Mineralocorticoid Receptor Antagonists Eplerenone and Spironolactone Modify Adrenal Cortex Morphology and Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Luxol Fast Blue Staining for Spironolactone Bodies Detection
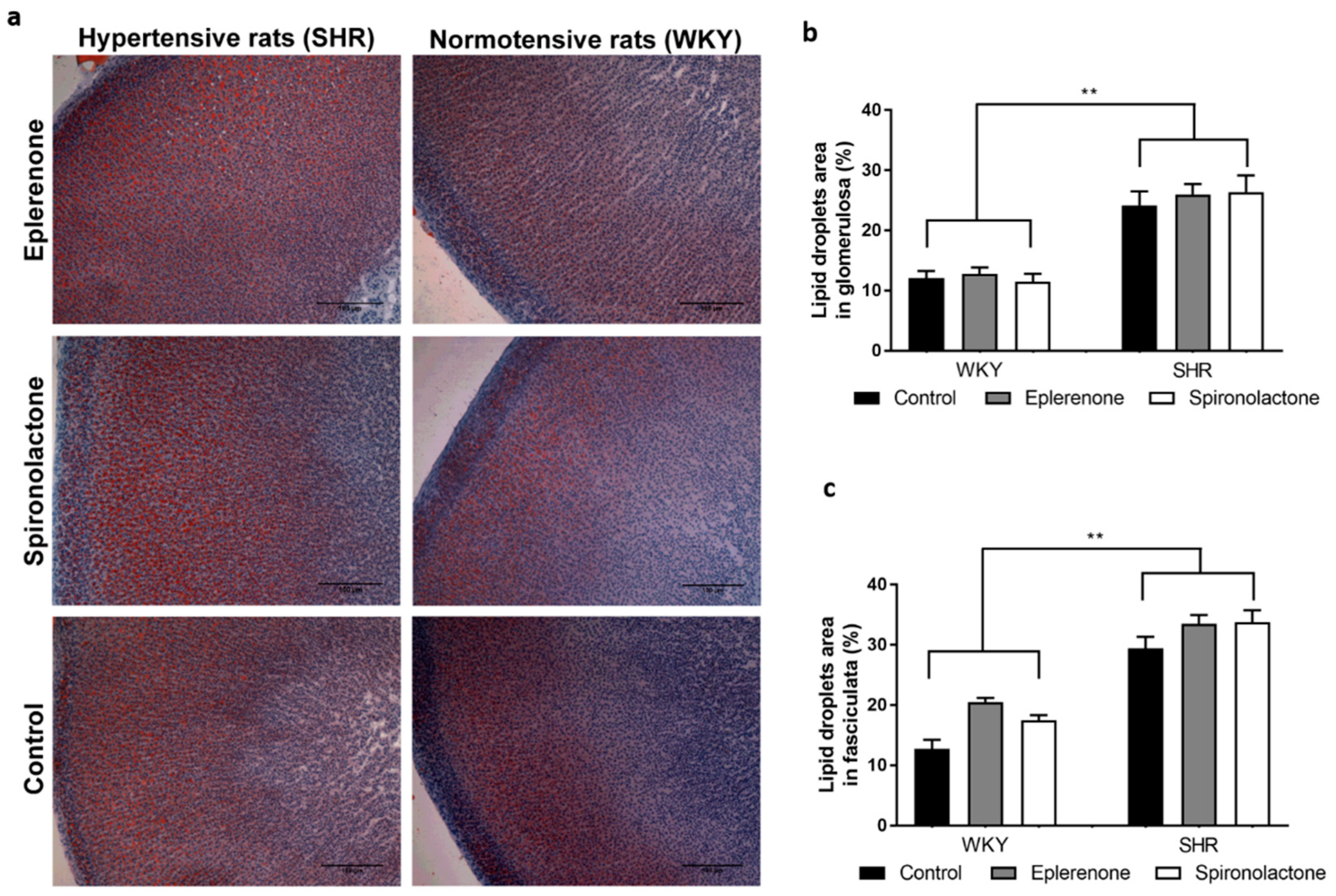
2.3. Oil Red O for Lipid Droplet Evaluation
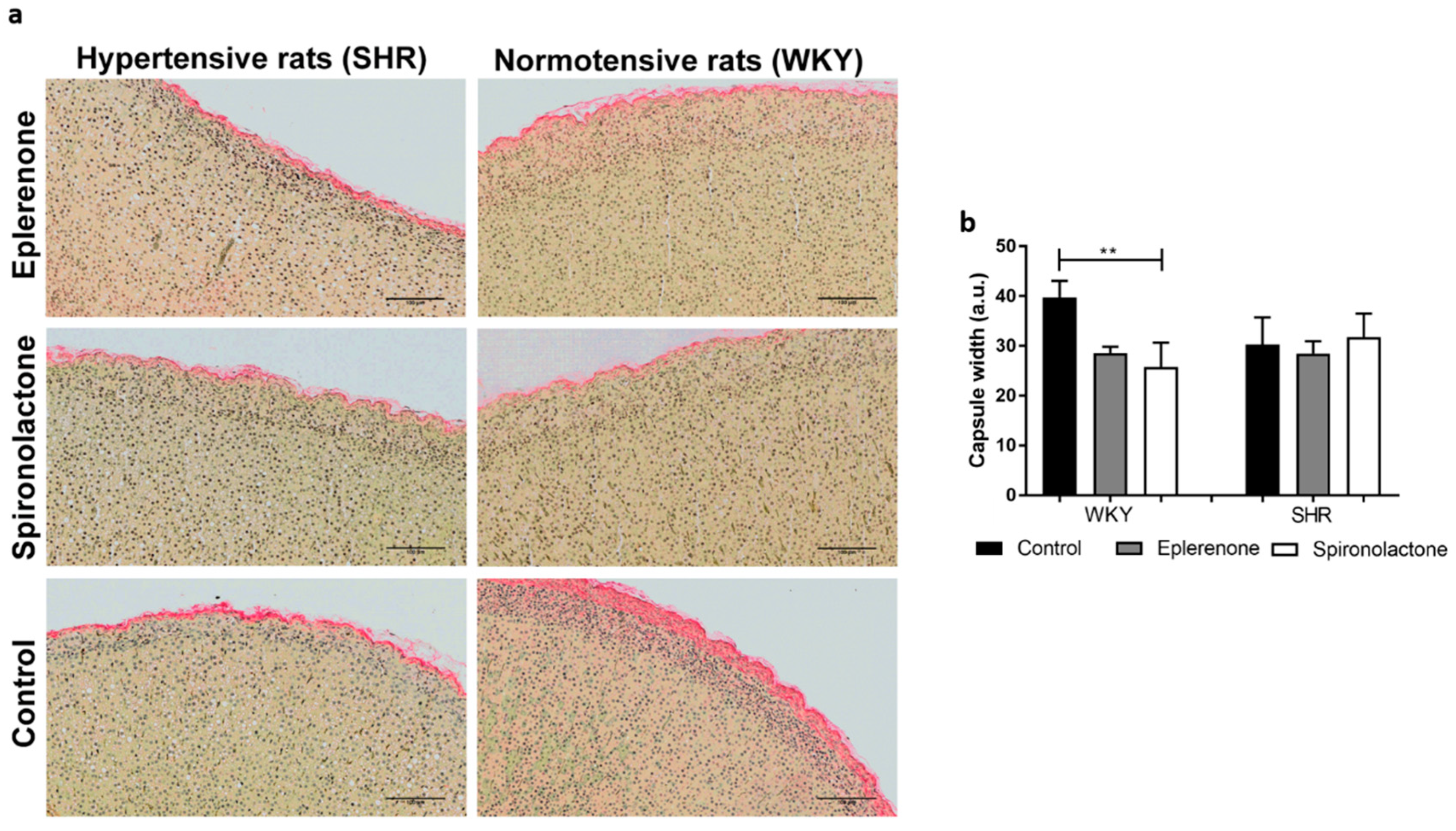
2.4. Sirius Red for Capsule Evaluation
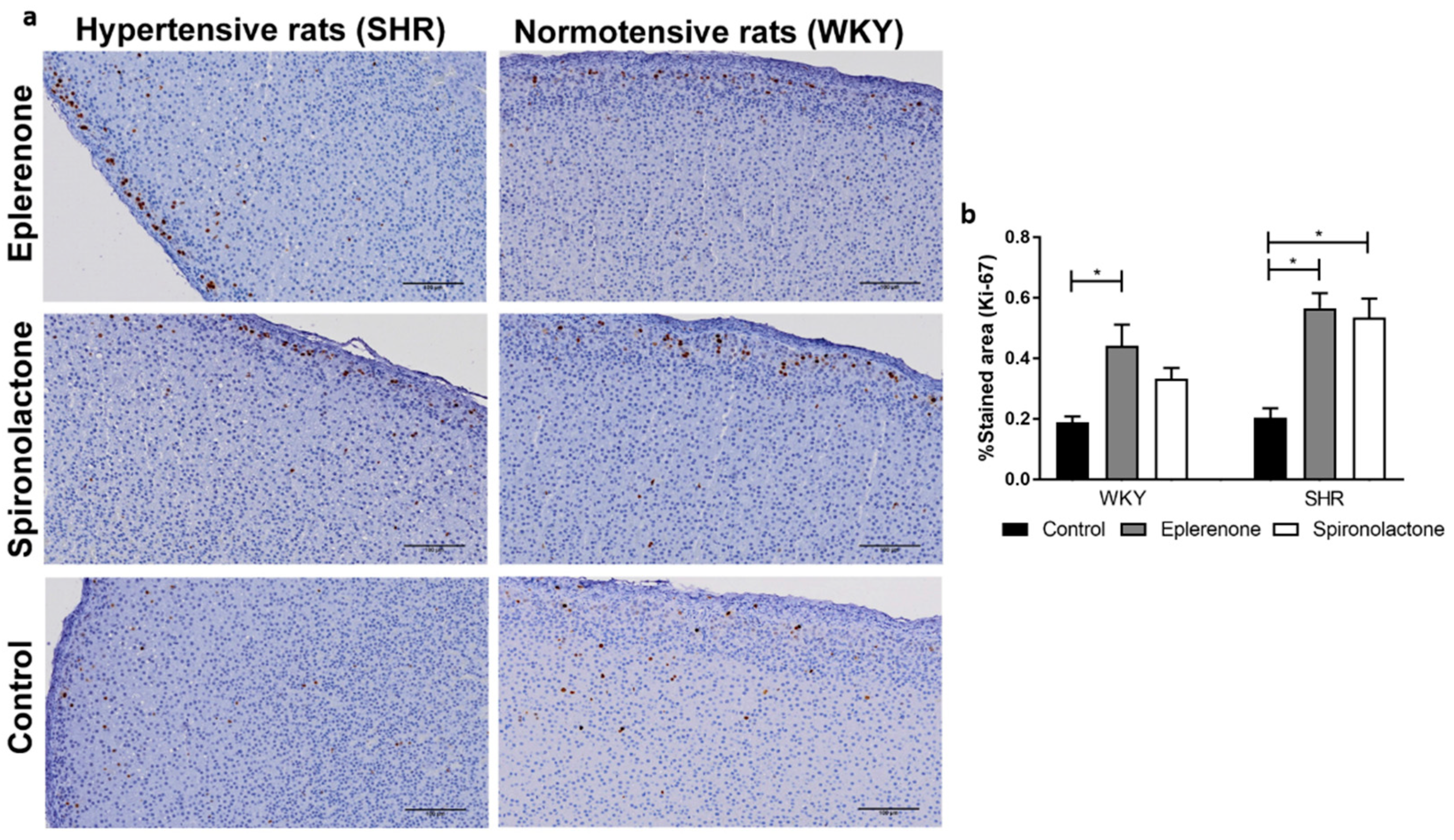
2.5. Immunohistochemistry Protocol
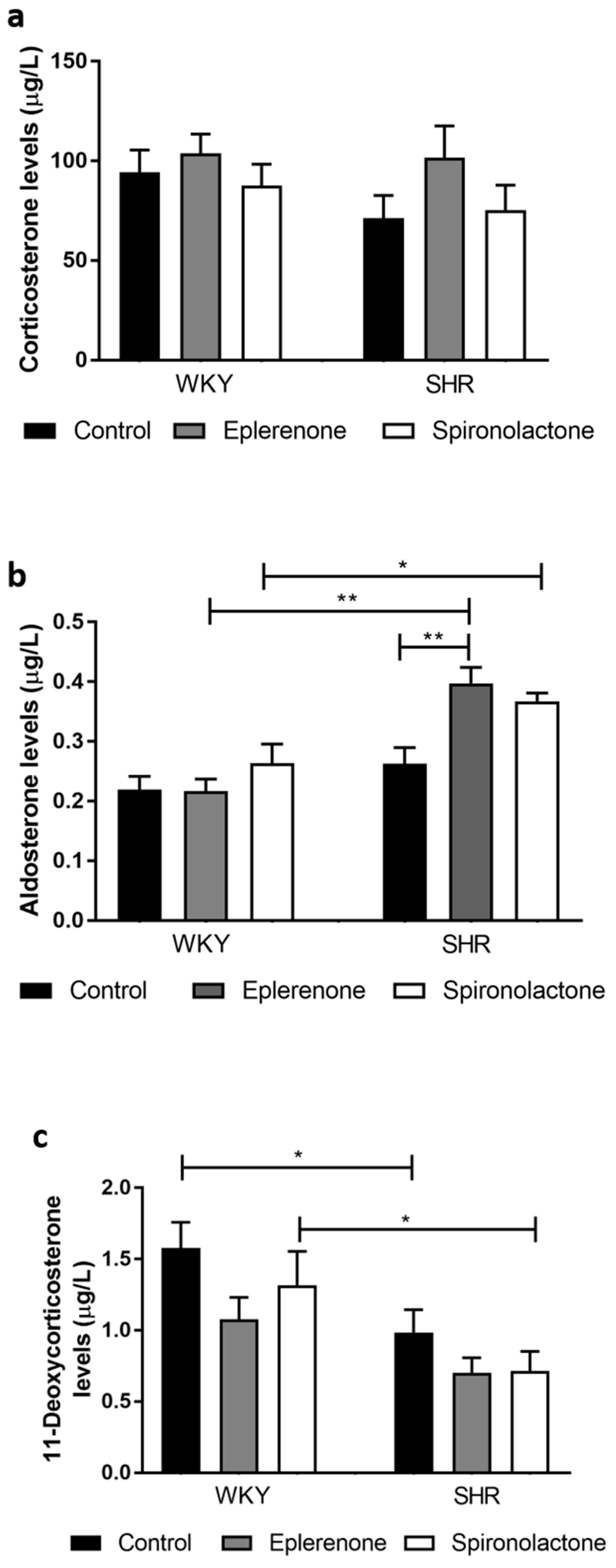
2.6. Corticosterone and 11-Deoxycorticosterone Extraction and Quantification
2.7. Aldosterone Extraction and Quantification
2.8. Statistical Analysis
3. Results
3.1. Arterial Blood Pressure
3.2. Adrenal Cortex Morphology
3.3. Lipid Droplets Evaluation
3.3.1. Adrenal Capsule Width
3.3.2. Cell Proliferation
3.3.3. CYP11B1, CYP11B2, and IZA Expression
3.3.4. Steroid Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connell, J.M.; Davies, E. The new biology of aldosterone. J. Endocrinol. 2005, 186, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Bärfacker, L. 30 Years of the Mineralocorticoid Receptor: Mineralocorticoid receptor antagonists: 60 years of research and development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef]
- Yugar-Toledo, J.C.; Modolo, R.; de Faria, A.P.; Moreno, H. Managing resistant hypertension: Focus on mineralocorticoid-receptor antagonists. Vasc. Health Risk Manag. 2017, 13, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A. Aldosteronism and Hypertension. Clin. J. Am. Soc. Nephrol. 2006, 1, 1039–1045. [Google Scholar] [CrossRef]
- Jeunemaitre, X.; Chatellier, G.; Kreft-Jais, C.; Charru, A.; DeVries, C.; Plouin, P.F.; Corvol, P.; Menard, J. Efficacy and tolerance of spironolactone in essential hypertension. Am. J. Cardiol. 1987, 60, 820–825. [Google Scholar] [CrossRef]
- Croom, K.F.; Perry, C.M. Eplerenone: A review of its use in essential hypertension. Am. J. Cardiovasc. Drugs 2005, 5, 51–69. [Google Scholar] [CrossRef]
- Garthwaite, S.M.; McMahon, E.G. The evolution of aldosterone antagonists. Mol. Cell Endocrinol. 2004, 217, 27–31. [Google Scholar] [CrossRef]
- Shrago, S.S.; Waisman, J.; Cooper, P.H. Spironolactone bodies in an adrenal adenoma. Arch. Pathol. 1975, 99, 416–420. [Google Scholar]
- Patel, K.A.; Calomeni, E.P.; Nadasdy, T.; Zynger, D.L. Adrenal gland inclusions in patients treated with aldosterone antagonists (Spironolactone/Eplerenone): Incidence, morphology, and ultrastructural findings. Diagn. Pathol. 2014, 9, 147. [Google Scholar] [CrossRef]
- Aiba, M.; Suzuki, H.; Kageyama, K.; Murai, M.; Tazaki, H.; Abe, O.; Saruta, T. Spironolactone bodies in aldosteronomas and in the attached adrenals. Enzyme histochemical study of 19 cases of primary aldosteronism and a case of aldosteronism due to bilateral diffuse hyperplasia of the zona glomerulosa. Am. J. Pathol. 1981, 103, 404–410. [Google Scholar]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Valentine, L.S. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr. Zur Pathol. 1973, 150, 174–187. [Google Scholar] [CrossRef]
- Pereira, S.S.; Morais, T.; Costa, M.M.; Monteiro, M.P.; Pignatelli, D. The emerging role of the molecular marker p27 in the differential diagnosis of adrenocortical tumors. Endocr. Connect. 2013, 2, 137–145. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 7th ed.; Pearson Education: London, UK, 2018. [Google Scholar]
- Ye, P.; Yamashita, T.; Pollock, D.M.; Sasano, H.; Rainey, W.E. Contrasting effects of eplerenone and spironolactone on adrenal cell steroidogenesis. Horm. Metab. Res. 2009, 41, 35–39. [Google Scholar] [CrossRef]
- Netchitailo, P.; Delarue, C.; Perroteau, I.; Leboulenger, F.; Capron, M.H.; Vaudry, H. Relative inhibitory potency of five mineralocorticoid antagonists on aldosterone biosynthesis in vitro. Biochem. Pharmacol. 1985, 34, 189–194. [Google Scholar] [CrossRef]
- Kovacs, K.; Horvath, E.; Singer, W. Fine structure and morphogenesis of spironolactone bodies in the zona glomerulosa of the human adrenal cortex. J. Clin. Pathol. 1973, 26, 949–957. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Martin, H.F. Spironolactone bodies. An immunoperoxidase study with biochemical correlation. Am. J. Clin. Pathol. 1981, 75, 92–95. [Google Scholar] [CrossRef]
- Russell, V.A. The Spontaneously Hypertensive Rat as a Model of Attention Deficit Hyperactivity Disorder. In Attention Deficit Hyperactivity Disorder; Springer: Berlin/Heidelberg, Germany, 2005; pp. 79–95. [Google Scholar]
- Sagvolden, T.; Hendley, E.D.; Knardahl, S. Behavior of hypertensive and hyperactive rat strains: Hyperactivity is not unitarily determined. Physiol. Behav. 1992, 52, 49–57. [Google Scholar] [CrossRef]
- Langen, B.; Dost, R. Comparison of SHR, WKY and Wistar rats in different behavioural animal models: Effect of dopamine D1 and alpha2 agonists. Atten. Deficit Hyperact. Disord. 2011, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto-Gomez, I.; Banegas, I.; Martínez-Cañamero, M.; Luna, J.d.D.; de Gasparo, M.; Ramírez-Sánchez, M. Functional and neurometabolic asymmetry in SHR and WKY rats following vasoactive treatments. Sci. Rep. 2019, 9, 16098. [Google Scholar] [CrossRef]
- Cowley, A.W., Jr.; Liard, J.F. Vasopressin and arterial pressure regulation. Special lecture. Hypertension 1988, 11, I25–I32. [Google Scholar] [CrossRef]
- Van Beusecum, J.; Inscho, E.W. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr. Opin. Pharmacol. 2015, 21, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, F.B.; Khor, V.K.; Shen, W.-J.; Azhar, S. Cholesterol ester droplets and steroidogenesis. Mol. Cell. Endocrinol. 2013, 371, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wexler, B.C.; McMurtry, J.P. Differences in adrenal cholesterol, ascorbic acid, circulating corticosterone and aldosterone during the onset of hypertension in SHR vs. WKy rats. Cardiovasc. Res. 1982, 16, 573–579. [Google Scholar] [CrossRef]
- Sowers, J.; Tuck, M.; Asp, N.D.; Sollars, E. Plasma aldosterone and corticosterone responses to adrenocorticotropin, angiotensin, potassium, and stress in spontaneously hypertensive rats. Endocrinology 1981, 108, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Makino, S.; Hirasawa, R.; Takao, T.; Sugawara, M.; Murakami, K.; Ono, K.; Ota, Z. Abnormalities in the hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats during development of hypertension. Endocrinology 1989, 125, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Vinson, G.P. Functional Zonation of the Adult Mammalian Adrenal Cortex. Front. Neurosci. 2016, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Mitani, F.; Mukai, K.; Suematsu, M.; Ishimura, Y. Studies on cytogenesis in adult rat adrenal cortex: Circadian and zonal variations and their modulation by adrenocorticotropic hormone. J. Biochem. 1999, 126, 1175–1183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nogueira, E.F.; Bollag, W.B.; Rainey, W.E. Angiotensin II regulation of adrenocortical gene transcription. Mol. Cell Endocrinol. 2009, 302, 230–236. [Google Scholar] [CrossRef][Green Version]
- Otis, M.; Campbell, S.; Payet, M.D.; Gallo-Payet, N. The growth-promoting effects of angiotensin II in adrenal glomerulosa cells: An interactive tale. Mol. Cell Endocrinol. 2007, 273, 1–5. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Roniker, B.; Krause, S.L.; Weiss, R.J. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am. J. Hypertens. 2002, 15, 709–716. [Google Scholar] [CrossRef]
- Eudy, R.J.; Sahasrabudhe, V.; Sweeney, K.; Tugnait, M.; King-Ahmad, A.; Near, K.; Loria, P.; Banker, M.E.; Piotrowski, D.W.; Boustany-Kari, C.M. The use of plasma aldosterone and urinary sodium to potassium ratio as translatable quantitative biomarkers of mineralocorticoid receptor antagonism. J. Transl. Med. 2011, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.M.; Graciano, M.L.; Seth, D.; Awayda, M.S.; Navar, L.G. Aldosterone receptor antagonism exacerbates intrarenal angiotensin II augmentation in ANG II-dependent hypertension. Am. J. Physiology. Ren. Physiol. 2007, 293, F139–F147. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Santos, M.; Almeida, S.; Marques, I.; Bettencourt, P.; Carvalho, H. High-dose spironolactone changes renin and aldosterone levels in acutely decompensated heart failure. Cor Vasa 2014, 56, e463–e470. [Google Scholar] [CrossRef]
- Milliez, P.; DeAngelis, N.; Rucker-Martin, C.; Leenhardt, A.; Vicaut, E.; Robidel, E.; Beaufils, P.; Delcayre, C.; Hatem, S.N. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur. Heart J. 2005, 26, 2193–2199. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am. J. Cardiol. 1993, 71, 12a–16a. [Google Scholar] [CrossRef]
- Du, L.; Qin, M.; Yi, Y.; Chen, X.; Jiang, W.; Zhou, L.; Zhang, D.; Xu, K.; Yang, Y.; Li, C.; et al. Eplerenone Prevents Atrial Fibrosis via the TGF-β Signaling Pathway. Cardiology 2017, 138, 55–62. [Google Scholar] [CrossRef] [PubMed]

| Groups | CYP11B1 (%) | CYP11B2 (%) | IZA (%) |
|---|---|---|---|
| WKY control | 42.42 ± 3.04 | 2.12 ± 0.70 | 31.63 ± 2.37 |
| WKY eplerenone | 42.14 ± 1.78 | 4.02 ± 0.34 | 30.88 ± 2.70 |
| WKY spironolactone | 46.01 ± 1.44 | 3.58 ± 0.71 | 35.18 ± 2.67 |
| SHR control | 49.21 ± 4.15 | 1.84 ± 0.38 | 35.08 ± 1.56 |
| SHR eplerenone | 43.27 ± 5.26 | 5.59 ± 2.57 | 35.19 ± 1.27 |
| SHR spironolactone | 51.80 ± 1.23 | 4.15 ± 1.67 | 37.77 ± 2.48 |
| p-value | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.S.; Carvalho, L.; Costa, M.M.; Melo, A.; Ferreira, I.M.P.L.V.O.; Gomez-Sanchez, C.E.; Monteiro, M.P.; Vinson, G.; Pignatelli, D. Mineralocorticoid Receptor Antagonists Eplerenone and Spironolactone Modify Adrenal Cortex Morphology and Physiology. Biomedicines 2021, 9, 441. https://doi.org/10.3390/biomedicines9040441
Pereira SS, Carvalho L, Costa MM, Melo A, Ferreira IMPLVO, Gomez-Sanchez CE, Monteiro MP, Vinson G, Pignatelli D. Mineralocorticoid Receptor Antagonists Eplerenone and Spironolactone Modify Adrenal Cortex Morphology and Physiology. Biomedicines. 2021; 9(4):441. https://doi.org/10.3390/biomedicines9040441
Chicago/Turabian StylePereira, Sofia S., Liliana Carvalho, Madalena M. Costa, Armindo Melo, Isabel M. P. L. V. O. Ferreira, Celso E. Gomez-Sanchez, Mariana P. Monteiro, Gavin Vinson, and Duarte Pignatelli. 2021. "Mineralocorticoid Receptor Antagonists Eplerenone and Spironolactone Modify Adrenal Cortex Morphology and Physiology" Biomedicines 9, no. 4: 441. https://doi.org/10.3390/biomedicines9040441
APA StylePereira, S. S., Carvalho, L., Costa, M. M., Melo, A., Ferreira, I. M. P. L. V. O., Gomez-Sanchez, C. E., Monteiro, M. P., Vinson, G., & Pignatelli, D. (2021). Mineralocorticoid Receptor Antagonists Eplerenone and Spironolactone Modify Adrenal Cortex Morphology and Physiology. Biomedicines, 9(4), 441. https://doi.org/10.3390/biomedicines9040441







