Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy
Abstract
1. Introduction
2. Definition of CSCs
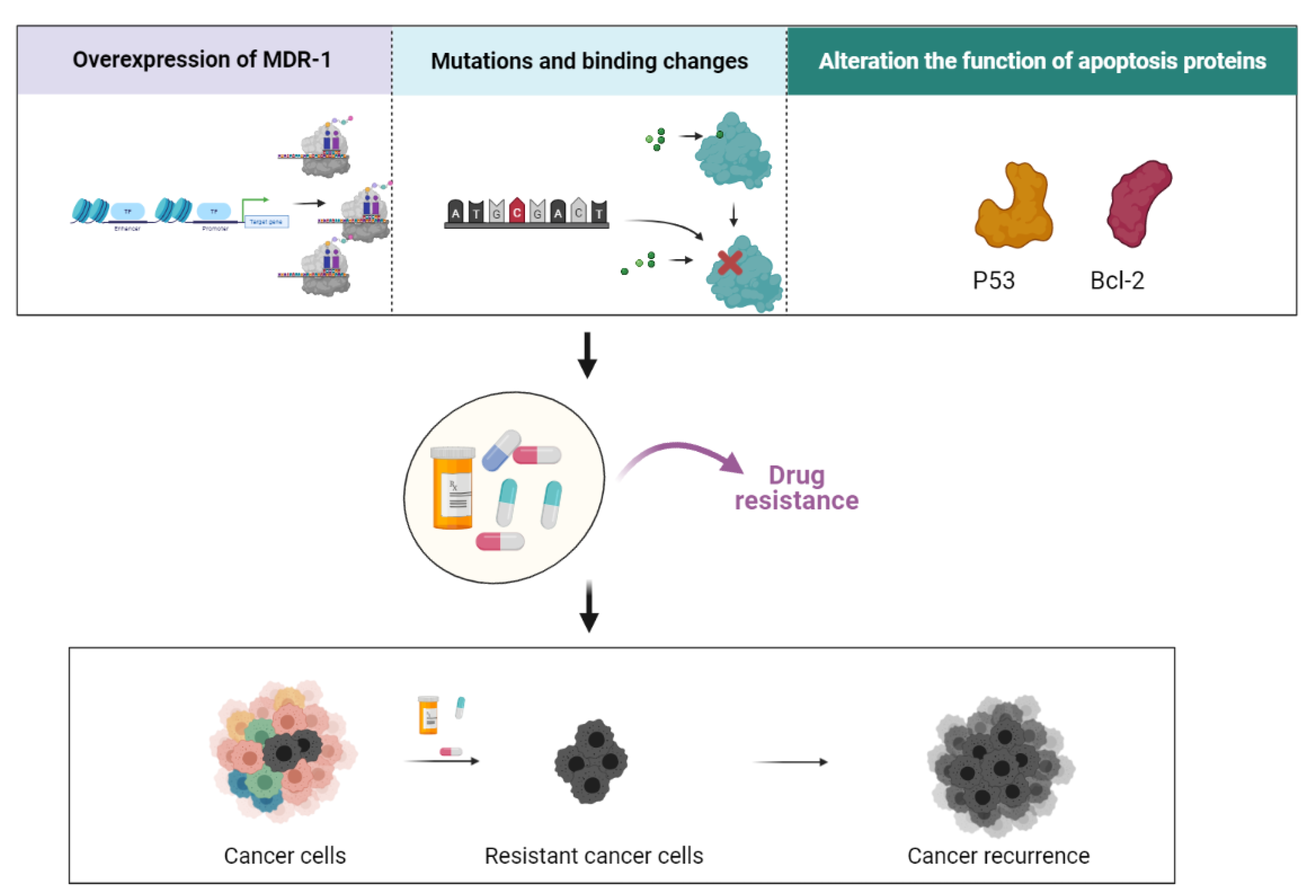
3. Mechanisms of Resistance in CSCs
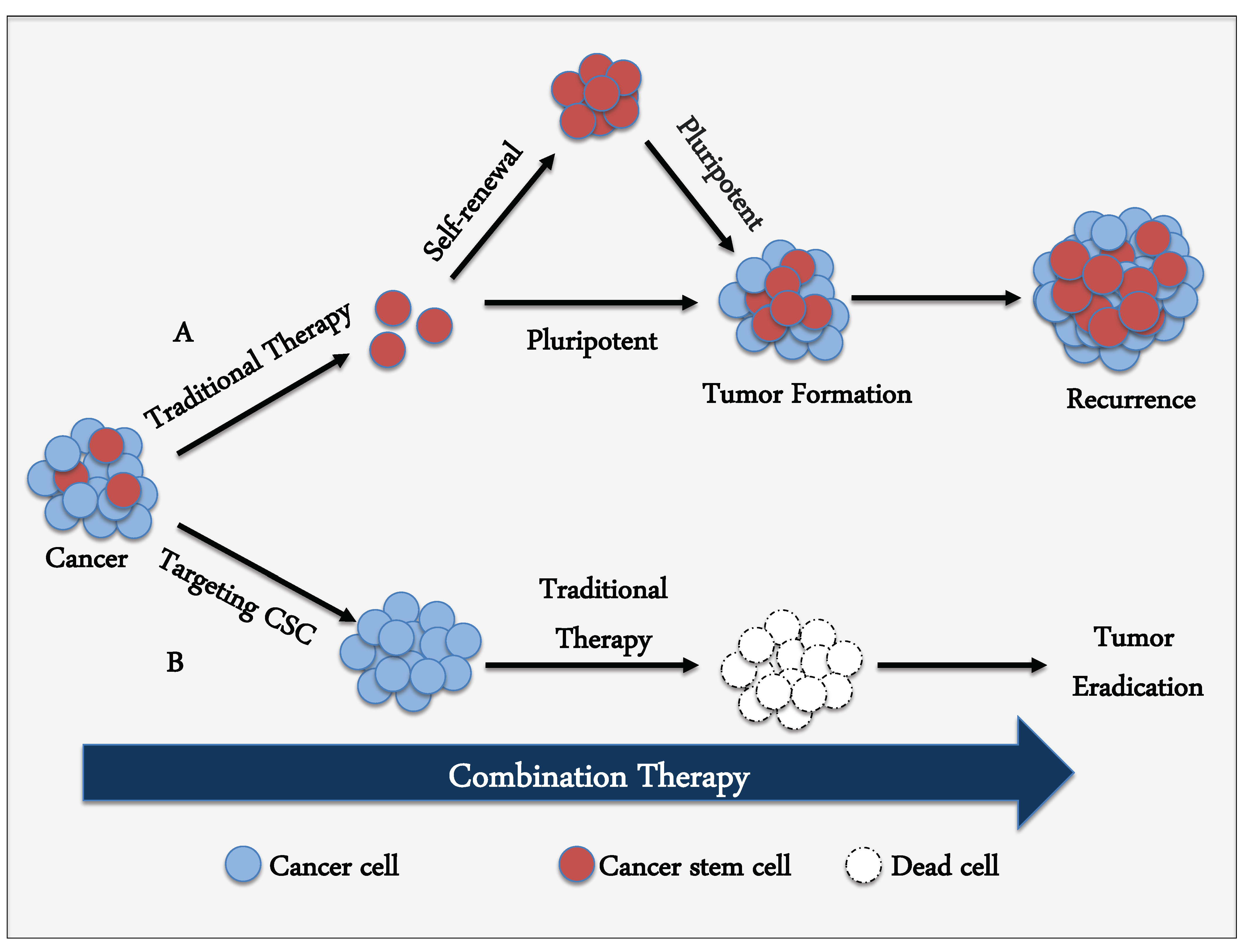
4. Targeting Strategy against CSCs
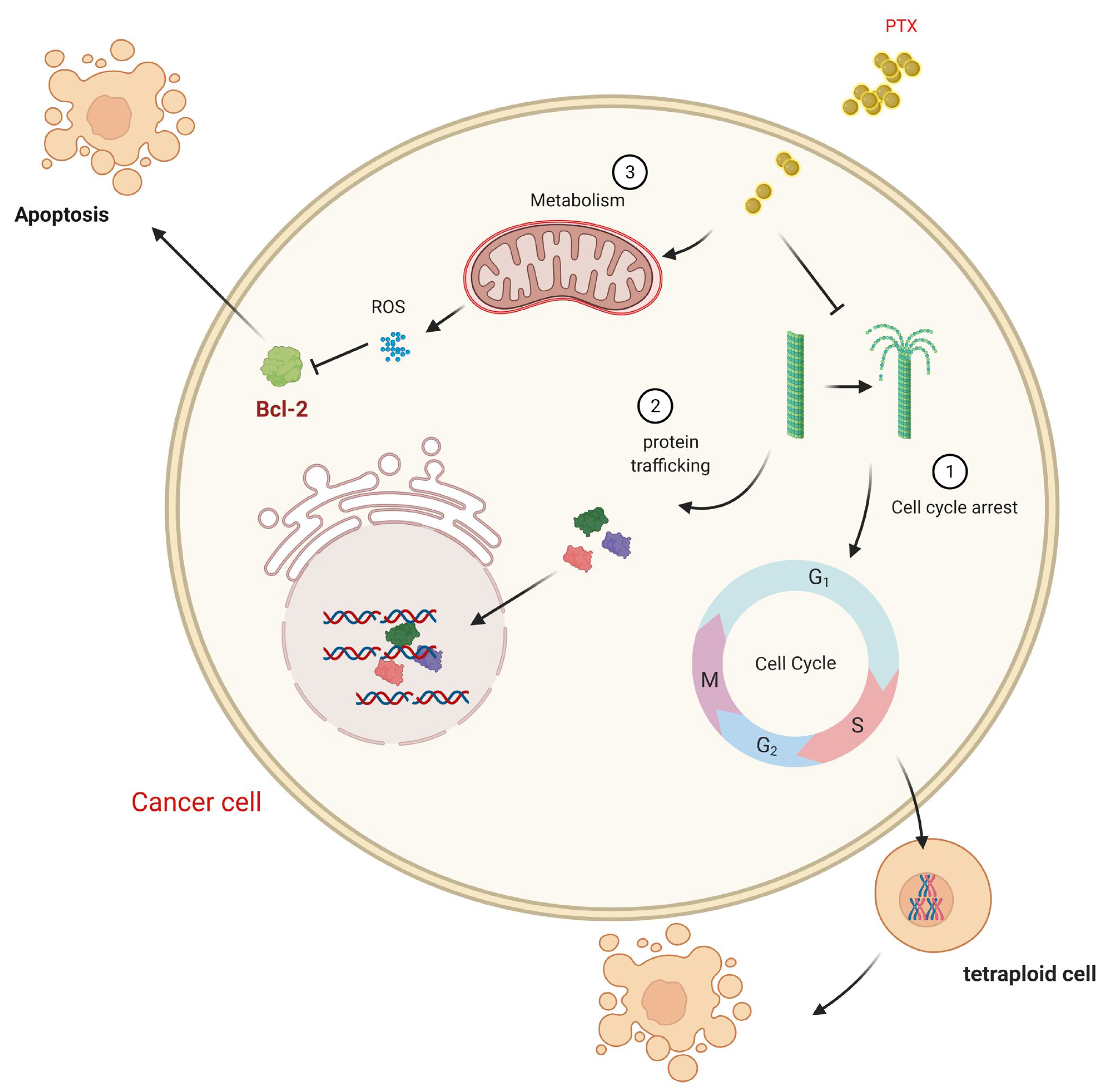
5. Pharmacology and the Mechanism of Action of Paclitaxel
6. Paclitaxel in Drug Resistance
7. Combinatorial Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Seno, M. Conversion of stem cells to cancer stem cells: Undercurrent of cancer initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nat. Cell Biol. 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise reviews: Cancer stem cell targeted therapies: Toward clinical success. Stem. Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv. Exp. Med. Biol. 2015, 890, 57–74. [Google Scholar]
- Singh, S.; Chellappan, S. Lung cancer stem cells: Molecular features and therapeutic targets. Mol. Asp. Med. 2014, 39, 50–60. [Google Scholar] [CrossRef]
- Leon, G.; MacDonagh, L.; Finn, S.P.; Cuffe, S.; Barr, M.P. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol. Ther. 2016, 158, 71–90. [Google Scholar] [CrossRef]
- Zakaria, N.; Satar, N.A.; Abu Halim, N.H.; Ngalim, S.H.; Yusoff, N.M.; Lin, J.; Yahaya, B.H. Targeting lung cancer stem cells: Research and clinical impacts. Front. Oncol. 2017, 7, 80. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Rosell, R. Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl. Lung Cancer Res. 2015, 4, 689–703. [Google Scholar] [PubMed]
- Gottschling, S.; Schnabel, P.A.; Herth, F.J.F.; Herpel, E. Are we missing the target? Cancer stem cells and drug resistance in non-small cell lung cancer. Cancer Genom. Proteom. 2012, 9, 275–286. [Google Scholar]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.K.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nat. Cell Biol. 2015, 517, 209–213. [Google Scholar] [CrossRef]
- Lu, H.; Chen, I.; Shimoda, L.A.; Park, Y.; Zhang, C.; Tran, L.; Zhang, H.; Semenza, G.L. Chemotherapy-induced Ca2+ release stimulates breast cancer stem cell enrichment. Cell Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.K.; Hoffmann, A.D.; Gerson, S.L.; Liu, H. New opportunities and challenges to defeat cancer stem cells. Trends Cancer 2017, 3, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Fiori, M.E.; Villanova, L.; de Maria, R. Cancer stem cells: At the fore front of personalized medicine and immuno-therapy. Curr. Opin. Pharmacol. 2017, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Horwitz, S.B.; Lothstein, L.; Manfredi, J.J.; Mellado, W.; Parness, J.; Roy, S.N.; Schiff, P.B.; Sorbara, L.; Zeheb, R. Taxol: Mechanisms of action and resistance. Ann. N. Y. Acad. Sci. 1986, 466, 733–744. [Google Scholar] [CrossRef]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nat. Cell Biol. 1979, 277, 665–667. [Google Scholar] [CrossRef]
- Beulz-Riché, D.; Robert, J.; Riché, C.; Ratanasavanh, D. Effects of paclitaxel, cyclophosphamide, ifosfamide, tamoxifen and cyclosporine on the metabolism of methoxymorpholino doxorubicin in human liver microsomes. Cancer Chemother. Pharmacol. 2002, 49, 274–280. [Google Scholar] [CrossRef]
- Hood, K.A.; West, L.M.; Rouwé, B.; Northcote, P.T.; Berridge, M.V.; Wakefield, S.J.; Miller, J.H. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule-stabilizing activity. Cancer Res. 2002, 62, 3356–3360. [Google Scholar] [PubMed]
- Lee, F.Y.; Borzilleri, R.; Fairchild, C.R.; Kim, S.H.; Long, B.H.; Reventos-Suarez, C.; Vite, G.D.; Rose, W.C.; A Kramer, R. BMS-247550: A novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin. Cancer Res. 2001, 7, 1429–1437. [Google Scholar] [PubMed]
- Minami, H.; Sasaki, Y.; Watanabe, T.; Ogawa, M. Pharmacodynamic Modeling of the Entire Time Course of Leukopenia after a 3-Hour Infusion of Paclitaxel. Jpn. J. Cancer Res. 2001, 92, 231–238. [Google Scholar] [CrossRef]
- Yasuda, M.; Kimura, E.; Ochiai, K.; Tada, S.; Udagawa, Y.; Aoki, D.; Nozawa, S.; Kikuchi, Y.; Kita, T.; Nishida, M.; et al. Dose finding study of paclitaxel and carboplatin for ovarian cancer (JKTB). Gan. Kagaku Ryoho. 2001, 28, 493–498. [Google Scholar]
- Yen, W.-C.; Corpuz, M.R.; Prudente, R.Y.; Cooke, T.A.; Bissonnette, R.P.; Negro-Vilar, A.; Lamph, W.W. A Selective retinoid X receptor agonist bexarotene (targretin) prevents and overcomes acquired paclitaxel (Taxol) resistance in human non–small cell lung cancer. Clin. Cancer Res. 2004, 10, 8656–8664. [Google Scholar] [CrossRef]
- Swain, S.M.; Honig, S.F.; Tefft, M.C.; Walton, L. A phase II trial of paclitaxel (Taxol) as first line treatment in advanced breast cancer. Investig. New Drugs 1995, 13, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M. Paclitaxel (Taxol): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Kingston, D.G. Taxol: The chemistry and structure-activity relationships of a novel anticancer agent. Trends Biotechnol. 1994, 12, 222–227. [Google Scholar] [CrossRef]
- Long, B.H.; Carboni, J.M.; Wasserman, A.J.; Cornell, L.A.; Casazza, A.M.; Jensen, P.R.; Lindel, T.; Fenical, W.; Fairchild, C.R. Eleutherobin, a novel cytotoxic agent that induces tubulin polymerization, is similar to paclitaxel (Taxol). Cancer Res. 1998, 58, 1111–1115. [Google Scholar]
- Plasswilm, L.; Cordes, N.; Fietkau, R.; Sauer, R. Cytotoxicity of fractionated paclitaxel (Taxol) administration in vitro. Strahlenther. Onkol. 1998, 174, 37–42. [Google Scholar] [CrossRef]
- Spencer, C.M.; Faulds, D. Paclitaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs 1994, 48, 794–847. [Google Scholar] [CrossRef] [PubMed]
- Sulkes, A.; Beller, U.; Peretz, T.; Shacter, J.; Hornreich, G.; McDaniel, C.; Winograd, B. Taxol: Initial Israeli experience with a novel anticancer agent. Isr. J. Med Sci. 1994, 30, 70–78. [Google Scholar]
- Wenk, M.R.; Fahr, A.; Reszka, R.; Seelig, J. Paclitaxel partitioning into lipid bilayers. J. Pharm. Sci. 1996, 85, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Willey, T.A.; Bekos, E.J.; Gaver, R.C.; Duncan, G.F.; Tay, L.K.; Beijnen, J.H.; Farmen, R.H. High-performance liquid chromatographic procedure for the quantitative determination of paclitaxel (Taxol®) in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 1993, 621, 231–238. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Mullins, D.W.; Burger, C.J.; Elgert, K.D. Paclitaxel enhances macrophage IL-12 production in tumor-bearing hosts through nitric oxide. J. Immunol. 1999, 162, 6811–6818. [Google Scholar] [PubMed]
- Glass-Marmor, L.; Beitner, R. Taxol (paclitaxel) induces a detachment of phosphofructokinase from cytoskeleton of melanoma cells and decreases the levels of glucose 1,6-bisphosphate, fructose 1,6-bisphosphate and ATP. Eur. J. Pharmacol. 1999, 370, 195–199. [Google Scholar] [CrossRef]
- Wang, T.; Shigdar, S.; Gantier, M.P.; Hou, Y.; Wang, L.; Li, Y.; Al Shamaileh, H.; Yingchun, H.; Zhou, S.-F.; Zhao, X.; et al. Cancer stem cell targeted therapy: Progress amid controversies. Oncotarget 2015, 6, 44191–44206. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Fan, Z.; Liu, H.; Zhang, X.; Cai, Z.; Xu, L.; Luo, J.; Huang, Y.; He, L.; et al. Single-cell sequencing reveals variants in ARID1A, GPRC5A and MLL2 driving self-renewal of human bladder cancer stem cells. Eur. Urol. 2017, 71, 8–12. [Google Scholar] [CrossRef]
- Barbuti, A.M.; Chen, Z.S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Lung cancers: Molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Li, R.; Liu, Z.; Zhang, J.; Luo, R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: A systematic review. Int. J. Clin. Exp. Pathol. 2013, 6, 2644–2650. [Google Scholar]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. Lung cancer stem cells: The root of resistance. Cancer Lett. 2016, 372, 147–156. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Liu, H. Overview of Cancer Stem Cells and Stemness for Community Oncologists. Target. Oncol. 2017, 12, 387–399. [Google Scholar] [CrossRef]
- Bauerschmitz, G.J.; Ranki, T.; Kangasniemi, L.; Ribacka, C.; Eriksson, M.; Porten, M.; Herrmann, I.; Ristimäki, A.; Virkkunen, P.; Tarkkanen, M.; et al. Tissue-specific promoters active in CD44+CD24−/low breast cancer cells. Cancer Res. 2008, 68, 5533–5539. [Google Scholar] [CrossRef]
- Lee, C.J.; Dosch, J.; Simeone, D.M. Pancreatic cancer stem cells. J. Clin. Oncol. 2008, 26, 2806–2812. [Google Scholar] [CrossRef]
- Matsui, W.; Wang, Q.; Barber, J.P.; Brennan, S.; Smith, B.D.; Borrello, I.; McNiece, I.; Lin, L.; Ambinder, R.F.; Peacock, C.; et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008, 68, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Yan, T.; Mizutani, A.; Sota, T.; Hiramoto, Y.; Prieto-Vila, M.; Chen, L.; Satoh, A.; Kudoh, T.; Kasai, T.; et al. Cancer stem cells maintain a hierarchy of differentiation by creating their niche. Cancer Cell Biol. 2014, 135, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Afify, S.M.; Nair, N.; Kumon, K.; Osman, A.; Du, J.; Mansour, H.; Quora, H.A.A.; Nawara, H.M.; Satoh, A. Hematopoietic cells derived from cancer stem cells generated from mouse induced pluripotent stem cells. Cancers 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Oze, M.; Afify, S.M.; Hassan, G.; L-Ghlban, S.E.; Nawara, H.M.; Fu, X.; Zahra, M.H.; Seno, A.; Winerh, I.; et al. Tumor-associated macrophages derived from cancer stem cells. Acta Histochem. 2020, 122, 151628. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Afify, S.M.; Hassan, G.; Fu, X.; Seno, A.; Seno, M. Revisiting cancer stem cells as the origin of cancer-associated cells in the tumor microenvironment: A hypothetical view from the potential of iPSCs. Cancers 2020, 12, 879. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radio resistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M. Association of reactive oxygen species levels and radio resistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Cheng, J.; Xie, B.; Wang, B.; Yi, J.; Tian, B.; Liu, Z.; Wang, F.; Zhang, Z. Characteristics of doxorubicin-selected multidrug-resistant human leukemia HL-60 cells with tolerance to arsenic trioxide and contribution of leukemia stem cells. Oncol. Lett. 2018, 15, 1255–1262. [Google Scholar] [CrossRef]
- Luzhna, L.; Lykkesfeldt, A.E.; Kovalchuk, O. Altered radiation responses of breast cancer cells resistant to hormonal therapy. Oncotarget 2015, 6, 1678–1694. [Google Scholar] [CrossRef]
- Alderton, G.K. Tumour evolution: Epigenetic and genetic heterogeneity in metastasis. Nat. Rev. Cancer 2017, 17, 141. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S.E. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Aoi, T. Biology of lung cancer: Genetic mutation, epithelial-mesenchymal transition, and cancer stem cells. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 517–523. [Google Scholar] [CrossRef]
- Shibata, M.; Hoque, M.O. Targeting cancer stem cells: A strategy for effective eradication of cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Zhang, J.-G.; Zhang, X.; Li, Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Chen, K.; Shi, W. Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel. Tumor Biol. 2016, 37, 10539–10544. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, D.; Yang, R.; Wang, S. Paclitaxel: New uses for an old drug. Drug Des. Dev. Ther. 2014, 8, 279–284. [Google Scholar] [CrossRef]
- Ferlini, C.; Raspaglio, G.; Mozzetti, S.; Distefano, M.; Filippetti, F.; Martinelli, E.; Ferrandina, G.; Gallo, D.; Ranelletti, F.O.; Scambia, G. Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol. Pharmacol. 2003, 64, 51–58. [Google Scholar] [CrossRef]
- Manfredi, J.J.; Parness, J.; Horwitz, S.B. Taxol binds to cellular microtubules. J. Cell Biol. 1982, 94, 688–696. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Horwitz, S.B.; Cohen, D.; Rao, S.; Ringel, I.; Shen, H.J.; Yang, C.P. Taxol: Mechanisms of action and resistance. J. Natl. Cancer Inst. Monogr. 1993, 1993, 55–61. [Google Scholar] [CrossRef]
- Samaan, T.M.A.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.; Fojo, T. Taxanes. Cancer Chemother. Biol. Response Modif. 1997, 17, 59–79. [Google Scholar] [PubMed]
- Snyder, J.P.; Nettles, J.H.; Cornett, B.; Downing, K.H. The binding conformation of Taxol in β-tubulin: A model based on electron crystallographic density. Proc. Natl. Acad. Sci. USA 2001, 98, 5312–5316. [Google Scholar] [CrossRef] [PubMed]
- Combeau, C.; Commercon, A.; Mioskowski, C.; Rousseau, B.; Aubert, F.; Goeldner, M. Predominant labeling of beta- over alpha-tubulin from porcine brain by a photoactivatable taxoid derivative. Biochemistry 1994, 33, 6676–6683. [Google Scholar] [CrossRef]
- Collins, C.; Vallee, R.B. Temperature-dependent reversible assembly of taxol-treated microtubules. J. Cell Biol. 1987, 105, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Giannakakou, P.; Robey, R.; Fojo, T.; Blagosklonny, M.V. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: Molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 2001, 20, 3806–3813. [Google Scholar] [CrossRef]
- Parekh, H.; Wiesen, K.; Simpkins, H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem. Pharmacol. 1997, 53, 461–470. [Google Scholar] [CrossRef]
- Tran, T.-A.; Gillet, L.; Roger, S.; Besson, P.; White, E.; Le Guennec, J.-Y. Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem. Biophys. Res. Commun. 2009, 379, 304–308. [Google Scholar] [CrossRef]
- Shetti, D.; Zhang, B.; Fan, C.; Mo, C.; Lee, B.H.; Wei, K. Low dose of paclitaxel combined with XAV939 attenuates metastasis, angiogenesis and growth in breast cancer by suppressing wnt signaling. Cells 2019, 8, 892. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Giannakakou, P.; El-Deiry, W.S.; Kingston, D.G.; Higgs, P.I.; Neckers, L.; Fojo, T. Raf-1/bcl-2 phosphorylation: A step from microtubule damage to cell death. Cancer Res. 1997, 57, 130–135. [Google Scholar]
- Haldar, S.; Basu, A.; Croce, C.M. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997, 57, 229–233. [Google Scholar] [PubMed]
- Ling, Y.H.; Yang, Y.; Tornos, C.; Singh, B.; Perez-Soler, R. Paclitaxel-induced apoptosis is associated with expression and activation of c-Mos gene product in human ovarian carcinoma SKOV3 cells. Cancer Res. 1998, 58, 3633–3640. [Google Scholar] [PubMed]
- Blagosklonny, M.V.; Fojo, T. Molecular effects of paclitaxel: Myths and reality (a critical review). Int. J. Cancer 1999, 83, 151–156. [Google Scholar] [CrossRef]
- Ding, A.H.; Porteu, F.; Sanchez, E.; Nathan, C.F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science 1990, 248, 370–372. [Google Scholar] [CrossRef]
- Manthey, C.L.; Brandes, M.E.; Perera, P.Y.; Vogel, S.N. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J. Immunol. 1992, 149, 2459–2465. [Google Scholar]
- O’Brien, J.M.; Wewers, M.D.; Moore, S.A.; Allen, J.N. Taxol and colchicine increase LPS-induced pro-IL-1 beta production, but do not increase IL-1 beta secretion. A role for microtubules in the regulation of IL-1 beta production. J. Immunol. 1995, 154, 4113–4122. [Google Scholar]
- Rosette, C.; Karin, M. Cytoskeletal control of gene expression: Depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 1995, 128, 1111–1119. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Schulte, T.W.; Nguyen, P.; Mimnaugh, E.G.; Trepel, J.; Neckers, L. Taxol induction of p21WAF1 and p53 requires c-raf-1. Cancer Res. 1995, 55, 4623–4626. [Google Scholar]
- Hari, M.; Loganzo, F.; Annable, T.; Tan, X.; Musto, S.; Morilla, D.B.; Nettles, J.H.; Snyder, J.P.; Greenberger, L.M. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol. Cancer Ther. 2006, 5, 270–278. [Google Scholar] [CrossRef]
- Yusuf, R.Z.; Duan, Z.; Lamendola, D.E.; Penson, R.T.; Seiden, M.V. Paclitaxel Resistance: Molecular Mechanisms and Pharmacologic Manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Bradley, G.; Ling, V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994, 13, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Sangrajrang, S.; Fellous, A. Taxol resistance. Chemotherapy 2000, 46, 327–334. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Mayer, U.; Wagenaar, E.; Mol, C.A.A.M.; Van Deemter, L.; Smit, J.J.M.; Van Der Valk, M.A.; Voordouw, A.C.; Spits, H.; Van Tellingen, O.; et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc. Natl. Acad. Sci. USA 1997, 94, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Paull, K.; Monks, A.; Hose, C.; Lee, J.S.; Weinstein, J.; Grever, M.; Bates, S.; Fojo, T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the National Cancer Institute Anticancer Drug Screen. J. Clin. Investig. 1995, 95, 2205–2214. [Google Scholar] [CrossRef]
- Schöndorf, T.; Scharl, A.; Kurbacher, C.M.; Bien, O.; Becker, M.; Neumann, R.; Kolhagen, H.; Rustemeyer, J.; Mallmann, P.; Göhring, U.-J. Amplification of the mdr1-gene is uncommon in recurrent ovarian carcinomas. Cancer Lett. 1999, 146, 195–199. [Google Scholar] [CrossRef]
- Duan, Z.; Feller, A.J.; Penson, R.T.; Chabner, B.A.; Seiden, M.V. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: Analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin. Cancer Res. 1999, 5, 3445–3453. [Google Scholar]
- Schöndorf, T.; Kurbacher, C.M.; Göhring, U.-J.; Benz, C.; Becker, M.; Sartorius, J.; Kolhagen, H.; Mallman, P.; Neumann, R. Induction of MDR1-gene expression by antineoplastic agents in ovarian cancer cell lines. Anticancer. Res. 2002, 22, 2199–2203. [Google Scholar]
- Haber, M.; Burkhart, C.A.; Regl, D.L.; Madafiglio, J.; Norris, M.D.; Horwitz, S.B. Altered expression of Mβ2, the class II β-tubulin isotype, in a murine J774.2 cell line with a high level of taxol resistance. J. Biol. Chem. 1995, 270, 31269–31275. [Google Scholar] [CrossRef]
- Bhalla, K.; Huang, Y.; Tang, C.; Self, S.; Ray, S.; Mahoney, M.E.; Ponnathpur, V.; Tourkina, E.; Ibrado, A.M.; Bullock, G. Characterization of a human myeloid leukemia cell line highly resistant to taxol. Leukemia 1994, 8, 465–475. [Google Scholar]
- Cabral, F.; Wible, L.; Brenner, S.; Brinkley, B.R. Taxol-requiring mutant of Chinese hamster ovary cells with impaired mitotic spindle assembly. J. Cell Biol. 1983, 97, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M.; Kuo, D.Y.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Investig. 1997, 100, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Miller, H.P.; Banerjee, A.; Luduena, R.F.; Wilson, L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl. Acad. Sci. USA 1994, 91, 11358–11362. [Google Scholar] [CrossRef]
- Derry, W.B.; Wilson, L.; Jordan, M.A. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry 1995, 34, 2203–2211. [Google Scholar] [CrossRef]
- Giannakakou, P.; Sackett, D.L.; Kang, Y.-K.; Zhan, Z.; Buters, J.T.M.; Fojo, T.; Poruchynsky, M.S. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J. Biol. Chem. 1997, 272, 17118–17125. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Nishio, K.; Kubota, N.; Ohmori, T.; Funayama, Y.; Ohira, T.; Nakajima, H.; Adachi, M.; Saijo, N. Characterization of a taxol-resistant human small-cell lung cancer cell line. Jpn. J. Cancer Res. 1994, 85, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Benetatos, C.A.; Colarusso, P.J.; Dexter, D.W.; Hudes, G.R. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br. J. Cancer 1998, 77, 562–566. [Google Scholar] [CrossRef]
- Jaffrézou, J.P.; Dumontet, C.; Derry, W.B.; Durán, G.; Chen, G.; Tsuchiya, E.; Wilson, L.; Jordan, M.A.; Sikic, B.I. Novel mechanism of resistance to paclitaxel (Taxol) in human K562 leukemia cells by combined selection with PSC 833. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 1995, 7, 517–527. [Google Scholar]
- Dumontet, C.; Duran, G.E.; Steger, K.A.; Beketic-Oreskovic, L.; Sikic, B.I. Resistance mechanisms in human sarcoma mutants derived by single-step exposure to paclitaxel (Taxol). Cancer Res. 1996, 56, 1091–1097. [Google Scholar]
- White, E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996, 10, 1–15. [Google Scholar] [CrossRef]
- Lanni, J.S.; Lowe, S.W.; Licitra, E.J.; Liu, J.O.; Jacks, T. p53-independent apoptosis induced by paclitaxel through an indirect mechanism. Proc. Natl. Acad. Sci. USA 1997, 94, 9679–9683. [Google Scholar] [CrossRef]
- Wahl, A.F.; Donaldson, K.L.; Faircnild, C.; Lee, F.Y.F.; Foster, S.A.; Demers, G.W.; Galloway, D.A. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat. Med. 1996, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Willingham, M.C.; Reed, J.C.; Miyashita, T.; Ray, S.; Ponnathpur, V.; Huang, Y.; Mahoney, M.E.; Bullock, G.; Bhalla, K. High levels of p26BCL-2 oncoprotein retard taxol-induced apoptosis in human pre-B leukemia cells. Leukemia 1994, 8, 1960–1969. [Google Scholar]
- McDonnell, T.J.; Troncoso, P.; Brisbay, S.M.; Logothetis, C.; Chung, L.W.; Hsieh, J.T.; Tu, S.M.; Campbell, M.L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992, 52, 6940–6944. [Google Scholar] [PubMed]
- Zhang, C.C.; Yang, J.-M.; White, E.; Murphy, M.; Levine, A.; Hait, W.N. The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene 1998, 16, 1617–1624. [Google Scholar] [CrossRef]
- Terasima, T.; Tolmach, L.J. X-Ray sensitivity and DNA synthesis in synchronous populations of hela cells. Science 1963, 140, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Tishler, R.B.; Schiff, P.B.; Geard, C.R.; Hall, E.J. Taxol: A novel radiation sensitizer. Int. J. Radiat. Oncol. 1992, 22, 613–617. [Google Scholar] [CrossRef]
- Lokeshwar, B.L.; Ferrell, S.M.; Block, N.L. Enhancement of radiation response of prostatic carcinoma by taxol: Therapeutic potential for late-stage malignancy. Anticancer. Res. 1995, 15, 93–98. [Google Scholar]
- Cividalli, A.; Arcangeli, G.; Cruciani, G.; Livdi, E.; Danesi, D.T. Enhancement of radiation response by paclitaxel in mice according to different treatment schedules. Int. J. Radiat. Oncol. 1998, 40, 1163–1170. [Google Scholar] [CrossRef]
- Erlich, E.; McCall, A.R.; Potkul, R.; Walter, S.; Vaughan, A. paclitaxel is only a weak radiosensitizer of human cervical carcinoma cell lines. Gynecol. Oncol. 1996, 60, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Sevin, B.-U.; Perras, J.; Nguyen, H.N.; Pham, C.; Steren, A.J.; Koechli, O.R.; Averette, H.E. Paclitaxel: A radiation sensitizer of human cervical cancer cells. Gynecol. Oncol. 1995, 57, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, J.; Fisher, J.; Teague, D.; Cook, J.A.; Mitchell, J.B. In vitro studies of taxol as a radiation sensitizer in human tumor cells. J. Natl. Cancer Inst. 1994, 86, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016, 37, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Kaur, J.; Tikoo, K. Dual drug-loaded paclitaxel–thymoquinone nanoparticles for effective breast cancer therapy. J. Nanoparticle Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; Ajabnoor, G.M.; Al-Abd, A.M. Thymoquinone enhances paclitaxel anti-breast cancer activity via inhibiting tumor-associated stem cells despite apparent mathematical antagonism. Molecules 2020, 25, 426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yoo, H.-I.; Kang, H.S.; Ro, J.; Yoon, S. Salinomycin sensitizes antimitotic drugs-treated cancer cells by increasing apoptosis via the prevention of G2 arrest. Biochem. Biophys. Res. Commun. 2012, 418, 98–103. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, G.; Dong, S.; Jiang, Z.-X.; Chena, M. An iTEP-salinomycin nanoparticle that specifically and effectively inhibits metastases of 4T1 orthotopic breast tumors. Biomaterials 2016, 93, 1–9. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Nandy, A.; Hor, P.; Mukhopadhyay, A. Breast cancer stem cells: A novel therapeutic target. Clin. Breast Cancer 2013, 13, 7–15. [Google Scholar] [CrossRef]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef]
- Tian, J.; Lo, C.; Al Raffa, F.; Dai, M.; Lebrun, J.-J. Abstract 144: Dasatinib enhances the effects of paclitaxel on chemotherapy-resistant breast cancer through targeting breast cancer stem cells. Tumor Biol. 2018, 78 (Suppl. 13), 144. [Google Scholar]
- Fornier, M.N.; Morris, P.G.; Abbruzzi, A.; D’Andrea, G.; Gilewski, T.; Bromberg, J.; Dang, C.; Dickler, M.; Modi, S.; Seidman, A.D.; et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann. Oncol. 2011, 22, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wei, J.; Su, G.H.; Lin, J. Dasatinib can enhance paclitaxel and gemcitabine inhibitory activity in human pancreatic cancer cells. Cancer Biol. Ther. 2019, 20, 855–865. [Google Scholar] [CrossRef]
- Merz, M.; Komljenovic, D.; Zwick, S.; Semmler, W.; Bäuerle, T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur. J. Cancer 2011, 47, 277–286. [Google Scholar] [CrossRef]
- Choi, K.H.; Jeon, J.Y.; Lee, Y.-E.; Kim, S.W.; Kim, S.Y.; Yun, Y.J.; Park, K.C. Synergistic activity of paclitaxel, sorafenib, and radiation therapy in advanced renal cell carcinoma and breast cancer. Transl. Oncol. 2019, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Calle, A.S.; Hassan, G.; Kumon, K.; Nawara, H.M.; Zahra, M.H.; Mansour, H.M.; Khayrani, A.C.; Alam, M.J.; Du, J.; et al. A novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br. J. Cancer 2020, 122, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Atallah, M.N.; Mansour, H.; Quora, H.A.A.; Alam, M.J.; Osman, A.; Kakuta, H.; et al. Paclitaxel and sorafenib: The effective combination of suppressing the self-renewal of cancer stem cells. Cancers 2020, 12, 1360. [Google Scholar] [CrossRef]
- Pashaei-Asl, F.; Pashaei-Asl, R.; Khodadadi, K.; Akbarzadeh, A.; Ebrahimie, E.; Pashaiasl, M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1483–1487. [Google Scholar] [CrossRef]
- Cheung, C.W.Y.; Gibbons, N.; Johnson, D.W.; Nicol, D.L. Silibinin—A promising new treatment for cancer. Anticancer. Agents Med. Chem. 2010, 10, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Tecklenburg, M.; Sclafani, R.A.; Agarwal, R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene 2003, 22, 8271–8282. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol. Biomark. Prev. 2003, 12, 933–939. [Google Scholar]
- Deep, G.; Singh, R.P.; Agarwal, C.; Kroll, D.J.; Agarwal, R. Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef]
- Ho, B.Y.; Lin, C.-H.; Apaya, M.K.; Chao, W.-W.; Shyur, L.-F. Silibinin and paclitaxel cotreatment significantly suppress the activity and lung metastasis of triple negative 4T1 mammary tumor cell in mice. J. Tradit. Complement Med. 2012, 2, 301–311. [Google Scholar] [CrossRef][Green Version]
- Tyagi, A.K.; Agarwal, C.; Chan, D.C.; Agarwal, R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol. Rep. 2004, 11, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, P.; Chen, B.; Wang, Y.; Wang, X.; Internati, M.C.; Wachtel, M.S.; Frezza, E.E. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer. Res. 2008, 28, 1119–1127. [Google Scholar] [PubMed]
- Liu, M.; Zhang, J.; Li, J.-F.; Wang, X.-X. Roles of curcumin combined with paclitaxel on growth inhibition and apoptosis of oral squamous cell carcinoma cell line CAL27 in vitro. Shanghai Kou Qiang Yi Xue 2016, 25, 538–541. [Google Scholar] [PubMed]
- Bava, S.V.; Puliyappadamba, V.T.; Deepti, A.; Nair, A.; Karunagaran, D.; John Anto, R. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J. Biol. Chem. 2005, 280, 6301–6308. [Google Scholar] [CrossRef]
- Bava, S.V.; Sreekanth, C.N.; Thulasidasan, A.K.T.; Anto, N.P.; Cheriyan, V.T.; Puliyappadamba, V.T.; Menon, S.G.; Ravichandran, S.D.; Anto, R.J. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int. J. Biochem. Cell Biol. 2011, 43, 331–341. [Google Scholar] [CrossRef]
- Dang, Y.-P.; Yuan, X.-Y.; Tian, R.; Li, D.-G.; Liu, W. Curcumin improves the paclitaxel-induced apoptosis of HPV-positive human cervical cancer cells via the NF-kappaB-p53-caspase-3 pathway. Exp. Ther. Med. 2015, 9, 1470–1476. [Google Scholar] [CrossRef]
- Punfa, W.; Suzuki, S.; Pitchakarn, P.; Yodkeeree, S.; Naiki, T.; Takahashi, S.; Limtrakul, P. Curcumin-loaded PLGA Nanoparticles Conjugated with Anti-P-glycoprotein Antibody to Overcome Multidrug Resistance. Asian Pac. J. Cancer Prev. 2014, 15, 9249–9258. [Google Scholar] [CrossRef]
- Sreekanth, C.N.; Bava, S.V.; Sreekumar, E.; Anto, R.J. Molecular evidences for the chemo sensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene 2011, 30, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Sethi, G.; Aggarwal, B.B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Boztas, A.O.; Karakuzu, O.; Galante, G.; Ugur, Z.; Kocabas, F.; Altuntas, C.Z.; Yazaydin, A.O. Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharm. 2013, 10, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Singh, P.; Panda, D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010, 277, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Faião-Flores, F.; Suarez, J.A.Q.; Pardi, P.C.; Maria, D.A. DM-1, sodium 4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxy-phenolate: A curcumin analog with a synergic effect in combination with paclitaxel in breast cancer treatment. Tumour Biol. 2012, 33, 775–785. [Google Scholar] [CrossRef]
- Aukunuru, J.; Thadakapally, R.; Aafreen, A.; Habibuddin, M.; Jogala, S. Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J. Pharm. Sci. 2016, 78, 65–72. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Li, F.; Zhang, W.; Shen, Y.; Zhou, D.; Guo, S. A multifunctional poly(curcumin) nanomedicine for dual-modal targeted delivery, intracellular responsive release, dual-drug treatment and imaging of multidrug resistant cancer cells. J. Mater. Chem. B 2016, 4, 2954–2962. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, Y.; Liu, R.; Zhang, H.; Zhang, Y. Potentiation of paclitaxel activity by curcumin in human breast cancer cell by modulating apoptosis and inhibiting EGFR signaling. Arch. Pharmacal Res. 2014, 37, 1086–1095. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Amiji, M. Curcumin Enhances Oral Bioavailability and Anti-Tumor Therapeutic Efficacy of Paclitaxel upon Administration in Nanoemulsion Formulation. J. Pharm. Sci. 2010, 99, 4630–4641. [Google Scholar] [CrossRef]
- Innamaa, A.; Jackson, L.; Asher, V.; Van Schalkwyk, G.; Warren, A.; Keightley, A.; Hay, D.; Bali, A.; Sowter, H.; Khan, R. Expression and effects of modulation of the K2P potassium channels TREK-1 (KCNK2) and TREK-2 (KCNK10) in the normal human ovary and epithelial ovarian cancer. Clin. Transl. Oncol. 2013, 15, 910–918. [Google Scholar] [CrossRef]
- Kar, R.; Sharma, C.; Sen, S.; Jain, S.K.; Gupta, S.D.; Singh, N. Response of primary culture of human ovarian cancer cells to chemotherapy: In vitro individualized therapy. J. Cancer Res. Ther. 2016, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Banik, N.L.; Ray, S.K. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem. Int. 2012, 61, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sharma, C.P.; Sreenivasan, K. Targeted coadministration of sparingly soluble paclitaxel and curcumin into cancer cells by surface engineered magnetic nanoparticles. J. Mater. Chem. 2011, 21, 15708–15717. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Huang, D.-M.; Chueh, S.-C.; Teng, C.-M.; Guh, J.-H. Alisol B acetate, a triterpene from Alismatis rhizoma, induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells. Cancer Lett. 2006, 231, 270–278. [Google Scholar] [CrossRef]
- Thomas, S.L.; Zhong, D.; Zhou, W.; Malik, S.; Liotta, D.; Snyder, J.P.; Hamel, E.; Giannakakou, P. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle 2008, 7, 2409–2417. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z.; Han, Z.; Tian, N. Paclitaxel-sensitization enhanced by curcumin involves down-regulation of nuclear factor-kappaB and Lin28 in Hep3B cells. J. Recept Signal Transduct Res. 2015, 35, 618–625. [Google Scholar] [CrossRef]
- Byam, J.; Renz, J.; Millis, J.M. Liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2013, 2, 22–30. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S.; Takada, Y.; Banerjee, S.; Newman, R.A.; Bueso-Ramos, C.E.; Price, J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005, 11, 7490–7498. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.-N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef]
- Kyakulaga, A.; Aqil, F.; Munagala, R.; Gupta, R.C. Synergistic combinations of paclitaxel and withaferin A against human non-small cell lung cancer cells. Oncotarget 2020, 11, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; McAndrew, N.P.; Troxel, A.; Feldman, M.; Lal, P.; Rosen, M.; Burrell, J.; Colleen Redlinger, C.; Gallagher, M.; Bradbury, A.R.; et al. Combination paclitaxel and palbociclib: Results of a Phase I trial in advanced breast cancer. Clin. Cancer Res. 2019, 25, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Zhou Sh Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel-doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Cancer/Tissue Type | Affected Tubulin | Modification | PTX Resistance | Reference |
|---|---|---|---|---|---|
| 1A9PTX10 &1A9PTX22 | Ovarian cancer | βI | mutation | accelerating | [106] |
| A549 | Non-small-cell lung cancer | βIVa, βIII, βI | Altered expression | accelerating | [103] |
| H69/Txl | Small-cell lung cancer | α-tubulin | acetylation | accelerating | [107] |
| Pac 10 | Prostate carcinoma | βIII, βIVa | Altered expression | accelerating | [108] |
| KPTA5 | Leukemia | βIVa | Altered expression | accelerating | [109] |
| MES-SA | Sarcoma | βIII, βIVa | Altered expression | reducing | [110] |
| Combination | Phase | Cancer Type | Clinical Trial Identifier |
|---|---|---|---|
| Napabucasin and Gemcitabine | 3 | Metastatic pancreatic cancer | NCT03721744 |
| Bevacizumab | 3 | Metastatic breast cancer | NCT00028990 |
| Fruquintinib | 3 | Gastric cancer | NCT03223376 |
| NovoTTF-100L | 3 | Ovarian cancer | NCT03940196 |
| Atezolizumab | 3 | Triple negative breast cancer | NCT02425891 |
| Cisplatin plus radiotherapy | 4 | Non-small-cell lung cancer | NCT00686322 |
| Chemotherapy (Carboplatin) | 4 | Her-2 negative breast cancer | NCT03799692 |
| RAD001 and Carboplatin | 4 | Carcinoma, large cell Neuroendocrine tumors | NCT01317615 |
| Bevacizumab and Carboplatin | 4 | Ovarian cancer | NCT01706120 |
| Bevacizumab | 4 | Triple negative breast cancer | NCT01094184 |
| Vantictumab | 1 | Metastatic breast cancer | NCT01973309 |
| Cisplatin | 2 | Esophageal cancer | NCT01444547 |
| Lapatinib | 2 | Urothelial cancer and bladder cancer | NCT01700010 |
| Reparixin | 2 | Metastatic breast cancer | NCT02370238 |
| Tegafur, Oxaliplatin and Capecitabine | 3 | Stomach cancer | NCT04135781 |
| DHP107 | 2 | Recurrent or metastatic breast cancer | NCT03326102 |
| Lenalidomide | 1 | Prostate cancer | NCT00933426 |
| LDE225 | 1 | Recurrent ovarian cancer | NCT02195973 |
| Cirmtuzumab | 1 | Breast neoplasms | NCT02776917 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawara, H.M.; Afify, S.M.; Hassan, G.; Zahra, M.H.; Seno, A.; Seno, M. Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy. Biomedicines 2021, 9, 500. https://doi.org/10.3390/biomedicines9050500
Nawara HM, Afify SM, Hassan G, Zahra MH, Seno A, Seno M. Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy. Biomedicines. 2021; 9(5):500. https://doi.org/10.3390/biomedicines9050500
Chicago/Turabian StyleNawara, Hend M., Said M. Afify, Ghmkin Hassan, Maram H. Zahra, Akimasa Seno, and Masaharu Seno. 2021. "Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy" Biomedicines 9, no. 5: 500. https://doi.org/10.3390/biomedicines9050500
APA StyleNawara, H. M., Afify, S. M., Hassan, G., Zahra, M. H., Seno, A., & Seno, M. (2021). Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy. Biomedicines, 9(5), 500. https://doi.org/10.3390/biomedicines9050500







