Prevalence of Hepatitis E Virus in Children with Acute Hepatitis: One Egyptian Center Study
Abstract
Introduction
Methods
Anti-HEV-IgM by ELISA (Adaltis)
HEV IgG-ELISA (Adaltis)
Detection of HEV RNA
HEV RNA extraction
Reverse-transcription real time PCR (RT-PCR)
Statistical analysis
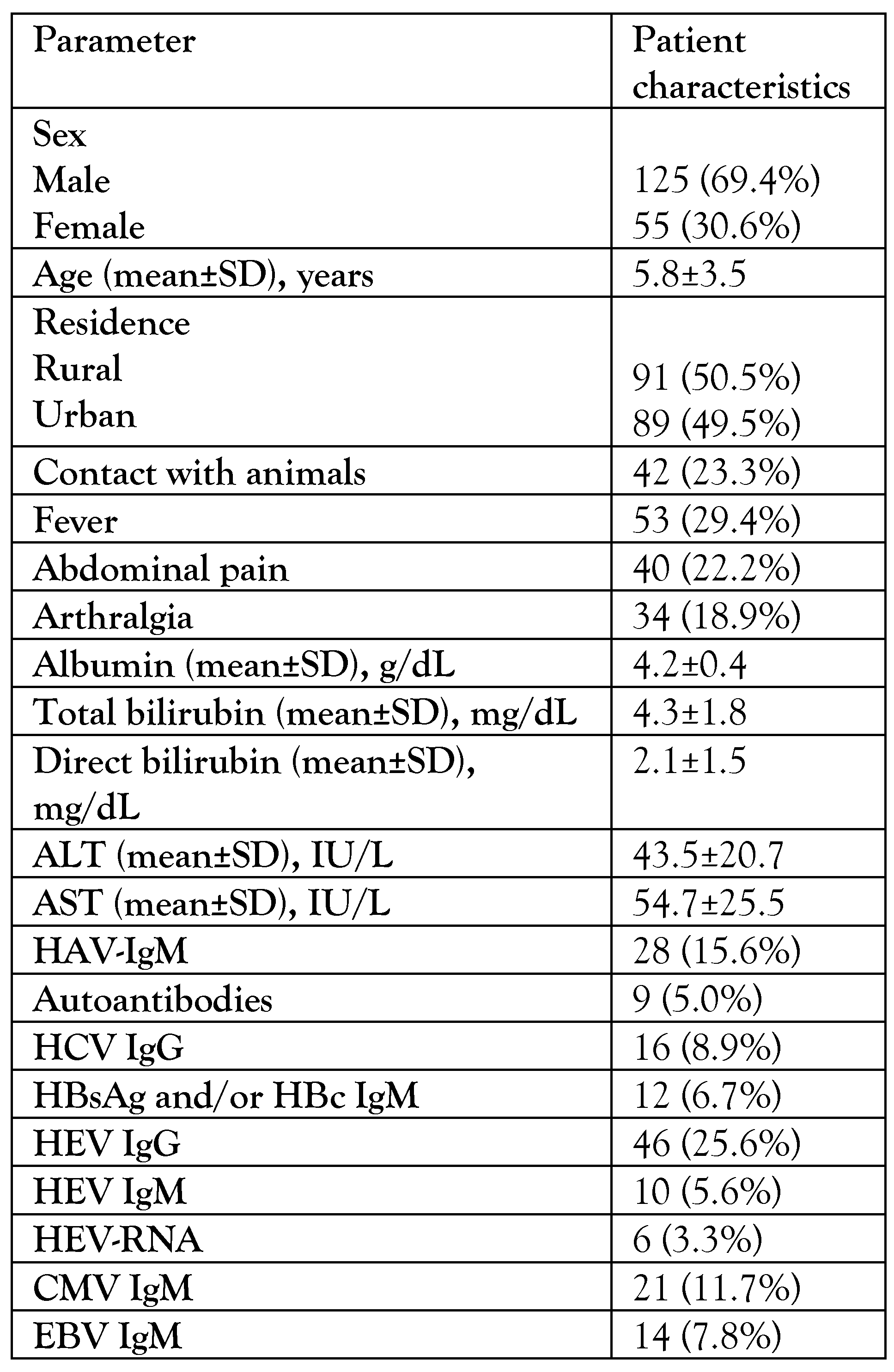
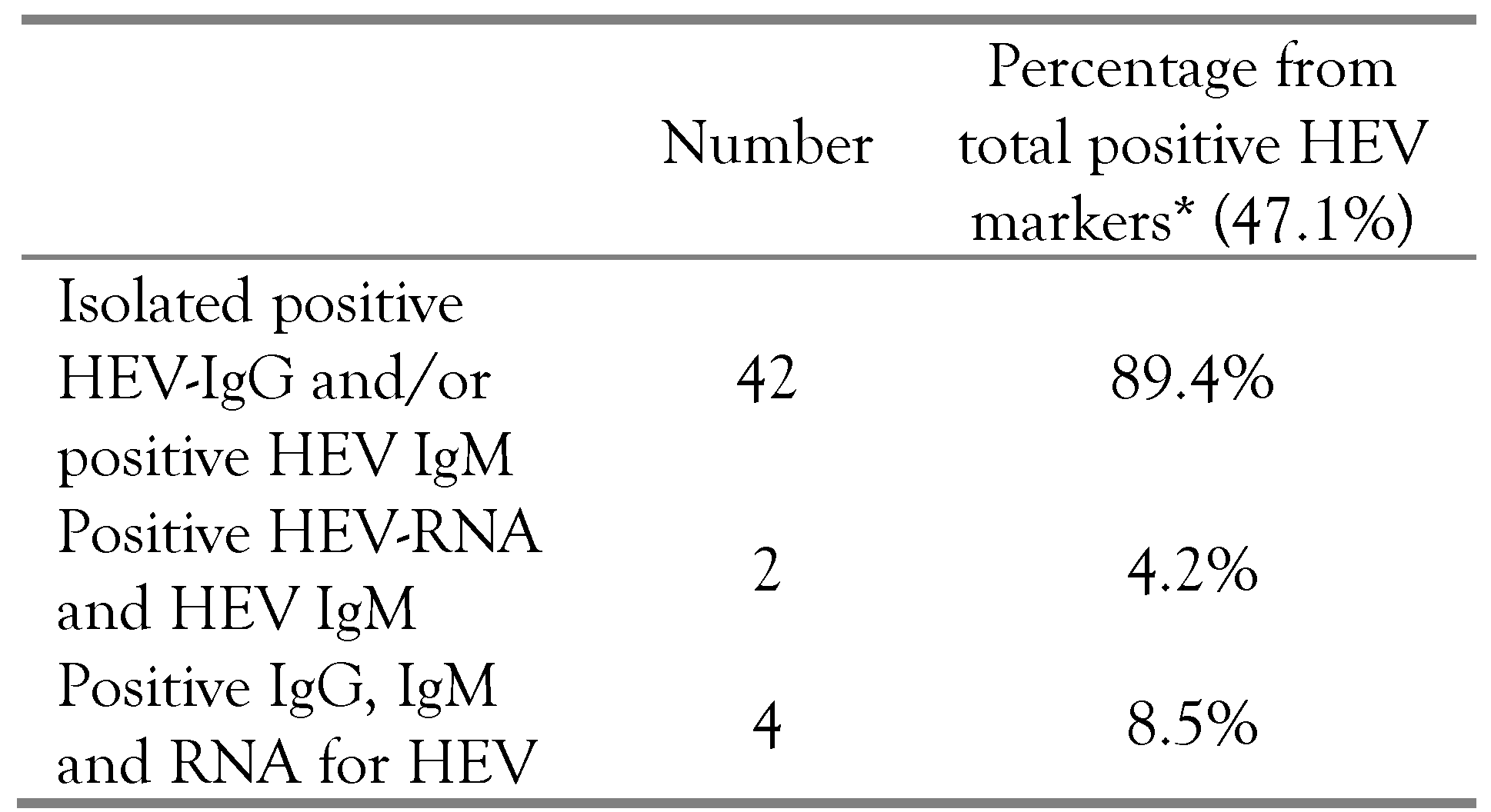
Results
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Di Bartolo, I.; Ponterio, E.; Angeloni, G.; Trevisani, M.; Ostanello, F. Zoonotic transmission of hepatitis E virus in industrialized countries. New Microbiol 2013, 36, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.W.; Dalton, H.R. Hepatitis E: an underestimated emerging threat. Ther Adv Infect Dis 2019, 6, 2049936119837162. [Google Scholar] [CrossRef]
- Pérez-Gracia, M.T.; Suay, B.; Mateos-Lindemann, M.L. Hepatitis E: an emerging disease. Infect Genet Evol 2014, 22, 40–59. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; et al. Hepatitis E virus infection. Nat Rev Dis Primers 2017, 3, 17086. [Google Scholar] [CrossRef]
- World Health Organization. Hepatitis E: Fact Sheet. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 30 October 2019).
- Murrison, L.B.; Sherman, K.E. The enigma of hepatitis E virus. Gastroenterol Hepatol (N Y) 2017, 13, 484–491. [Google Scholar]
- Xu, C.; Wang, R.Y.; Schechterly, C.A.; Ge, S.; Shih, J.W.; Xia, N.S.; et al. An assessment of hepatitis E virus (HEV) in US blood donors and recipients: no detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion 2013, 53, 2505–2511. [Google Scholar] [CrossRef]
- Stoszek, S.K.; Abdel-Hamid, M.; Saleh, D.A.; et al. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg 2006, 100, 95–101. [Google Scholar] [CrossRef]
- Abdelmawla, D.; Moemen, D.; Darwish, A.; Mowafy, W. Hepatitis E virus prevalence in Egyptian children with transfusion-dependent thalassemia. Braz J Infect Dis 2019, 23, 40–44. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, I.A.; Ghazal, H.; Fazili, J.; Nusrat, S. Mystery of hepatitis E virus: recent advances in its diagnosis and management. Int J Hepatol 2015, 2015, 872431. [Google Scholar] [CrossRef] [PubMed]
- Aboulata, A.A.; Ahmad, M.S.; Shaban, M.M.; Zayd, K.M.; Abd El-Moktader, A.M. Prevalence of hepatitis E virus in Egyptian children presented with minor hepatic disorders. Egypt J Immunol 2005, 12, 71–76. [Google Scholar]
- Hasan, G.; Assiri, A.; Marzuuk, N.; et al. Incidence and characteristics of hepatitis E virus infection in children in Assiut, Upper Egypt. J Int Med Res 2016, 44, 1115–1122. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Romanò, L.; Paladini, S.; Tagliacarne, C.; Canuti, M.; Bianchi, S.; Zanetti, A.R. Hepatitis E in Italy: a long-term prospective study. J Hepatol 2011, 54, 34–40. [Google Scholar] [CrossRef]
- Bayhan, G.İ.; Demiören, K.; Güdücüoğlu, H. Epidemiology of hepatitis E virus in children in the province of Van, Turkey. Turk Pediatri Ars 2016, 51, 148–151. [Google Scholar] [CrossRef]
- Zakaria, S.; Fouad, R.; Shaker, O.; et al. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis 2007, 44, e30–e36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blackard, J.T.; Rouster, S.D.; Nady, S.; et al. Genotypic characteristics of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol 2009, 46, 140–144. [Google Scholar] [CrossRef]
- Labrique, A.B.; Thomas, D.L.; Stoszek, S.K.; Nelson, K.E. Hepatitis E: an emerging infectious disease. Epidemiol Rev 1999, 21, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.P.; Tsarev, S.A.; Iqbal, M.; et al. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J Infect Dis 1994, 170, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol 2013, 10, 24–33. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Li, Y.; et al. The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis. Liver Int 2020. [CrossRef]
- Wu, J.; Guo, N.; Zhu, L.; et al. Seroprevalence of AIH-related autoantibodies in patients with acute hepatitis E viral infection: a prospective case-control study in China. Emerg Microbes Infect 2020, 9, 332–340. [Google Scholar] [CrossRef]
- Wang, L.; Li, M. Study on hepatitis A virus and hepatitis E virus co-infection. Int J Inf Dis 2018, 73, 221. [Google Scholar] [CrossRef]
- van Gerven, N.M.; van der Eijk, A.A.; Pas, S.D.; et al. Seroprevalence of hepatitis E virus in autoimmune hepatitis patients in the Netherlands. J Gastrointestin Liver Dis 2016, 25, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Cevahir, N.; Demir, M.; Bozkurt, A.I.; Ergin, A.; Kaleli, I. Seroprevalence of hepatitis E virus among primary school children. Pak J Med Sci 2013, 29, 629–632. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
© GERMS 2020.
Share and Cite
Zaki, M.E.S.; Alsayed, M.A.L.; Abbas, H.R.R.; Ahmed, D.M.; El Ashry, A.Y. Prevalence of Hepatitis E Virus in Children with Acute Hepatitis: One Egyptian Center Study. Germs 2020, 10, 88-94. https://doi.org/10.18683/germs.2020.1189
Zaki MES, Alsayed MAL, Abbas HRR, Ahmed DM, El Ashry AY. Prevalence of Hepatitis E Virus in Children with Acute Hepatitis: One Egyptian Center Study. Germs. 2020; 10(2):88-94. https://doi.org/10.18683/germs.2020.1189
Chicago/Turabian StyleZaki, Maysaa El Sayed, Mona Abdel Latif Alsayed, Hoda Ramadan Ryad Abbas, Doaa Mabrouk Ahmed, and Amany Yusif El Ashry. 2020. "Prevalence of Hepatitis E Virus in Children with Acute Hepatitis: One Egyptian Center Study" Germs 10, no. 2: 88-94. https://doi.org/10.18683/germs.2020.1189
APA StyleZaki, M. E. S., Alsayed, M. A. L., Abbas, H. R. R., Ahmed, D. M., & El Ashry, A. Y. (2020). Prevalence of Hepatitis E Virus in Children with Acute Hepatitis: One Egyptian Center Study. Germs, 10(2), 88-94. https://doi.org/10.18683/germs.2020.1189




