Abstract
Introduction: Acute disseminated encephalomyelitis (ADEM) is a rare neurological complication seen in association with severe dengue infection. Here we report a case of a six-year-old male child with ADEM following dengue fever. Case report: A six-year-old boy was admitted with fever, hematuria, and melena of five days duration. On evaluation, the child had shock and features of coagulopathy. Dengue NS 1 antigen and IgM ELISA were positive. The child received treatment as per the 2009 WHO dengue protocol. On day seven of illness, he developed neurological symptoms. Magnetic resonance imaging (MRI) done on day eight of illness showed T2/FLAIR hyperintensities in bilateral frontoparietal subcortical deep white matter, occipital periventricular white matter, pons and cerebellar hemispheres, diagnostic of ADEM. He responded to intravenous methylprednisolone. He was discharged 2 weeks after hospitalization. His neurological examination was normal at follow up after a month. Conclusions: Early recognition and prompt treatment of ADEM in dengue can lead to a successful outcome without neurological deficits.
Introduction
Dengue infection is a flavivirus infection. The transmission to humans is by the Aedes aegypti mosquito. The incidence of dengue infection is increasing over the past 50 years period, and it has become a global health burden [1]. Between 1990-2013, the global burden of dengue was estimated to be about 50-100 million cases per year, and dengue illness was responsible for 1.14 million disability-adjusted life years [1]. In severe dengue, studies have reported case fatality rates ranging between 3.8% to 18.6% [2]. Ending the epidemic of dengue by 2030 by all health reforms is the target of sustainable development goals three [3].
The reported incidence of neurological problems in dengue ranges between 0.5% and 6.2% [4,5]. The serotypes 2 and 3 of dengue virus are mostly associated with neurological complications, which include encephalopathy, meningitis, encephalitis, stroke, immune-mediated complications like acute disseminated encephalomyelitis (ADEM), neuromuscular complications (myositis), Guillain-Barré syndrome (GBS) [5,6]. ADEM, an acute demyelinating disorder affecting the central nervous system, has been infrequently seen in association with dengue virus infection [7].
We report a rare case of a 6-year-old male child with ADEM following severe dengue.
Case report
A six-year-old boy was admitted with high-grade fever, hematuria, and melena of five days duration. On examination, he was febrile (100 °F/37.8 °C), heart rate was 108/min, respiratory rate was 24/min, and blood pressure 84/50 mmHg. The systemic examination was normal.
Investigations done revealed hemoglobin 12.4 g/dL, total leukocyte count 10,100 cells/µL (neutrophils – 94%, lymphocytes – 4%, monocytes – 1%, eosinophils – 1%); platelet count 61,000 cells/µL, blood urea 23 mg/dL; serum creatinine 0.4 mg/dL; serum glutamate oxaloacetic transaminase (SGOT) 164 IU/L; serum glutamate pyruvic transaminase (SGPT) 47 IU/L and normal prothrombin time and activated thromboplastin time. Serum dengue Ns1 antigen done by immunochromatographic lateral flow rapid card test was positive. Serum IgM dengue antibody test done by Pan Bio enzyme-linked immunosorbent assay (ELISA) technique was positive on the day of admission (day 5 of fever). Blood culture showed no growth. Coagulopathy was diagnosed based on elevated fibrin degradation products (FDP >3.29 mg/dL) and low platelet count at admission. The child was treated with IV fluids as per the 2009 WHO dengue protocol for hypotensive shock [8]. Initially, two 20 mL/kg isotonic crystalloid boluses were given, and fluid was started at 10 mL/kg for one hour and then gradually tapered to 5 mL/kg/hr and 3 mL/kg/hr.
On the seventh day of illness, he developed an episode of seizure that was treated with intravenous fosphenytoin, and on day eight of illness, the child developed altered sensorium, left-sided hemiparesis with right upper motor neuron facial palsy.
Magnetic resonance imaging (MRI) done on the eighth day of illness showed T2/FLAIR hyperintensities in bilateral frontoparietal subcortical deep white matter, occipital periventricular white matter, pons and cerebellar hemispheres with no post-contrast enhancement, diagnostic of ADEM (Figure 1, Figure 2 and Figure 3).
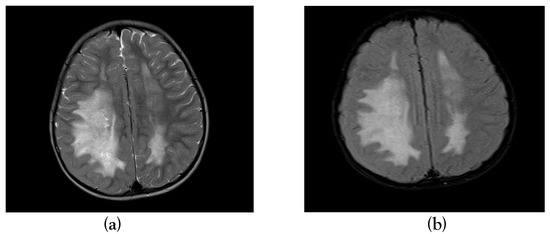
Figure 1.
T2 axial and T2 FLAIR sequence showing hyper intensity in bilateral frontoparietal white matter (a) & (b).
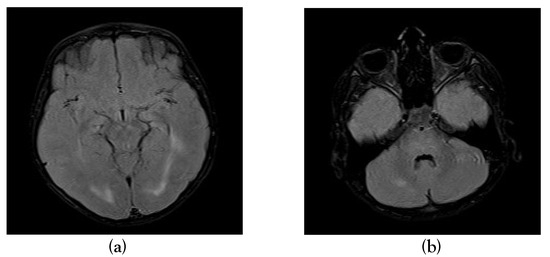
Figure 2.
T2 axial and T2 FLAIR sequence showing hyper intensity in occipital periventricular white matter (a), pons and cerebellar hemispheres (b).
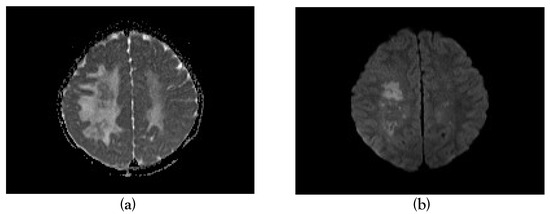
Figure 3.
Apparent diffusion coefficient (a) and diffusion-weighted imaging (b) images showing restricted diffusion in a few areas of bilateral frontoparietal white matter.
The child was treated with high dose intravenous methylprednisolone 30 mg/kg/day for five days, followed by 2 mg/kg/day of oral prednisolone for the next three weeks. Following intravenous methylprednisolone, his sensorium normalized, and left-sided weakness gradually improved. The child was discharged after two weeks of hospitalization. At discharge, he was able to sit unsupported. Parents were taught shoulder girdle strengthening, pelvic bridging, and lower limb strengthening exercises and advised to continue at home. On follow up at one month, his neurological examination was found to be normal.
Discussion
Dengue virus was initially considered as a non-neurotropic virus. An autopsy done on a fatal case of dengue showed an immunological reaction in astrocytes, microglia, and other brain tissue indicating viral invasion [9].
The neurological manifestations in dengue infection can be due to the direct neurotropic effect of the virus on the central nervous system, metabolic or systemic manifestations of dengue virus, and immune-mediated complications [4,10].
ADEM is most commonly seen after a viral infection but may also appear after parasitic infection, bacterial infection, vaccination, or without any specific precipitating event. Viral infections associated with ADEM include measles, influenza virus, rubella, mumps, enterovirus, varicella, Epstein-Barr virus, herpes simplex virus, cytomegalovirus, hepatitis A and rarely dengue [11].
ADEM is an uncommon neurological manifestation following dengue with a reported prevalence ranging from 0.4-0.8/100,000/year [12]. Dengue ADEM involves transient immune response directed at myelin or other self-antigens [13]. ADEM in dengue is a multifocal white matter illness with clinical features like altered sensorium, convulsions, and focal neurological deficits. ADEM following dengue fever is commonly reported among adults with a mean age group of 21 years [10].
ADEM has an abrupt onset, and the course of illness is monophasic. WA Wan Sulaiman et al. opined that the onset of neurological symptoms of ADEM was at day 8 of illness, and most of the cases of ADEM were seen in cases of classical dengue [10]. In a meta-analysis study done by Kamel MG et al., the onset of neurological manifestations ranged from three to nineteen days after the initial dengue symptoms [13]. Neurological manifestations before the tenth day of dengue symptoms and a fever higher than 101.2 °F (38.4 °C) at presentation were predictors of poor outcome in ADEM following dengue infection [13]. Our patient had developed features of ADEM on day seven of illness of severe dengue.
Demyelinating lesions with or without hemorrhagic foci on brain MRI are characteristic of ADEM resulting from dengue virus infection. The lesions can also involve the spinal cord extensively with several levels of involvement with spinal cord edema [14]. However, our patient had typical demyelinating lesions involving the brain on MRI with no involvement of the spinal cord. Therapy consists of the use of steroids in high doses, intravenous immunoglobulin, and plasma exchange [15]. Our patient responded to high dose intravenous methylprednisolone followed by oral steroids.
The recovery period of dengue associated ADEM is usually between one to four weeks as ADEM in general and the earliest recovery reported was as early as three days [16]. Reports on long-term outcomes of ADEM following dengue infection are not available as per our knowledge.
ADEM, as a neurological presentation following dengue infection, is rarely described. ADEM must be included in the differential diagnosis if the patient has any evidence of neurological involvement in the recovery phase of dengue infection. As dengue ADEM is seen in the convalescent phase of dengue illness, presentation may be in the absence of fever, this entity needs to be considered in endemic areas or in returning travelers from these areas.
Conclusions
In a child with dengue fever, the development of neurological manifestations may hint towards ADEM. Early recognition and prompt treatment of ADEM can lead to a successful outcome without neurological deficits.
Consent
Written informed consent was obtained from the patient’s legal guardians for publication of this case report and images.
Author Contributions
All authors were involved in the case management, manuscript draft, and final approval. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Conflicts of interest
All authors – none to declare.
References
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Md-Sani, S.S.; Md-Noor, J.; Han, W.H.; et al. Prediction of mortality in severe dengue cases. BMC Infect Dis 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Sustainable Development Goals (SDGs). Available online: https://sustainabledevelopment.un.org/topics/sustainab ledevelopmentgoals (accessed on 16 December 2019).
- Murthy, J.M. Neurological complication of dengue infection. Neurol India 2010, 58, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J.; Wichmann, O.; Farrar, J.; Gascón, J. Neurological complications of dengue virus infection. Lancet Neurol 2013, 12, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.B.; Kuster, G.W.; Onuki-Castro, F.L.; Souza, V.A.; Levi, J.E.; Pannuti, C.S. Involvement of the central nervous system in patients with dengue virus infection. J Neurol Sci 2008, 267, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, C.; Uppin, S.G.; Dakshinamurthy, K.; Borgahain, R. Acute disseminated encephalomyelitis following dengue hemorrhagic fever. Neurol India 2010, 58, 599–601. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control; WHO Press: Geneva, Switzerland, 2009. [Google Scholar]
- Ramos, C.; Sánchez, G.; Pando, R.H.; et al. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol 1998, 4, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wan Sulaiman, W.A.; Inche Mat, L.N.; Hashim, H.Z.; et al. Acute disseminated encephalomyelitis in dengue viral infection. J Clin Neurosci 2017, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M. Post infectious encephalomyelitis. Curr Neurol Neurosci Rep 2003, 3, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, L.A.; Babur, W.; Chaudhry, G.A.; Al-Atawi, F.E.; Robert, A.A. Acute disseminated encephalomyelitis: a call to the clinicians for keeping this rare condition on clinical radar. Pan Afr Med J 2018, 29, 138. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.G.; Nam, N.T.; Han, N.H.B.; et al. Post-dengue acute disseminated encephalomyelitis: A case report and meta-analysis. PLoS Negl Trop Dis 2017, 11, e0005715. [Google Scholar] [CrossRef] [PubMed]
- Jugpal, T.S.; Dixit, R.; Garg, A.; et al. Spectrum of findings on magnetic resonance imaging of the brain in patients with neurological manifestations of dengue fever. Radiol Bras 2017, 50, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Karoli, R.; Siddiqi, Z.; Fatima, J.; Maini, S. Was it a case of acute disseminated encephalomyelitis? A rare association following dengue fever. J Neurosci Rural Pract 2013, 4, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Gala, H.C.; Avasthi, B.S.; Lokeshwar, M.R. Dengue shock syndrome with two atypical complications. Indian J Pediatr 2012, 79, 386–388. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2020.