Eggmanone Effectively Overcomes Prostate Cancer Cell Chemoresistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cell Scratch-Wound Assay
2.3. Cell Proliferation Assay
2.4. Transfection
2.5. Cell Apoptosis Assay
2.6. Modified Boyden Chamber Invasion Assay
2.7. Sphere Formation Assay
2.8. Real-Time PCR
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
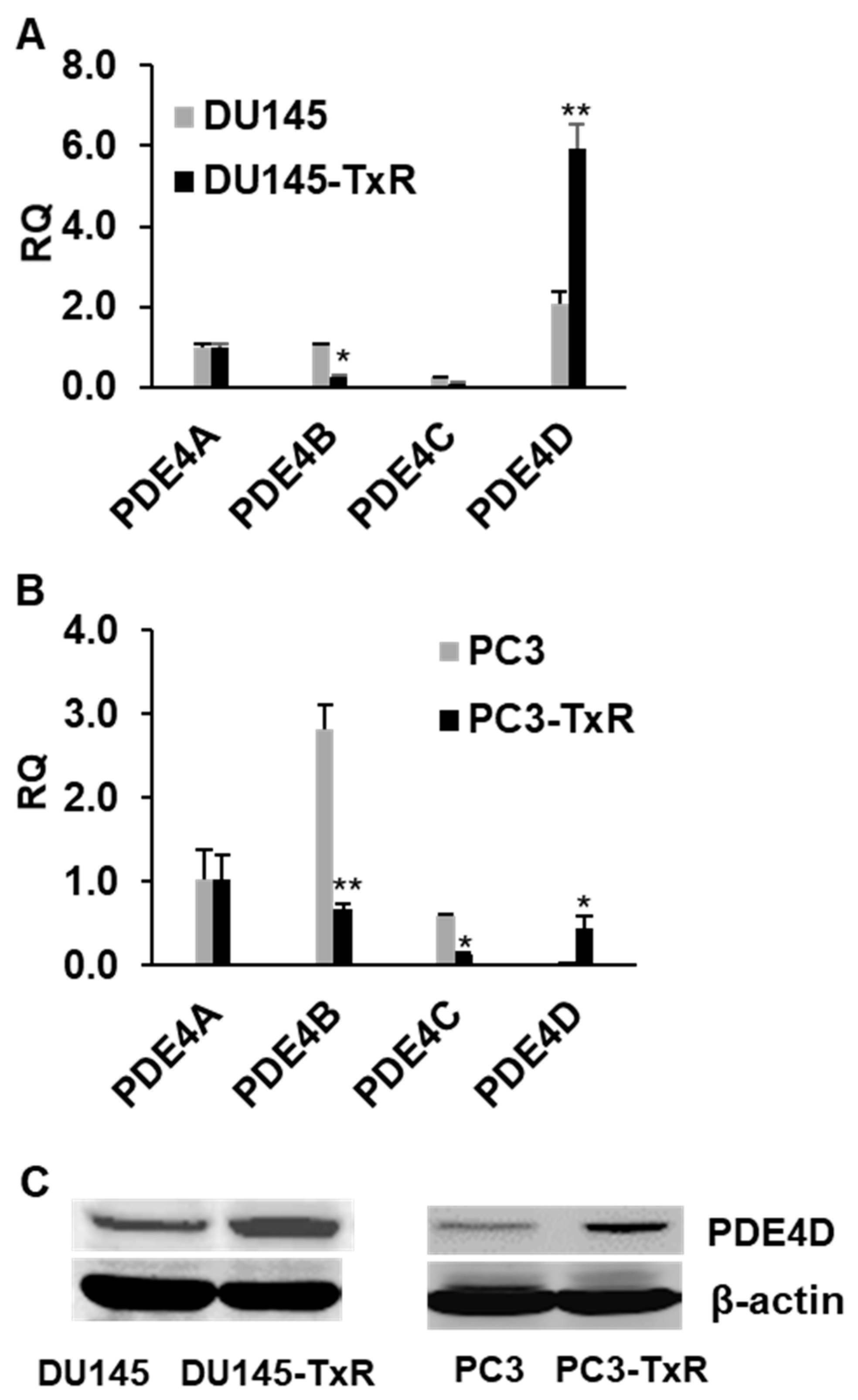
3.1. PDE4D Expression Is Highly Upregulated in Chemo-Resistant Prostate Cancer Cells
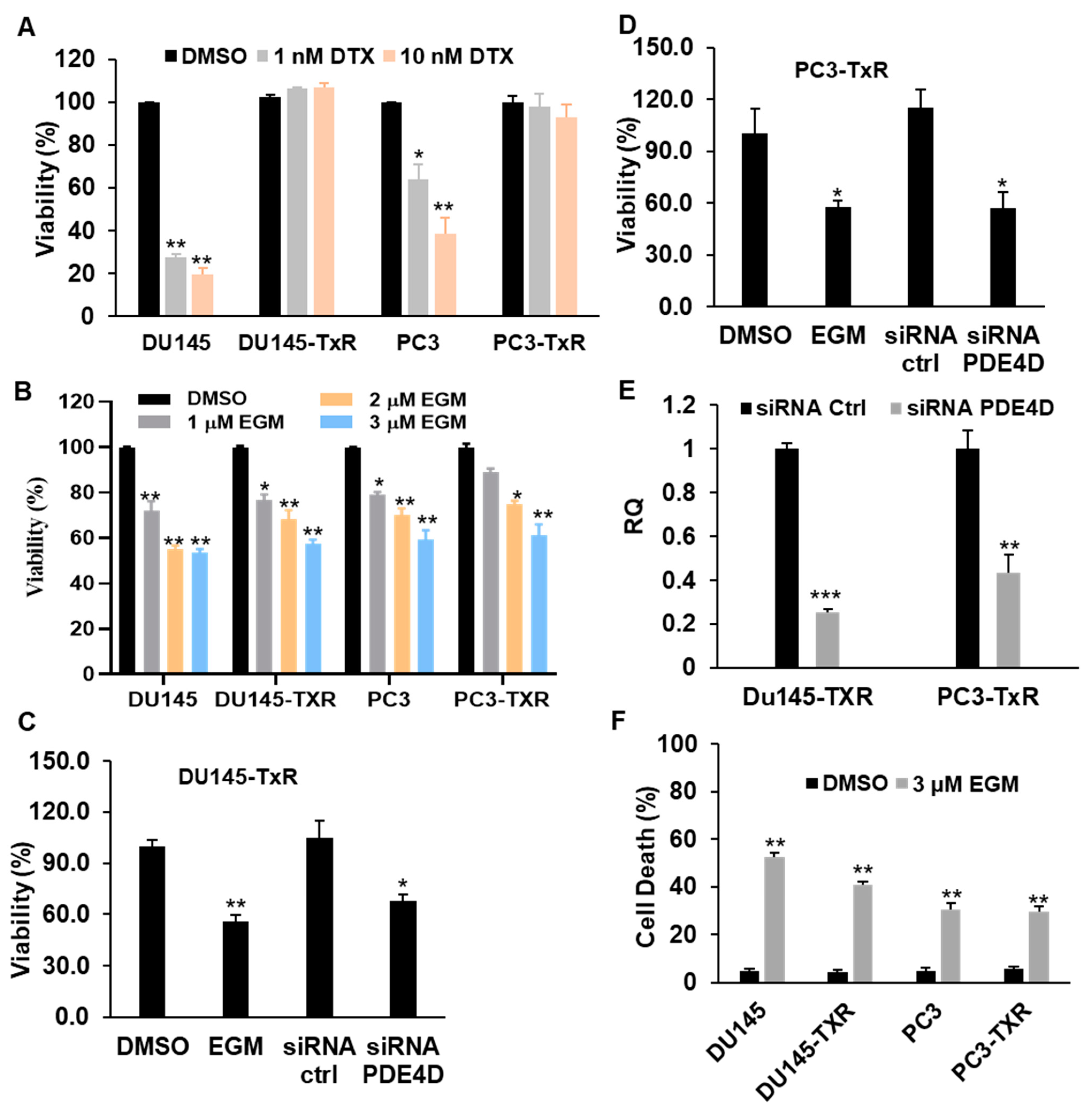
3.2. Eggmanone Decreases the Invasion of Chemo-Resistant Prostate Cancer Cells
3.3. Eggmanone Reduces Proliferation and Induces Cell Death of Chemo-Resistant Prostate Cancer Cells
3.4. Eggmanone Overcomes Prostate Cancer Cell Chemo-Resistance via Sonic Hedgehog Signaling
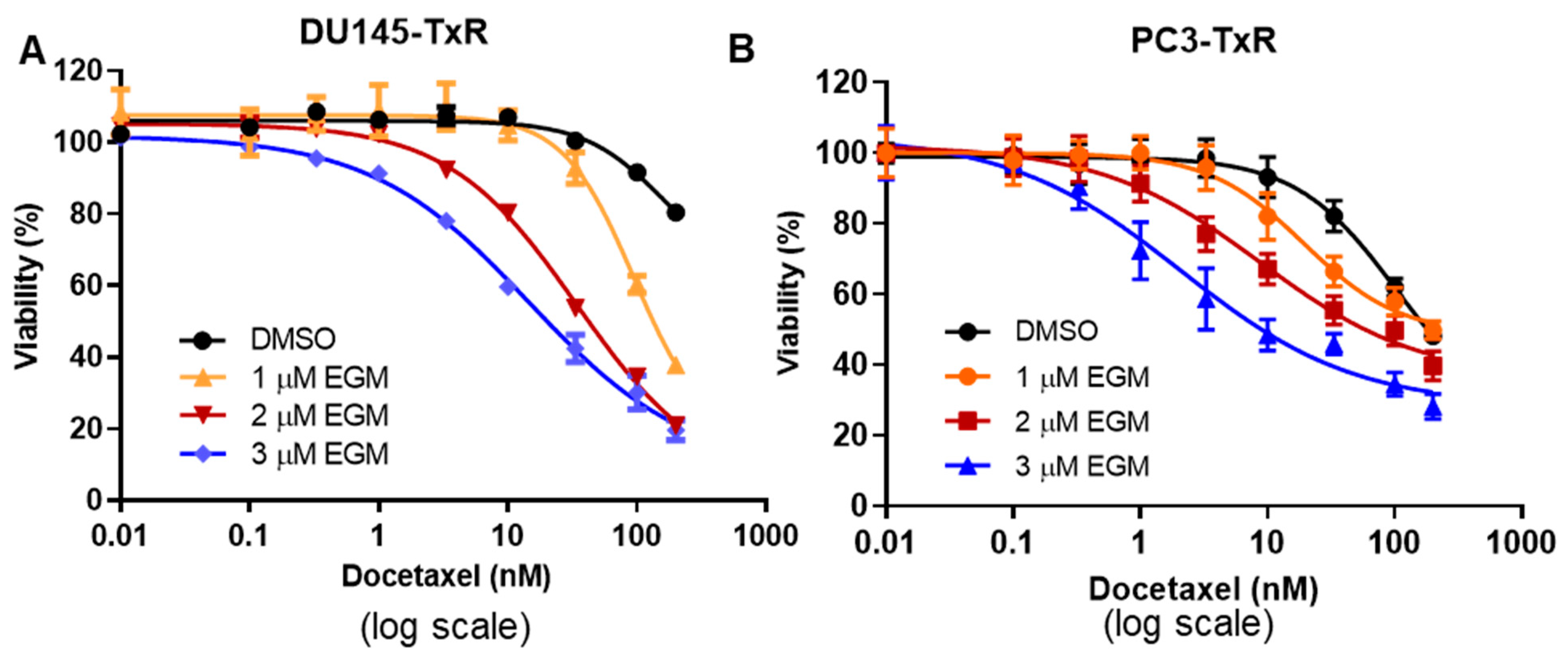
3.5. Treatment with the Combination of Eggmanone and Docetaxel Dramatically Overcomes Prostate Cancer Cell Chemo-Resistance
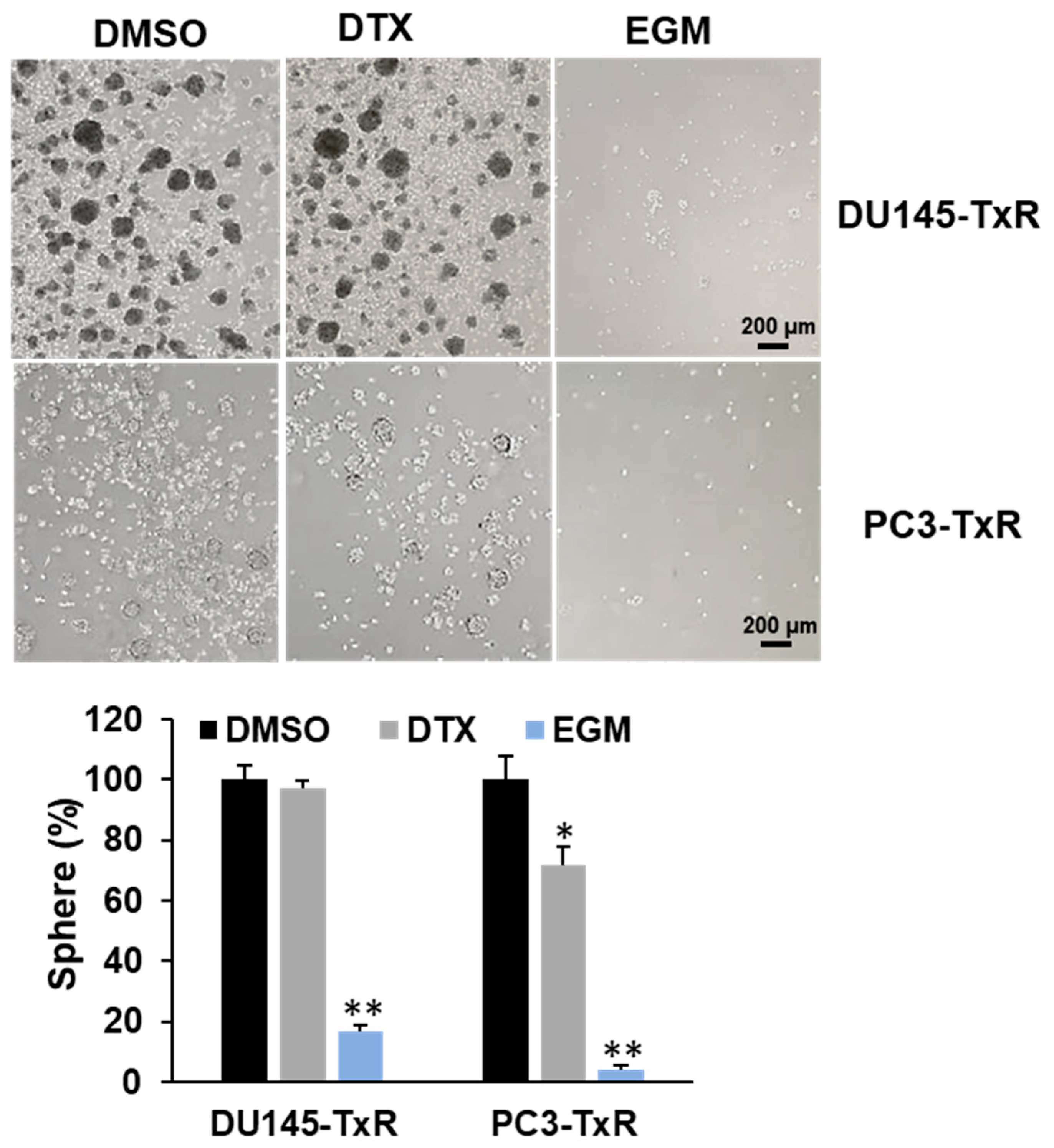
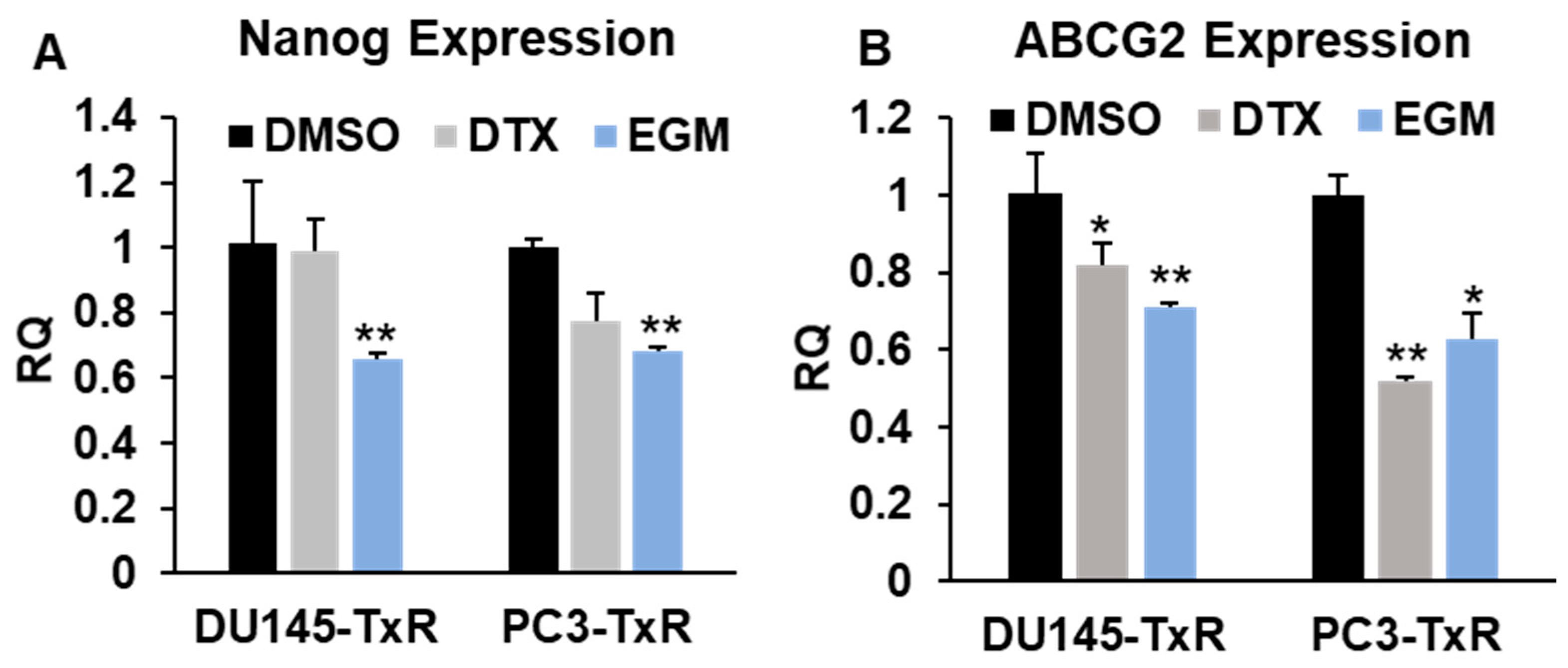
3.6. Eggmanone Attenuates Stem-Like Properties of Chemo-Resistant Prostate Cancer Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bishr, M.; Saad, F. Overview of the latest treatments for castration-resistant prostate cancer. Nat. Rev. Urol. 2013, 10, 522–528. [Google Scholar] [CrossRef]
- Huggins, C. The Hormone-Dependent Cancers. JAMA 1963, 186, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer: I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Lohiya, V.; Aragon-Ching, J.B.; Sonpavde, G. Role of Chemotherapy and Mechanisms of Resistance to Chemotherapy in Metastatic Castration-Resistant Prostate Cancer. Clin. Med. Insights Oncol. 2016, 10, 57–66. [Google Scholar] [CrossRef]
- Cánovas, V.; Puñal, Y.; Maggio, V.; Redondo, E.; Marín, M.; Mellado, B.; Olivan, M.; Lleonart, M.; Planas, J.; Morote, J.; et al. Prostate Tumor Overexpressed-1 (PTOV1) promotes docetaxel-resistance and survival of castration resistant prostate cancer cells. Oncotarget 2017, 8, 59165–59180. [Google Scholar] [CrossRef]
- Lin, D.-C.; Xu, L.; Ding, L.-W.; Sharma, A.; Liu, L.-Z.; Yang, H.; Tan, P.; Vadgama, J.; Karlan, B.Y.; Lester, J.; et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc. Natl. Acad. Sci. USA 2013, 110, 6109–6114. [Google Scholar] [CrossRef]
- Rahrmann, E.P.; Collier, L.S.; Knutson, T.P.; Doyal, M.E.; Kuslak, S.L.; Green, L.E.; Malinowski, R.L.; Roethe, L.; Akagi, K.; Waknitz, M.; et al. Identification of PDE4D as a Proliferation Promoting Factor in Prostate Cancer Using a Sleeping Beauty Transposon-Based Somatic Mutagenesis Screen. Cancer Res. 2009, 69, 4388–4397. [Google Scholar] [CrossRef] [PubMed]
- Goldhoff, P.; Warrington, N.M.; Limbrick, D.D.; Hope, A.; Woerner, B.M.; Jackson, E.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Targeted Inhibition of Cyclic AMP Phosphodiesterase-4 Promotes Brain Tumor Regression. Clin. Cancer Res. 2008, 14, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lerner, A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood 1998, 92, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Streiff, M.B.; Bugayenko, A.; Kato, G.J. Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21WAF1/CIP1 proteins in human acute lymphoblastic leukemia cells. Blood 2002, 99, 3390–3397. [Google Scholar] [CrossRef]
- Bolger, G.B.; Bizzi, M.F.; Pinheiro, S.V.; Trivellin, G.; Smoot, L.; Accavitti, M.-A.; Korbonits, M.; Ribeiro-Oliveira, A. cAMP-specific PDE4 phosphodiesterases and AIP in the pathogenesis of pituitary tumors. Endocr.-Relat. Cancer 2016, 23, 419–431. [Google Scholar] [CrossRef]
- Smith, P.G.; Wang, F.; Wilkinson, K.N.; Savage, K.J.; Klein, U.; Neuberg, D.S.; Bollag, G.; Shipp, M.A.; Aguiar, R.C.T. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT–dependent apoptosis in diffuse large B-cell lymphoma. Blood 2005, 105, 308–316. [Google Scholar] [CrossRef]
- Nagy, Z.S.; Ross, J.A.; Rodriguez, G.; Balint, B.L.; Szeles, L.; Nagy, L.; Kirken, R.A. Genome Wide Mapping Reveals PDE4B as an IL-2 Induced STAT5 Target Gene in Activated Human PBMCs and Lymphoid Cancer Cells. PLoS ONE 2013, 8, e57326. [Google Scholar] [CrossRef]
- Bao, Z.; Feng, Y.; Wang, H.; Zhang, C.; Sun, L.; Yan, Z.; Liu, Q.; Guo, T.; Li, M.; Yang, X.; et al. Integrated analysis using methylation and gene expression microarrays reveals PDE4C as a prognostic biomarker in human glioma. Oncol. Rep. 2014, 32, 250–260. [Google Scholar] [CrossRef]
- Powers, G.L.; Hammer, K.D.; Domenech, M.; Frantskevich, K.; Malinowski, R.L.; Bushman, W.; Beebe, D.J.; Marker, P.C. Phosphodiesterase 4D Inhibitors Limit Prostate Cancer Growth Potential. Mol. Cancer Res. 2014, 13, 149–160. [Google Scholar] [CrossRef]
- Williams, C.H.; Hempel, J.E.; Hao, J.; Frist, A.Y.; Williams, M.M.; Fleming, J.T.; Sulikowski, G.A.; Cooper, M.K.; Chiang, C.; Hong, C.C. An In Vivo Chemical Genetic Screen Identifies Phosphodiesterase 4 as a Pharmacological Target for Hedgehog Signaling Inhibition. Cell Rep. 2015, 11, 43–50. [Google Scholar] [CrossRef]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–70. [Google Scholar] [CrossRef]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Bruno, O.; Romussi, A.; Spallarossa, A.; Brullo, C.; Schenone, S.; Bondavalli, F.; Vanthuyne, N.; Roussel, C. New Selective Phosphodiesterase 4D Inhibitors Differently Acting on Long, Short, and Supershort Isoforms. J. Med. Chem. 2009, 52, 6546–6557. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ramirez, A.; Wang, Z.; Chow, M.S.; Hao, J. A simple and sensitive HPLC–MS/MS method for quantification of eggmanone in rat plasma and its application to pharmacokinetics. J. Pharm. Biomed. Anal. 2018, 153, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cory, G. Scratch-Wound Assay. Methods Mol. Biol. 2011, 769, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Kambe, M.; Tajima, O.; Moriya, S.; Sawaki, H.; Hotta, H.; Kondo, Y.; Narimatsu, H.; Miyagi, T.; Furukawa, K.; et al. Membrane sialidase NEU3 is highly expressed in human melanoma cells promoting cell growth with minimal changes in the composition of gangliosides. Cancer Sci. 2011, 102, 2139–2149. [Google Scholar] [CrossRef]
- Po, A.; Citarella, A.; Catanzaro, G.; Besharat, Z.M.; Trocchianesi, S.; Gianno, F.; Sabato, C.; Moretti, M.; De Smaele, E.; Vacca, A.; et al. Hedgehog-GLI signalling promotes chemoresistance through the regulation of ABC transporters in colorectal cancer cells. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Shi, J.; Chai, F.; Zhou, J.; Chen, C.; Xie, S.; Chen, X.; Su, P. The Hedgehog inhibitor cyclopamine antagonizes chemoresistance of breast cancer cells. OncoTargets Ther. 2013, 6, 1643–1647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, S.; Chitkara, D.; Mehrazin, R.; Behrman, S.W.; Wake, R.W.; Mahato, R.I. Chemoresistance in Prostate Cancer Cells Is Regulated by miRNAs and Hedgehog Pathway. PLoS ONE 2012, 7, e40021. [Google Scholar] [CrossRef] [PubMed]
- Steg, A.D.; Katre, A.A.; Bevis, K.S.; Ziebarth, A.; Dobbin, Z.C.; Shah, M.M.; Alvarez, R.D.; Landen, C.N. Smoothened Antagonists Reverse Taxane Resistance in Ovarian Cancer. Mol. Cancer Ther. 2012, 11, 1587–1597. [Google Scholar] [CrossRef]
- Ni, J.; Cozzi, P.; Hao, J.; Duan, W.; Graham, P.; Kearsley, J.; Li, Y. Cancer stem cells in prostate cancer chemoresistance. Curr. Cancer Drug Targets 2014, 14, 225–240. [Google Scholar] [CrossRef]
- Yun, E.-J.; Lo, U.-G.; Hsieh, J.-T. The evolving landscape of prostate cancer stem cell: Therapeutic implications and future challenges. Asian J. Urol. 2016, 3, 203–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bahmad, H.F.; Cheaito, K.; Chalhoub, R.M.; Hadadeh, O.; Monzer, A.; Ballout, F.; El-Hajj, A.; Mukherji, D.; Liu, Y.-N.; Daoud, G.; et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Front. Oncol. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Tanaka, T.; Nakai, D.; Morita, N.; Suzuki, K. Immunohistochemical expression of four different stem cell markers in prostate cancer: High expression of NANOG in conjunction with hypoxia-inducible factor-1α expression is involved in prostate epithelial malignancy. Oncol. Lett. 2014, 8, 985–992. [Google Scholar] [CrossRef]
- Bae, K.-M.; Su, Z.; Frye, C.; McClellan, S.; Allan, R.W.; Andrejewski, J.T.; Kelley, V.; Jorgensen, M.; Steindler, D.A.; Vieweg, J.; et al. Expression of Pluripotent Stem Cell Reprogramming Factors by Prostate Tumor Initiating Cells. J. Urol. 2010, 183, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, A.; Chumakova, A.; Hitomi, M.; Lathia, J. Enrichment and Interrogation of Cancer Stem Cells. In Cancer Stem Cells; Elsevier BV: San Leandro, CA, USA, 2016; Volume 2016, pp. 59–98. [Google Scholar]
- Sabnis, N.G.; Miller, A.; Titus, M.A.; Huss, W.J. The Efflux Transporter ABCG2 Maintains Prostate Stem Cells. Mol. Cancer Res. 2016, 15, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.M.; Canettieri, G.; Montminy, M.R. Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc. Natl. Acad. Sci. USA 2001, 98, 10936–10941. [Google Scholar] [CrossRef]
- Mayr, B.; Montminy, M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001, 2, 599–609. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; A Banat, G.; Schmall, A.; Szibor, M.; Pomagruk, D.; Hänze, J.; Kolosionek, E.; Wilhelm, J.; Braun, T.; Grimminger, F.; et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene 2012, 32, 1121–1134. [Google Scholar] [CrossRef]
- Cao, B.; Wang, K.; Liao, J.-M.; Zhou, X.; Liao, P.; Zeng, S.X.; He, M.; Chen, L.; He, Y.; Li, W.; et al. Inactivation of oncogenic cAMP-specific phosphodiesterase 4D by miR-139-5p in response to p53 activation. eLife 2016, 5, e15978. [Google Scholar] [CrossRef]
- Feldmann, G.; Dhara, S.; Fendrich, V.; Bedja, D.; Beaty, R.; Mullendore, M.; Karikari, C.; Alvarez, H.; Iacobuzio-Donahue, C.; Jimeno, A.; et al. Blockade of Hedgehog Signaling Inhibits Pancreatic Cancer Invasion and Metastases: A New Paradigm for Combination Therapy in Solid Cancers. Cancer Res. 2007, 67, 2187–2196. [Google Scholar] [CrossRef]
- Gao, Q.; Yuan, Y.; Gan, H.-Z.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387. [Google Scholar] [CrossRef]
- Vidal, S.J.; Rodriguezbravo, V.; Galsky, M.D.; Cordoncardo, C.; Domingodomenech, J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene 2014, 33, 4451–4463. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Lottaz, C.; Beier, D.; Meyer, K.; Kumar, P.; Hermann, A.; Schwarz, J.; Junker, M.; Oefner, P.J.; Bogdahn, U.; Wischhusen, J.; et al. Transcriptional Profiles of CD133+ and CD133− Glioblastoma-Derived Cancer Stem Cell Lines Suggest Different Cells of Origin. Cancer Res. 2010, 70, 2030–2040. [Google Scholar] [CrossRef]
- Qin, J.; Liu, X.; Laffin, B.; Chen, X.; Choy, G.; Jeter, C.R.; Calhoun-Davis, T.; Li, H.; Palapattu, G.S.; Pang, S.; et al. The PSA−/lo Prostate Cancer Cell Population Harbors Self-Renewing Long-Term Tumor-Propagating Cells that Resist Castration. Cell Stem Cell 2012, 10, 556–569. [Google Scholar] [CrossRef]
- Kang, T.-W.; Choi, S.W.; Yang, S.-R.; Shin, T.-H.; Kim, H.-S.; Yu, K.-R.; Hong, I.-S.; Ro, S.; Cho, J.M.; Kang, K.-S. Growth arrest and forced differentiation of human primary glioblastoma multiforme by a novel small molecule. Sci. Rep. 2014, 4, srep05546. [Google Scholar] [CrossRef][Green Version]


| DTX IC50 (nM) | ||||
|---|---|---|---|---|
| Cell Line | DMSO | 1 µM EGM | 2 µM EGM | 3 µM EGM |
| DU145-TxR | 161.5 | 94.84 | 34.16 | 14.58 |
| PC3-TxR | 103 | 19.41 | 8.85 | 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Lin, P.-J.; Hao, J. Eggmanone Effectively Overcomes Prostate Cancer Cell Chemoresistance. Biomedicines 2021, 9, 538. https://doi.org/10.3390/biomedicines9050538
Xie C, Lin P-J, Hao J. Eggmanone Effectively Overcomes Prostate Cancer Cell Chemoresistance. Biomedicines. 2021; 9(5):538. https://doi.org/10.3390/biomedicines9050538
Chicago/Turabian StyleXie, Chen, Pen-Jen Lin, and Jijun Hao. 2021. "Eggmanone Effectively Overcomes Prostate Cancer Cell Chemoresistance" Biomedicines 9, no. 5: 538. https://doi.org/10.3390/biomedicines9050538
APA StyleXie, C., Lin, P.-J., & Hao, J. (2021). Eggmanone Effectively Overcomes Prostate Cancer Cell Chemoresistance. Biomedicines, 9(5), 538. https://doi.org/10.3390/biomedicines9050538







