Fulminant Cryptococcal Meningoencephalitis After Successful Treatment of Primary Cutaneous Cryptococcosis
Abstract
Introduction
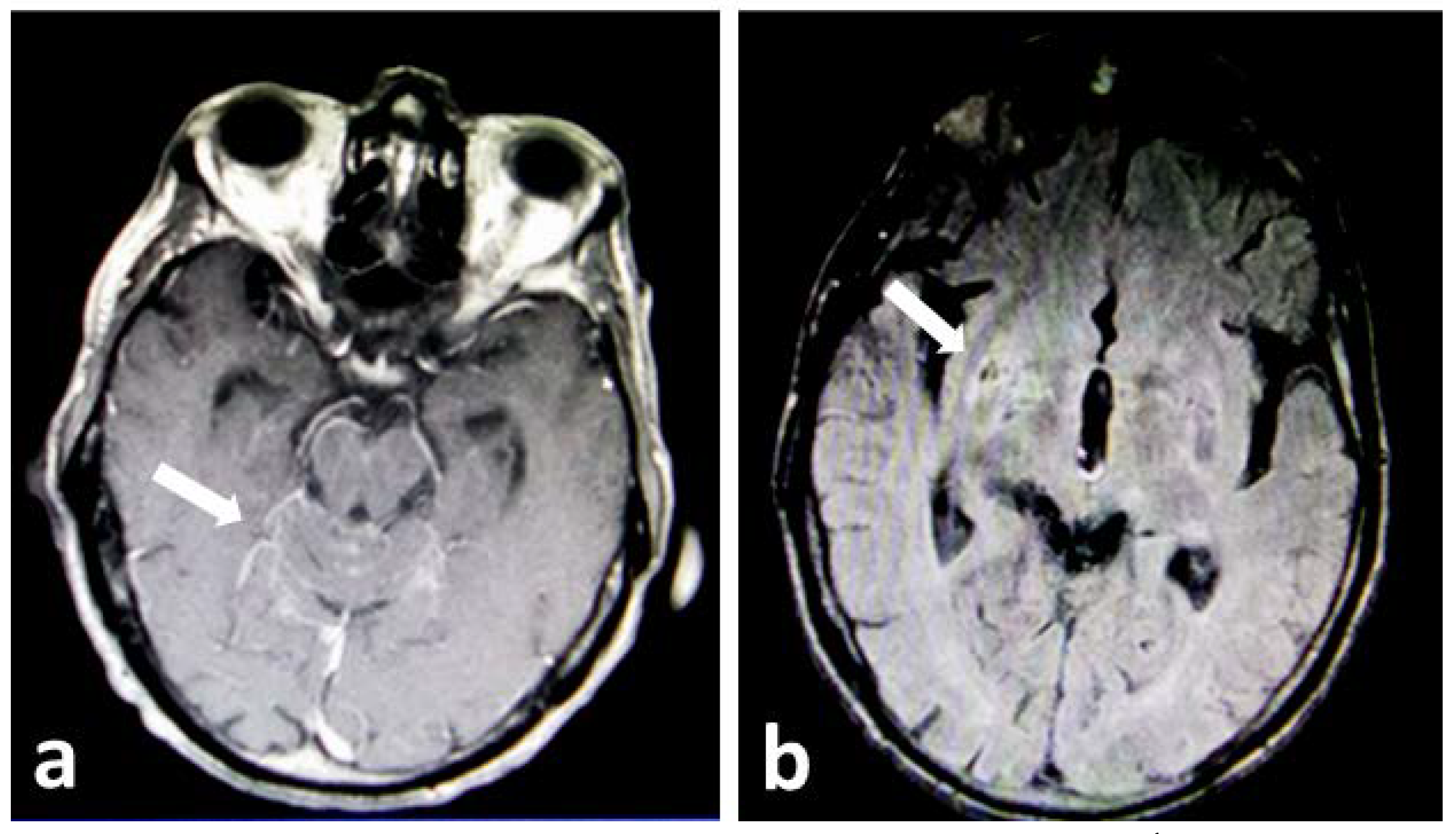
Case report
Discussion
Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Spring Harb Perspect Med. 2014, 4, a019760. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lainez, M.T.; Deliz-Aguirre, R.; Antunez, D.; et al. Cryptococcus laurentii meningitis in a non-HIV patient. IDCases. 2019, 18, e00612. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Wang, J.; Zou, L.L. Innate immune evasion strategies against Cryptococcal meningitis caused by Cryptococcus neoformans. Exp Ther Med. 2017, 14, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Bocca, A.L.; Casadevall, A. The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol. 2014, 87, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.P.; Dreyer, S. Ulcerative cellulitis of the arm: A case of primary cutaneous cryptococcosis. Dermatol Online J. 2018, 24, 9. [Google Scholar] [CrossRef]
- Srivastava, G.N.; Tilak, R.; Yadav, J.; Bansal, M. Cutaneous Cryptococcus: Marker for disseminated infection. BMJ Case Rep. 2015, 2015, bcr2015210898. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Gu, J.; Chen, J.; Liao, W.; Zhu, Y. Systemic review of published reports on primary cutaneous cryptococcosis in immunocompetent patients. Mycopathologia. 2015, 180, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Aguilera, C.; Jiménez-Gallo, D.; Collantes-Rodríguez, C.; Linares-Barrios, M. Primary cutaneous cryptococcosis: A new case of this rare entity. Open Forum Infect Dis. 2017, 4, ofw276. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.M.; Rocha, R.C.; Carneiro, L.E.; Vasconcelos, D.M.; Abreu, M.A. Disseminated cryptococcosis manifested as a single tumor in an immunocompetent patient, similar to the cutaneous primary forms. An Bras Dermatol. 2016, 91 (Suppl. S1), 29–31. [Google Scholar] [CrossRef] [PubMed]
- Tilak, R.; Prakash, P.; Nigam, C.; Tilak, V.; Gambhir, I.S.; Gulati, A.K. Cryptococcal meningitis with an antecedent cutaneous cryptococcal lesion. Dermatol Online J. 2009, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses. 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2020.
Share and Cite
Romiopoulos, I.; Pana, Z.D.; Pyrpasopoulou, A.; Linardou, I.; Avdelidi, E.; Sidiropoulou, M.; Chatzidrosou, E.; Ioannides, D.; Karagiannis, A.; Roilides, E. Fulminant Cryptococcal Meningoencephalitis After Successful Treatment of Primary Cutaneous Cryptococcosis. GERMS 2020, 10, 388-391. https://doi.org/10.18683/germs.2020.1232
Romiopoulos I, Pana ZD, Pyrpasopoulou A, Linardou I, Avdelidi E, Sidiropoulou M, Chatzidrosou E, Ioannides D, Karagiannis A, Roilides E. Fulminant Cryptococcal Meningoencephalitis After Successful Treatment of Primary Cutaneous Cryptococcosis. GERMS. 2020; 10(4):388-391. https://doi.org/10.18683/germs.2020.1232
Chicago/Turabian StyleRomiopoulos, Iordanis, Zoi Dorothea Pana, Athina Pyrpasopoulou, Ioanna Linardou, Eugenia Avdelidi, Maria Sidiropoulou, Eleni Chatzidrosou, Dimitrios Ioannides, Asterios Karagiannis, and Emmanuel Roilides. 2020. "Fulminant Cryptococcal Meningoencephalitis After Successful Treatment of Primary Cutaneous Cryptococcosis" GERMS 10, no. 4: 388-391. https://doi.org/10.18683/germs.2020.1232
APA StyleRomiopoulos, I., Pana, Z. D., Pyrpasopoulou, A., Linardou, I., Avdelidi, E., Sidiropoulou, M., Chatzidrosou, E., Ioannides, D., Karagiannis, A., & Roilides, E. (2020). Fulminant Cryptococcal Meningoencephalitis After Successful Treatment of Primary Cutaneous Cryptococcosis. GERMS, 10(4), 388-391. https://doi.org/10.18683/germs.2020.1232



