostly as high and recurrent fevers) after initial improvement, as in this case. This is often interpreted as immune reconstitution inflammatory syndrome (IRIS) with PCR assay for
T. whipplei being often negative, indicating absence of vital bacteria, and this re-inflammation responds to corticosteroids therapy. In a recent study, IRIS was found in approximately 10% of patients with Whipple’s disease, with an outcome that varied from mild to fatal [
8]. Furthermore, the previous immunosuppressive treatment for a misdiagnosis of a rheumatology disease, most of the times seronegative arthritis, constitutes a risk factor of IRIS.
However, it was not our case as it did not respond to corticosteroid treatment. In the present case, apart from the first recurrence of fever under meropenem treatment, a possible explanation of recurrent disease might be the resistance of
T. whipplei to TMP-SMX. Indeed, there are several reports in the literature of
T. whipplei infection resistant to TMP-SMX with the responsible mutation being detected in the position N4S of the gene encoding dihydropteroate synthase (DHPS), the target of SMX [
9].