Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Included and Study Design
2.2. Data Sources
2.3. Identification of Patients on Calcitriol Supplementation
2.4. Identification of Control Subjects through Propensity Score Matching
2.5. Outcome Variables
2.6. Assessment of Additional Covariates
2.7. Statistical Analysis
2.8. Ethical Issues and Confidenciality
3. Results
3.1. Association between Calcitriol Supplementation and SARS-CoV2 Infection
3.2. Association between Calcitriol Supplementation and Risk of Severe COVID-19 or COVID-19 Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Ferreira, A.J.; Silva, E.A.C.S. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008, 93, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.S.; Oliveira Sampaio, W.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/Mas axis of the renin-angiotensin System: Focus on angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Ima, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin convertint enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Anguiano, L.; Clotet, S.; Roca-Ho, H.; Rebull, M.; Pascual, J.; Soler, M.J. Paricalcitol modulates ACE2 shedding and renal AD-AM17 in NOD mica beyond proteinuria. Am. J. Physiol. Renal. Physiol. 2016, 310, F534–F546. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin, D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR Attenuates Acute Lung Injury by Blocking Ang-2-Tie-2 Pathway and Renin-Angiotensin System. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef]
- Dancer, R.C.A.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Machado, C.D.S.; Aissa, A.F.; Ribeiro, D.L.; Antunes, L.M.G. Vitamin D supplementation alters the expression of genes associated with hypertension and did not induce DNA damage in rats. J. Toxicol. Environ. Health Part A 2019, 82, 299–313. [Google Scholar] [CrossRef]
- Teymoori-Rad, M.; Marashi, S.M. Vitamin D and Covid-19: From potential therapeutic effects to unanswered questions. Rev. Med. Virol. 2021, 31, e2159. [Google Scholar] [CrossRef]
- Sekhon, J.S. Multivariate and propensity score matching software with automated balance Ootimization: The matching pack-age for R. J. Statist. Soft. 2011, 42, 1–52. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Yoshida, K.; Bohn, J. Package ‘tableone’ for R. Available online: https://cran.r-project.org/web/packages/tableone/tableone.pdf (accessed on 4 May 2021).
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.2-7. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 4 May 2021).
- Kassambara, A.; Kosinski, M.; Biecek, P.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using ‘ggplot2’. Available online: https://CRAN.R-project.org/package=survminer (accessed on 4 May 2021).
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and respiratory tract infections: A systematic review and me-ta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrition 2020, 12, 3377. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Díaz, J.F.A.; Miranda, J.L.; Bouillon, R.; Gomez, J.M.Q. Effect of calcifediol treatment and best available tehrapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2020. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.; Andress, D. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- KDIGO Guidelines. CKD-Mineral and Bone Disorder (CKD-MBD). Available online: https://kdigo.org/guidelines/ckd-mbd/ (accessed on 3 April 2021).
- Hansdottir, S.; Monick, M.M.; Lovan, N.; Powers, L.; Gerke, A.; Hunnighakw, G.W. Vitamin D decreases respiratory syncytial virus induction of NF-kB chemokines and cytokines in airway epithelium while maintaining the antiviral state. J. Immunol. 2010, 184, 965–974. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.B.; Peehl, D.M.; Feldman, D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: Potential com-bination therapy to treat prostate cancer. J. Nutr. 2007, 13, 205S–210S. [Google Scholar] [CrossRef]
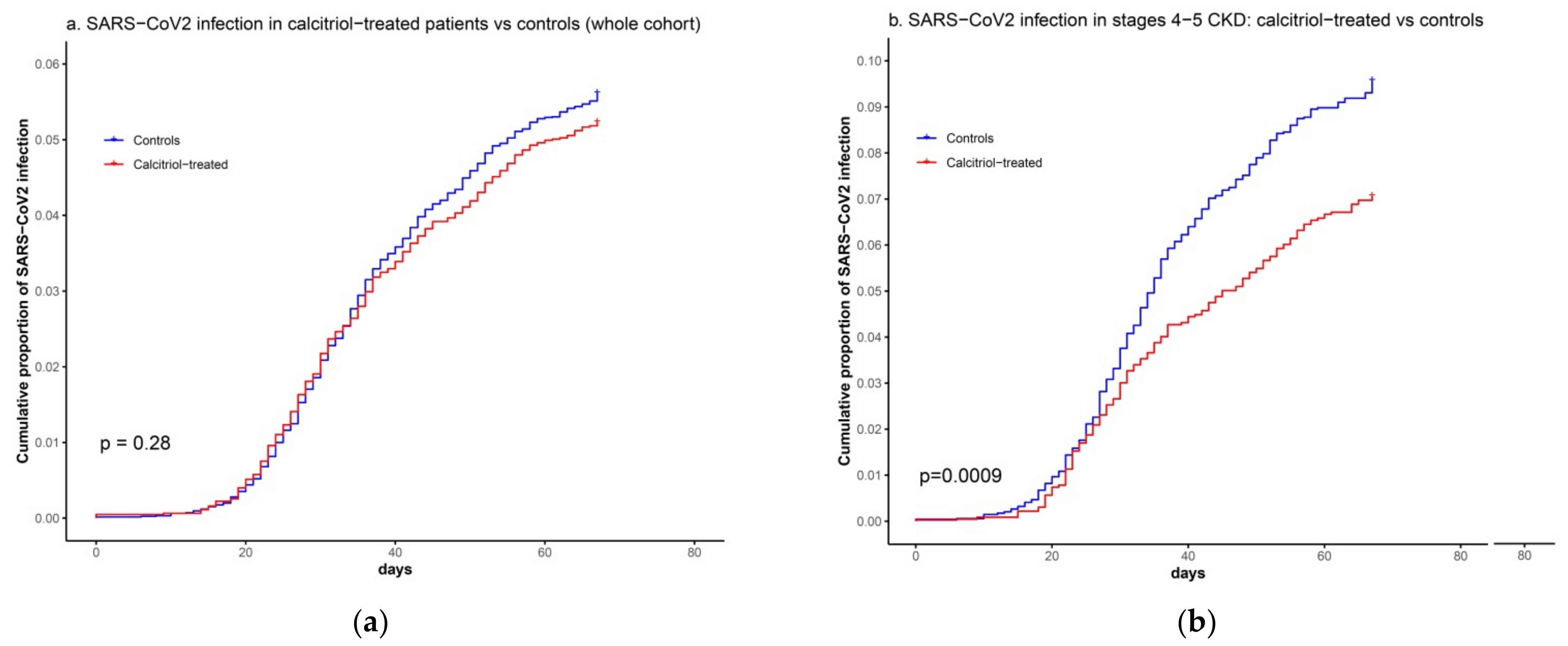
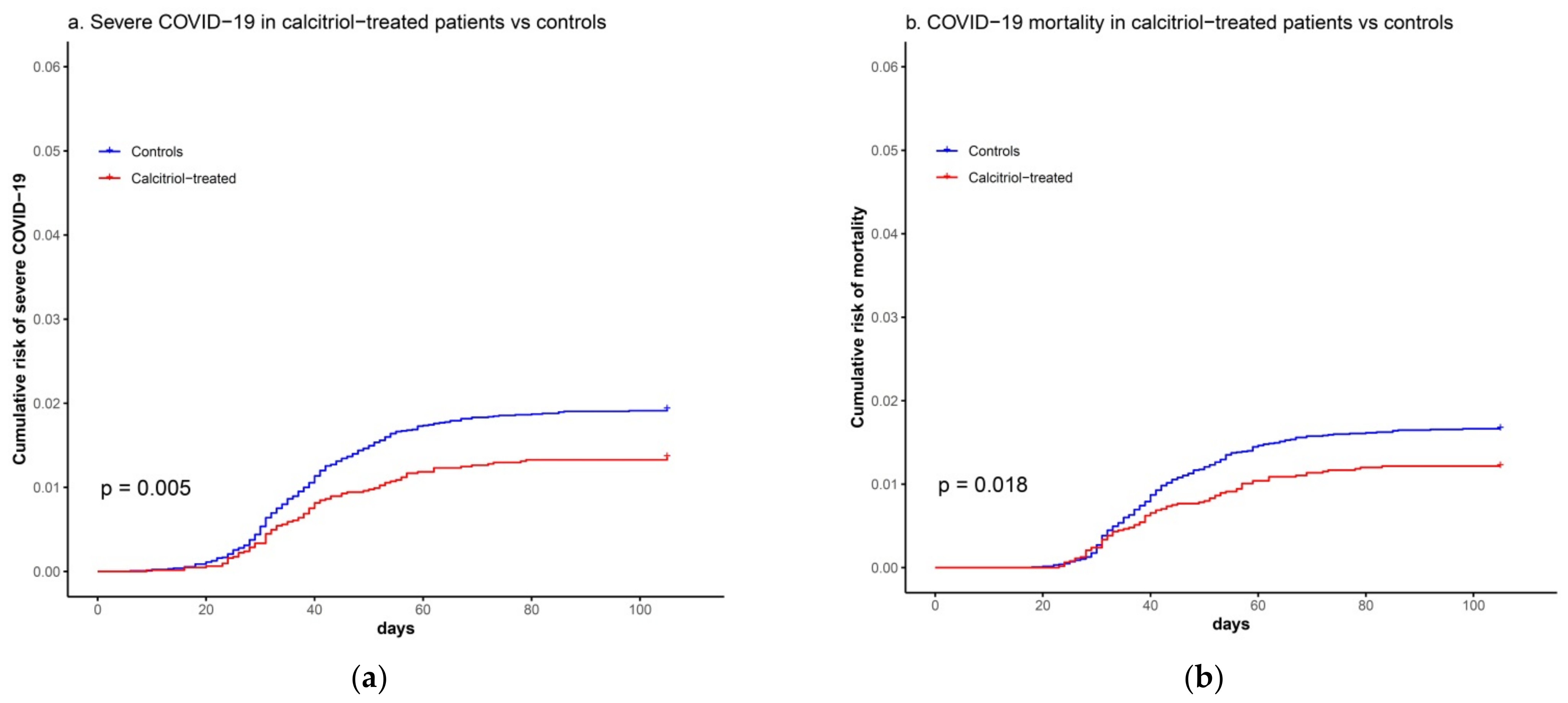
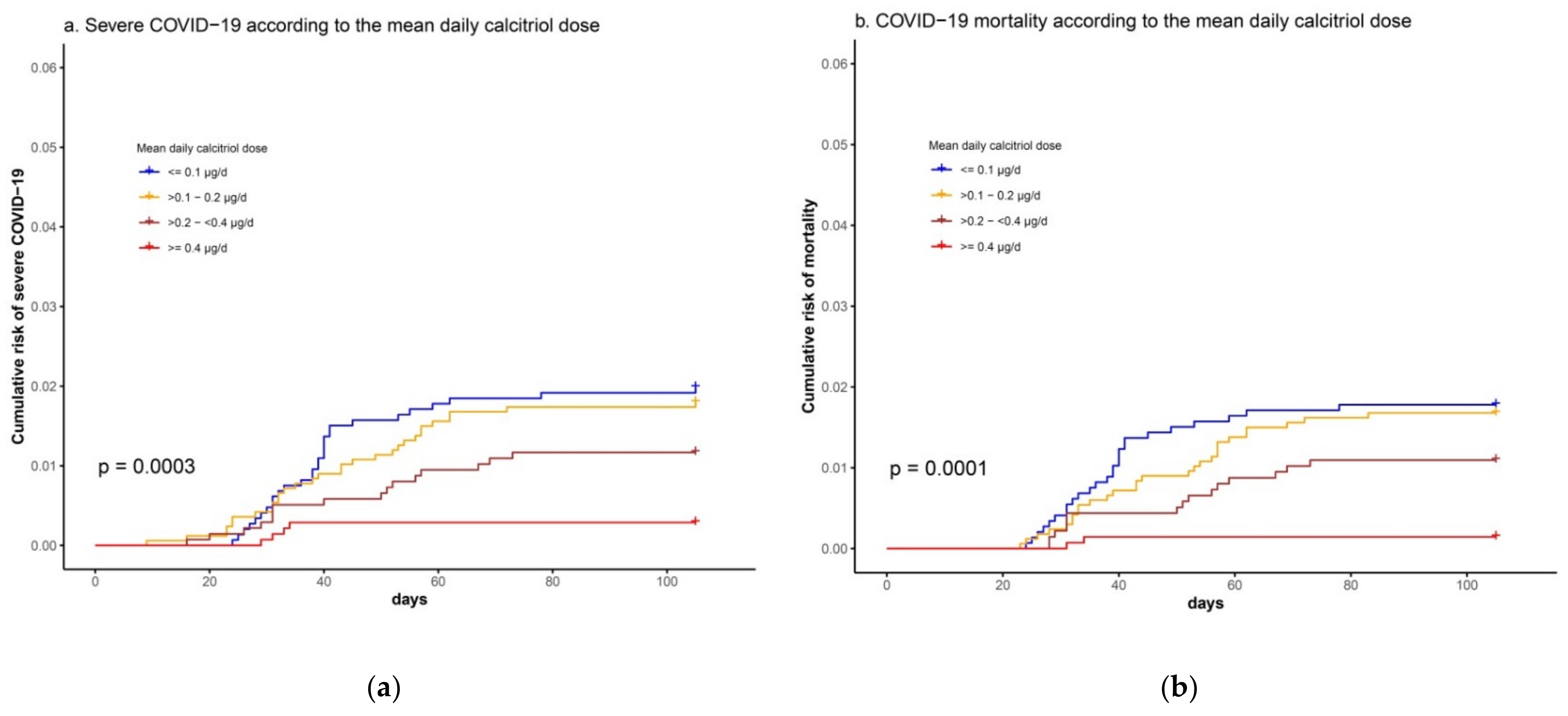
| Variables | Calcitriol-Treated (n = 6252) | Matched Controls (n = 12,504) | P 1 | SMD 2 |
|---|---|---|---|---|
| Female gender, n(%) | 3596 (57.5) | 7185 (57.5) | 0.954 | 0.001 |
| Age, mean (SD) | 70.2 (15.6) | 70.7 (14.7) | 0.022 | 0.035 |
| Cigarette smoking, n(%) | 1762 (28.2) | 3562 (28.5) | 0.676 | 0.007 |
| Nursing home residence, n(%) | 136 (2.2) | 270 (2.2) | 0.986 | 0.001 |
| Hypertension, n(%) | 4308 (68.9) | 8686 (69.5) | 0.443 | 0.012 |
| Obesity, n(%) | 2637 (42.2) | 5430 (43.4) | 0.107 | 0.025 |
| Diabetes, n(%) | 2355 (37.7) | 4857 (38.8) | 0.123 | 0.024 |
| Heart failure, n(%) | 1691 (27.0) | 3633 (29.1) | 0.004 | 0.045 |
| COPD, n(%) | 1255 (20.1) | 2638 (21.1) | 0.107 | 0.025 |
| Asthma, n(%) | 581 (9.3) | 1228 (9.8) | 0.259 | 0.018 |
| eGFR, mean (SD) | 49.0 (30.8) | 48.7 (26.6) | 0.379 | 0.013 |
| Cerebrovascular disease, n(%) | 696 (11.1) | 1487 (11.9) | 0.132 | 0.024 |
| Dementia, n(%) | 345 (5.5) | 744 (6.0) | 0.246 | 0.019 |
| Malignant neoplasia, n(%) | 2451 (39.2) | 5215 (41.7) | 0.001 | 0.051 |
| Liver cirrhosis, n(%) | 106 (1.7) | 235 (1.9) | 0.406 | 0.014 |
| Variables | Overall Cohort 2 | Subjects Diagnosed with CKD, Stages 4–5 3 | ||
|---|---|---|---|---|
| Univariate 4 HR (CI 95%)p 6 | Multivariate 5 HR (CI 95%)p 6 | Univariate 4 HR (CI 95%)p 6 | Multivariate 5 HR (CI 95%)p 6 | |
| Calcitriol treatment | 0.93 (0.82–1.06) | 0.73 (0.60–0.88) *** | 0.78 (0.64–0.94) ** | |
| Female sex | 0.85 (0.75–0.96) ** | 0.87 (0.72–1.03) | 0.78 (0.65–0.93) ** | |
| Age 7 | 1.13 (1.08–1.18) *** | 1.07 (0.99–1.16) | ||
| Cigarette smoking | 1.12 (0.99–1.28) | 1.11 (0.92–1.34) | ||
| Nursing home residence | 6.06 (4.99–7.37) *** | 4.23 (3.42–5.22) *** | 4.55 (3.50–5.92) *** | 4.03(3.03–5.34) *** |
| Hypertension | 1.21 (1.05–1.39) ** | 0.89 (0.70–1.14) | ||
| Obesity | 1.11 (0.98–1.25) | 1.24 (1.04–1.48) * | 1.37 (1.14–1.64) *** | |
| Diabetes | 1.30 (1.15–1.47) *** | 1.10 (0.92–1.32) | ||
| Heart failure | 1.75 (1.54–1.98) *** | 1.24 (1.08–1.42) ** | 1.36 (1.14–1.62) *** | 1.21 (1.01–1.45) * |
| COPD | 1.48 (1.29–1.69) *** | 1.21 (1.05–1.39) ** | 1.19 (0.98–1.44) | |
| Asthma | 1.06 (0.86–1.29) | 0.97 (0.71–1.32) | ||
| eGFR 8 | 0.88 (0.86–0.90) *** | 0.93 (0.90–0.96) *** | 0.74 (0.66–0.83) *** | 0.74 (0.66–0.83) *** |
| Cerebrovascular disease | 1.53 (1.30–1.81) *** | 1.20 (1.02–1.42) * | 1.13 (0.89–1.42) | |
| Dementia | 2.54 (2.12–3.04) *** | 1.64 (1.35–1.99) *** | 2.16 (1.69–2.75) *** | 1.66 (1.28–2.16) *** |
| Malignant neoplasia | 1.17 (1.03–1.32) * | 1.17 (0.98–1.40) | ||
| Liver cirrhosis | 1.02 (0.65–1.61) | 1.28 (0.78–2.11) | ||
| Osteoporosis | 0.97 (0.79–1.20) | 0.88 (0.64–1.22) | ||
| Dyslipidemia | 1.05 (0.93–1.19) | 1.12 (0.93–1.34) | ||
| Ischemic heart disease | 1.18 (1.01–1.38) * | 0.97 (0.79–1.20) | ||
| Peripheral arteriopathy | 1.46 (1.20–1.77) *** | 1.17 (0.91–1.49) | ||
| Hypoparathyroidism | 0.55 (0.40–0.77) *** | 0.22 (0.09–0.54) *** | ||
| Use of PPI | 1.43 (1.26–1.63) *** | 1.22 (1.00–1.50) * | ||
| Use oral corticosteroids | 1.35 (1.15–1.56) *** | 1.25 (1.06–1.46) ** | 1.15 (0.91–1.44) | |
| Use of DPP4-inhibitors | 1.22 (1.02–1.46) * | 0.83 (0.66–1.06) | ||
| Use of statins | 0.94 (0.83–1,06) | 0.81 (0.71–0.92) *** | 0.75 (0.63–0.89) *** | 0.78 (0.65–0.94) ** |
| Use of ACE inhibitors | 0.84 (0.72–0.99) * | 0.85 (0.66–1.09) | ||
| Use of ARB | 0.89 (0.77–1.03) | 0.76 (0.62–0.95) * | ||
| Use of immunosuppressants | 1.03 (0.84–1.28) | 1.24 (0.92–1.68) | 1.53 (1.13–2.07) ** | |
| Renal replacement therapy | 2.38 (1.97–2.86) *** | 1.68 (1.36–2.06) *** | 1.53 (1.25–1.87) *** | |
| Kidney transplant carrier | 0.91 (0.68–1.22) | 1.07 (0.71–1.61) | ||
| Variables | SARS-CoV2 Infection 1 | Severe COVID-19 2 | COVID-19 Mortality | |||
|---|---|---|---|---|---|---|
| Univariate 3 HR (CI 95%)p 5 | Multivariate 4 HR (CI 95%)p 5 | Univariate 3 HR (CI 95%)p 5 | Multivariate 4 HR (CI 95%)p 5 | Univariate 3 HR (CI 95%)p 5 | Multivariate 4 HR (CI 95%)p 5 | |
| Calcitriol dose | 0.90 (0.78–1.03) | 0.40 (0.25–0.62) *** | 0.45 (0.27–0.72) *** | 0.35 (0.21–0.58) *** | 0.53 (0.30–0.94) * | |
| Female sex | 1.03 (0.82–1.29) | 0.78 (0.50–1.21) | 0.87 (0.55–1.39) | |||
| Age 6 | 1.12 (1.04–1.21) ** | 1.84 (1.49–2.26) *** | 1.38 (1.11–1.72) ** | 2.17 (1.71–2.77) *** | 1.42 (1.07–1.87) * | |
| Cigarette smoking | 1.16 (0.91–1.47) | 1.06 (0.65–1.72) | 1.15 (0.70–1.91) | |||
| Nursing home residence | 8.51 (6.17–11.76) *** | 8.02 (5.78–11.12)*** | 12.42 (7.17–21.50) *** | 6.03 (3.30–11.01) *** | 14.31 (8.20–24.98) *** | 6.45 (3.51–11.85) *** |
| Hypertension | 1.09 (0.85–1.39) | 1.92 (1.09–3-36) * | 2.01 (1.10–3.66) * | |||
| Obesity | 1.13 (0.90–1.42) | 1.47 (0.95–2.29) | 1.66 (1.04–2.65) * | |||
| Diabetes | 1.12 (0.89–1.41) | 1.53 (0.98–2.37) | 1.60 (1.01–2.55) * | |||
| Heart failure | 1.75 (1.39–2.21) *** | 1.50 (1.18–1.90)*** | 3.19 (2.05–4.97) *** | 1.66 (1.04–2.65) * | 3.65 (2.28–5.85) *** | 1.79 (1.09–2.94) * |
| COPD | 1.30 (1.00–1.69) * | 1.27 (0.76–2.13) | 1.36 (0.80–2.33) | |||
| Asthma | 1.14 (0.79–1.64) | 0.79 (0.34–1.82) | 0.89 (0.39–2.05) | |||
| eGFR 7 | 0.94 (0.91–0.98) ** | 0.77 (0.69–0.86) *** | 0.68 (0.60–0.78) *** | 0.79 (0.67–0.94) ** | ||
| Cerebrovascular disease | 1.43 (1.04–1.95) * | 1.85 (1.06–3.25) * | 1.95 (1.08–3.49) * | |||
| Dementia | 2.33 (1.64–3.31) *** | 4.91 (2.87–8.39) *** | 2.32 (1.30–4.16) ** | 5.64 (3.27–9.72) *** | 2.37 (1.32–4.25) ** | |
| Malignant neoplasia | 1.06 (0.84–1.34) | 1.47 (0.94–2.28) | 1.57 (1.00–2.44) * | 1.63 (1.02–2.59) * | 1.72 (1.07–2.78) * | |
| Liver cirrhosis | 0.90 (0.37–2.18) | 0.05 (0–75.08) | 0.05 (0–112.61) | |||
| Osteoporosis | 0.90 (0.60–1.34) | 0.94 (0.43–2.04 | 1.06 (0.48–2.30) | |||
| Dyslipidemia | 0.92 (0.74–1.16) | 1.64 (1.04–2.60) * | 1.76 (1.08–2.86) * | |||
| Ischemic heart disease | 1.00 (0.74–1.37) | 1.05 (0.58–1.91) | 1.09 (0.58–2.02) | |||
| Peripheral arteriopathy | 1.27 (0.87–1.85) | 2.07 (1.12–3.83) * | 1.90 (0.97–3.71 | |||
| Hypoparathyroidism | 0.62 (0.43–0.88) ** | 0.19 (0.06–0.60) | 0.07 (0.10–0.49) ** | |||
| Use of PPI | 1.27 (1.01–1.60) * | 1.76 (1.09–2.85) * | 1.83 (1.10–3.05) * | |||
| Use oral corticosteroids | 1.53 (1.16–2.01) ** | 2.24 (1.38–3.64) *** | 2.28 (1.39–3.73) *** | 1.99 (1.18–3.36) ** | ||
| Use of DPP4-inhibitors | 1.02 (0.71–1.46) | 1.50 (0.81–2.77) | 1.53 (0.81–2.92) | |||
| Use of statins | 0.89 (0.71–1.12) | 1.27 (0.81–1.97) | 1.15 (0.72–1.84) | |||
| Use of ACE inhibitors | 0.92 (0.69–1.23) | 0.84 (0.47–1.50) | 0.72 (0.38–1.36) | |||
| Use of ARB | 0.81 (0.61–1.06) | 0.99 (0.59–1.64) | 0.92 (0.53–1.58) | |||
| Use of immunosuppressants | 1.25 (0.89–1.76) | 1.56 (0.84–2.88) | 1.26 (0.63–2.53) | |||
| Renal replacement therapy | 4.14 (2.84–6.04) *** | 3.83(2.61–5.61) *** | 1.88 (0.69–5.16) | 2.12 (0.77–5.82) | ||
| Kidney transplant carrier | 1.23 (0.77–1.96) | 1.44 (0.63–3.33) | 1.06 (0.38–2.91) | |||
| Variables | Overall Cohort 2 | Subjects Diagnosed with CKD, Stages 4–5 3 | ||
|---|---|---|---|---|
| Univariate 4 HR (CI 95%)p 6 | Multivariate 5 HR (CI 95%)p 6 | Univariate 4 HR (CI 95%)p 6 | Multivariate 5 HR (CI 95%)p 6 | |
| Calcitriol treatment | 0.70 (0.55–0.90) ** | 0.68 (0.53–0.87) ** | 0.51 (0.37–0.70) *** | 0.57 (0.41–0.79) *** |
| Female sex | 0.54 (0.43–0.67) *** | 0.68 (0.54–0.86) *** | 0.82 (0.61–1.08) | |
| Age 7 | 1.57 (1.43–1.73) *** | 1.18 (1.06–1.32) ** | 1.19 (1.04–1.35) *** | |
| Cigarette smoking | 1.12 (0.88–1.42) | 1.09 (0.81–1.47) | ||
| Nursing home residence | 6.86 (4.96–9.48) *** | 3.58 (2.52–5.08) *** | 5.01 (3.38–7.43) *** | 3.64 (2.39–5.55) *** |
| Hypertension | 1.82 (1.39–2.40) *** | 0.81 (0.55–1.17) | ||
| Obesity | 1.19 (0.96–1.48) | 1.21(0.91–1.60) | ||
| Diabetes | 1.73 (1.39–2.15) *** | 1.02 (0.77–1.35) | ||
| Heart failure | 2.74 (2.21–3.41) *** | 1.44 (1.15–1.81) ** | 1.61 (1.21–2.13) *** | 1.45 (1.09–1.93) ** |
| COPD | 1.82 (1.45–2.30) *** | 1.24 (0.92–1.66) | ||
| Asthma | 1.02 (0.71–1.47) | 1.20 (0.77–1.89) | ||
| eGFR 8 | 0.72 (0.68–0.76) *** | 0.77 (0.73–0.82) *** | 0.76 (0.63–0.91) ** | 0.77 (0.64–0.93) ** |
| Cerebrovascular disease | 1.83 (1.39–2.41) *** | 1.14 (0.79–1.64) | ||
| Dementia | 4.19 (3.19–5.48) *** | 2.37 (1.76–3.10) *** | 3.04 (2.16–4.27) *** | 2.33 (1.61–3.37) *** |
| Malignant neoplasia | 1.54 (1.24–1.92) *** | 1.26 (1.00–1-58) * | 1.42 (1.08–1.88) ** | 1.41 (1.07–1.87) * |
| Liver cirrhosis | 1.02 (0.45–2.29) | 1.19 (0.53–2.68) | ||
| Osteoporosis | 0.91 (0.62–1.34) | 0.88 (0.53–1.47) | ||
| Dyslipidemia | 1.32 (1.06–1.64) * | 1.36 (1.01–1.82) * | 1.51 (1.11–2.04) ** | |
| Ischemic heart disease | 1.61 (1.25–2.08) *** | 1.00 (0.72–1.39) | ||
| Peripheral arteriopathy | 2.16 (1.61–2.91) *** | 1.42 (0.99–2.03) | ||
| Hypoparathyroidism | 0.19 (0.07–0.50) *** | 0.12 (0.02–0.87) * | ||
| Use of PPI | 2.04 (1.60–2.61) *** | 1.22 (0.89–1.67) | ||
| Use oral corticosteroids | 1.41 (1.07–1.86) ** | 1.12 (0.78–1.60) | ||
| Use of DPP4-inhibitors | 1.16 (0.93–1.44) | 0.67 (0.45–1.01) | ||
| Use of statins | 1.37 (1.01–1.87) * | 0.74 (0.56–0.98) * | 0.74 (0.55–0.99) * | |
| Use of ACE inhibitors | 0.86 (0.65–1.14) | 0.79 (0.52–1.19) | ||
| Use of ARB | 0.88 (0.68–1.14) | 0.68 (0.48–0.97) * | ||
| Use of immunosuppressants | 1.36 (0.97–1.90) | 1.65 (1.17–2.33) ** | 1.47 (0.94–2.29) | 1.83 (1.17–2.86) ** |
| Renal replacement therapy | 1.97 (1.39–2.79) *** | 0.91 (0.63–1.32) | ||
| Kidney transplant carrier | 1.25 (0.80–1.97) | 1.22 (0.66–2.24) | ||
| Variables | Overall Cohort 1 | Subjects Diagnosed with CKD, Stages 4–5 2 | ||
|---|---|---|---|---|
| Univariate 3 HR (CI 95%)p 5 | Multivariate 4 HR (CI 95%)p 5 | Univariate 3 HR (CI 95%)p 5 | Multivariate 4 HR (CI 95%)p 5 | |
| Calcitriol treatment | 0.73 (0.56–0.95) * | 0.66 (0.51–0.86) ** | 0.55 (0.39–0.76) *** | 0.57 (0.41–0.80) *** |
| Female sex | 0.56 (0.45–0.71 )*** | 0.70 (0.54–0.89) ** | 0.83 (0.61–1.11) | |
| Age 6 | 1.92 (1.71–2.15) *** | 1.39 (1.22–1.58) *** | 1.42 (1.22–1.65) *** | 1.42 (1.20–1.67) *** |
| Cigarette smoking | 1.15 (0.90–1.48) | 1.13 (0.83–1.55) | 1.44 (1.04–2.00) * | |
| Nursing home residence | 8.08 (5.82–11.21) *** | 3.63 (2.54–5.18) *** | 5.72 (3.84–8.51) *** | 4.16 (2.70–6.42) *** |
| Hypertension | 2.08 (1.54–2.83) *** | 0.79 (0.54–1.18) | ||
| Obesity | 1.26 (0.99–1.58) | 1.24 (0.93–1.67) | 1.47 (1.09–1.99) * | |
| Diabetes | 1.88 (1.49–2.37) *** | 1.06 (0.79–1.43) | ||
| Heart failure | 3.24 (2.57–4.10) *** | 1.55 (1.21–1.98) *** | 1.76 (1.30–2.38) *** | |
| COPD | 2.02 (1.59–2.59) *** | 1.36 (1.00–1.85) * | ||
| Asthma | 1.11 (0.76–1.62) | 1.21 (0.75–1.95) | ||
| eGFR 7 | 0.69 (0.65–0.73) *** | 0.72 (0.66–0.78) *** | 0.78 (0.64–0.94) ** | 0.73 (0.60–0.89) ** |
| Cerebrovascular disease | 1.87 (1.40–2.51) *** | 1.12 (0.76–1.65) | ||
| Dementia | 4.91 (3.72–6.47) *** | 2.37 (1.75–3.21) *** | 3.39 (2.39–4.81) *** | 2.35 (1.60–3.43) *** |
| Malignant neoplasia | 1.67 (1.32–2.11) *** | 1.34 (1.05–1.72) * | 1.57 (1.17–2.11) ** | 1.48 (1.10–2.00) ** |
| Liver cirrhosis | 0.58 (0.18–1.79) | 0.64 (0.20–2.00) | ||
| Osteoporosis | 0.93 (0.62–1.41) | 0.79 (0.45–1.39) | ||
| Dyslipidemia | 1.38 (1.09–1.75) ** | 1.39 (1.02–1.90) * | ||
| Ischemic heart disease | 1.67 (1.28–2.20) *** | 1.02 (0.73–1.44) | ||
| Peripheral arteriopathy | 2.14 (1.56–2.95) *** | 1.29 (0.87–1.91) | ||
| Hypoparathyroidism | 0.11 (0.03–0.43) ** | 0.14 (0.02–0.98) * | ||
| Use of PPI | 2.21 (1.70–2.88) *** | 1.36 (0.97–1.92) | ||
| Use oral corticosteroids | 1.38 (1.03–1.86) * | 1.10 (0.75–1.62) | ||
| Use of DPP4-inhibitors | 1.46 (1.06–2.01) * | 0.70 (0.46–1.07) | ||
| Use of statins | 1.11 (0.88–1.41) | 0.70 (0.52–0.94) * | ||
| Use of ACE inhibitors | 0.81 (0.59–1.10) | 0.73 (0.47–1.14) | ||
| Use of ARB | 0.9 1 (0.69–1.19) | 0.70 (0.50–0.98) * | ||
| Use of immunosuppressants | 1.16 (0.79–1.71) | 1.60 (1.08–2.37) * | 1.23 (0.75–2.04) | 1.96 (1.17–3.28) ** |
| Renal replacement therapy | 1.78 (1.21–2.63) ** | 0.64 (0.42–0.99) * | 0.79 (0.53–1.19) | |
| Kidney transplant carrier | 0.92 (0.53–1.60) | 0.84 (0.40–1.80) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509. https://doi.org/10.3390/biomedicines9050509
Oristrell J, Oliva JC, Subirana I, Casado E, Domínguez D, Toloba A, Aguilera P, Esplugues J, Fafián P, Grau M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines. 2021; 9(5):509. https://doi.org/10.3390/biomedicines9050509
Chicago/Turabian StyleOristrell, Joaquim, Joan Carles Oliva, Isaac Subirana, Enrique Casado, Didier Domínguez, Andrea Toloba, Patricia Aguilera, Joan Esplugues, Pilar Fafián, and Maria Grau. 2021. "Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study" Biomedicines 9, no. 5: 509. https://doi.org/10.3390/biomedicines9050509
APA StyleOristrell, J., Oliva, J. C., Subirana, I., Casado, E., Domínguez, D., Toloba, A., Aguilera, P., Esplugues, J., Fafián, P., & Grau, M. (2021). Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines, 9(5), 509. https://doi.org/10.3390/biomedicines9050509







