177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. 177Lu-PSMA as a Therapeutic Agent
3. Patient Preparation for 177Lu-PSMA Therapy
3.1. Patient Selection and Preparation
3.2. Radionuclide Preparation
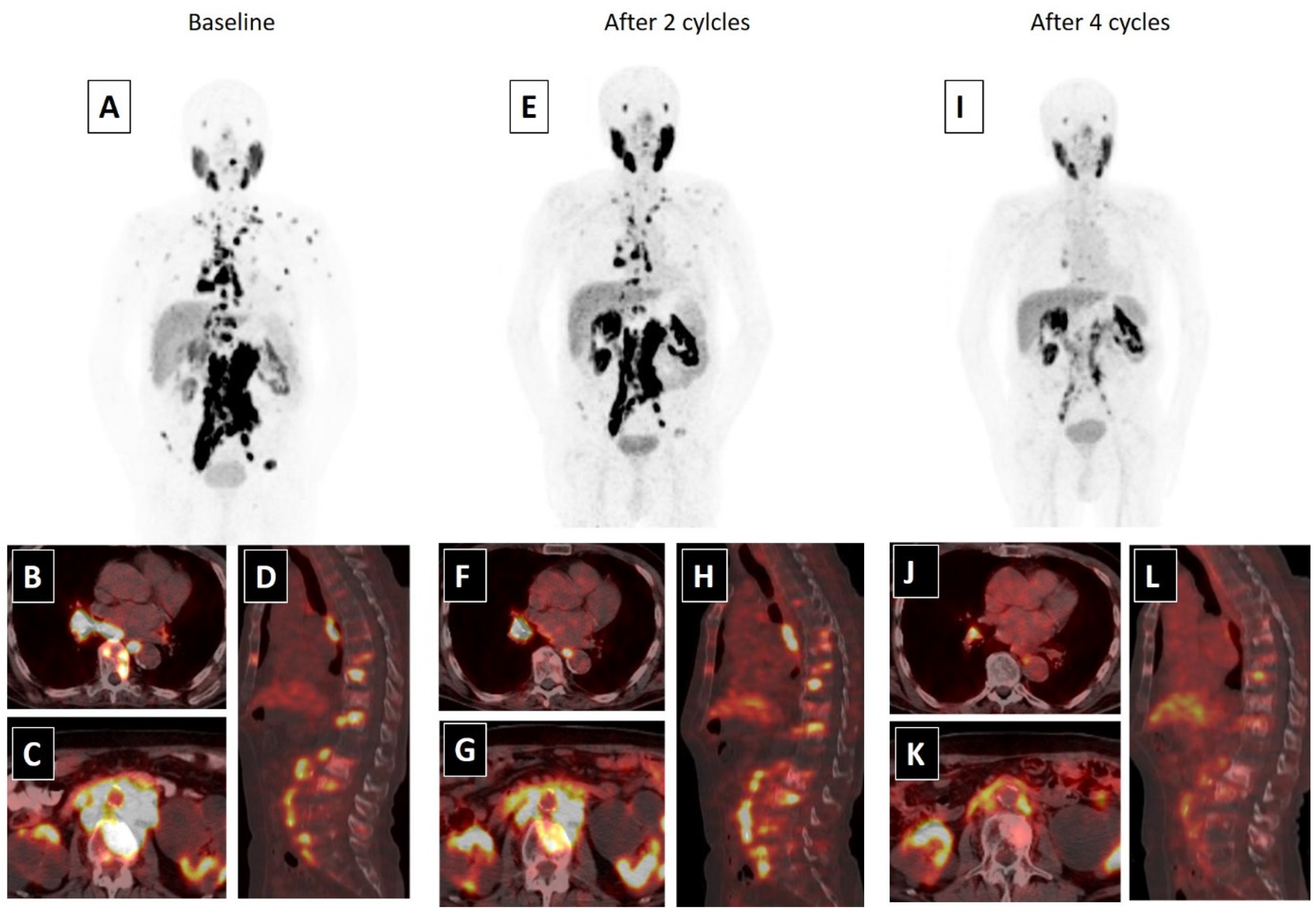
4. Therapy-Related Issues
4.1. Performing Therapy
4.2. Release of Patients
4.3. Post-Therapy Imaging
5. Dosimetry
6. Adverse Events
7. Efficacy
7.1. Prospective Trials
7.2. Meta-Analyses
7.3. Major Retrospective Trials
7.4. Predictors of Efficacy
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Khan, R.; Jemal, A. Cancer Statistics. Cancer Rehabil. 2018, 70, 7–30. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Murad, M.H.; Oh, W.K.; Jarrard, D.F.; Resnick, M.J.; Cookson, M.S. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2018. J. Urol. 2018, 200, 1264–1272. [Google Scholar] [CrossRef]
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. Semin. Orig. Investig. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Farolfi, A.; Ceci, F.; Castellucci, P.; Graziani, T.; Siepe, G.; Lambertini, A.; Schiavina, R.; Lodi, F.; Morganti, A.G.; Fanti, S. (68)Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA. Eur. J. Nucl. Med. Mol. Imaging 2019. [Google Scholar] [CrossRef]
- 1Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M. Diagnostic performance of 68 Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef]
- Farolfi, A.; Gafita, A.; Calais, J.; Eiber, M.; Afshar-Oromieh, A.; Spohn, F.; Barbato, F.; Weber, M.; Ilhan, H.; Cervati, V. 68Ga-PSMA-11 PET Detects Residual Prostate Cancer after Prostatectomy in a Multicenter Retrospective Study. J. Urol. 2019. [Google Scholar] [CrossRef]
- Kratochwil, C.; Afshar-Oromieh, A.; Kopka, K.; Haberkorn, U.; Giesel, F.L. Current Status of Prostate-Specific Membrane Antigen Targeting in Nuclear Medicine: Clinical Translation of Chelator Containing Prostate-Specific Membrane Antigen Ligands Into Diagnostics and Therapy for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 405–418. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Eppard, E.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gärtner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488. [Google Scholar] [CrossRef]
- Yordanova, A.; Becker, A.; Eppard, E.; Kürpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2016, 8, 3581–3590. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.-J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177 Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 58, 85–90. [Google Scholar] [CrossRef]
- Beauregard, J.-M.; Hofman, M.S.; Kong, G.; Hicks, R.J. The tumour sink effect on the biodistribution of 68Ga-DOTA-octreotate: Implications for peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 50–56. [Google Scholar] [CrossRef]
- Taïeb, D.; Foletti, J.-M.; Bardies, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter? J. Nucl. Med. 2018, 59, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium177PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Stegger, L.; Schäfers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32. [Google Scholar] [CrossRef]
- Demir, M.; Abuqbeitah, M.; Uslu-Beşli, L.; Yıldırım, Ö.; Yeyin, N.; Çavdar, İ.; Vatankulu, B.; Gündüz, H.; Kabasakal, L. Evaluation of radiation safety in 177Lu-PSMA therapy and development of outpatient treatment protocol. J. Radiol. Prot. 2016, 36, 269. [Google Scholar] [CrossRef] [PubMed]
- Kendi, A.T.; Halfdanarson, T.R.; Packard, A.; Dundar, A.; Subramaniam, R.M. Therapy With177Lu-DOTATATE: Clinical Implementation and Impact on Care of Patients with Neuroendocrine Tumors. Am. J. Roentgenol. 2019, 213, 309–317. [Google Scholar] [CrossRef]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hohberg, M.; Eschner, W.; Schmidt, M.; Dietlein, M.; Kobe, C.; Fischer, T.; Drzezga, A.; Wild, M. Lacrimal Glands May Represent Organs at Risk for Radionuclide Therapy of Prostate Cancer with [177Lu]DKFZ-PSMA-617. Mol. Imaging Biol. 2016, 18, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.-J.; Tamaki, N.; et al. Radiation Dosimetry for 177 Lu-PSMA I&T in Metastatic Castration-Resistant Prostate Cancer: Absorbed Dose in Normal Organs and Tumor Lesions. J. Nucl. Med. 2016, 58, 445–450. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Tripathi, M.; Damle, N.A.; Sahoo, R.K.; Seth, A.; Bal, C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl. Med. Commun. 2017, 38, 91–98. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; de Wit-van der Veen, B.J.; Verheij, M.; Stokkel, M.P.M. Dosimetry methods and clinical applications in peptide receptor radionuclide therapy for neuroendocrine tumours: A literature review. EJNMMI Res. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.; Shank, B.; Solin, L.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Hey, J.; Setz, J.; Gerlach, R.; Janich, M.; Hildebrandt, G.; Vordermark, D.; Gernhardt, C.R.; Kuhnt, T. Parotid gland-recovery after radiotherapy in the head and neck region–36 months follow-up of a prospective clinical study. Radiat. Oncol. 2011, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, N.; Moiseenko, V.; Song, W.Y.; Morris, C.G.; Bhatti, M.T.; Mendenhall, W.M. Severe Dry Eye Syndrome After Radiotherapy for Head-and-Neck Tumors. Int. J. Radiat. Oncol. 2012, 82, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, H.; Konijnenberg, M.W.; Van Der Zwan, W.A.; Kam, B.L.R.; Teunissen, J.J.M.; Kooij, P.P.; Mauff, K.A.L.; Krenning, E.P.; Kwekkeboom, D.J. Nephrotoxicity after PRRT with 177Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Rathke, H.; Giesel, F.L.; Flechsig, P.; Kopka, K.; Mier, W.; Hohenfellner, M.; Haberkorn, U.; Kratochwil, C. Repeated177Lu-Labeled PSMA-617 Radioligand Therapy Using Treatment Activities of Up to 9.3 GBq. J. Nucl. Med. 2017, 59, 459–465. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, D.S.; Heinrich, S.D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177–Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.K.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J. Clin. Oncol. 2020, 38, 5500. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.I. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Calopedos, R.J.S.; Chalasani, V.; Asher, R.; Emmett, L.; Woo, H.H. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Bauman, G.; Von Eyben, R.; Rahbar, K.; Soydal, C.; Haug, A.R.; Virgolini, I.; Kulkarni, H.; Baum, R.; Paganelli, G. Optimizing PSMA Radioligand Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer. A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 9054. [Google Scholar] [CrossRef]
- Von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Singh, A.; Schuchardt, C.; Niepsch, K.; Sayeg, M.; Leshch, Y.; Wester, H.-J.; Baum, R.P. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J. Nucl. Med. 2016, 57, 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Schlolaut, S.; Fimmers, R.; Yordanova, A.; Hirzebruch, S.; Schlenkhoff, C.; Gaertner, F.C.; Awang, Z.H.; Hauser, S.; Essler, M. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu]Lu-PSMA-617 radioligand therapy. Oncotarget 2017, 8, 103108–103116. [Google Scholar] [CrossRef]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef]
- Rahbar, K.; Bögeman, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bode, A.; Weckesser, M.; Avramovic, N.; Claesener, M.; Stegger, L.; Bögemann, M. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients With Metastatic Castration Resistant Prostate Cancer. Clin. Nucl. Med. 2016, 41, 522–528. [Google Scholar] [CrossRef]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of (177)Lu-PSMA radioligand therapy in taxane chemotherapy pretreated and taxane chemotherapy naive patients with metastatic castration resistant prostate cancer. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef]
- Emmett, L.M.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Rahbar, K.; Herrmann, K.; Kratochwil, C.; Eiber, M. 177 Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017, 58, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Eppard, E.; Gaertner, F.C.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Hauser, S.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177 Lu-PSMA-617. J. Nucl. Med. 2016, 58, 312–319. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu–PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef]
- Michalski, K.; Ruf, J.; Goetz, C.; Seitz, A.K.; Buck, A.K.; Lapa, C.; Hartrampf, P.E. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–7. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Schäfers, M.; Weckesser, M.; Schlack, K.; Boegemann, M.; Rahbar, K. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics 2019, 9, 4841. [Google Scholar] [CrossRef]
- Rathke, H.; Holland-Letz, T.; Mier, W.; Flechsig, P.; Mavriopoulou, E.; Röhrich, M.; Kopka, K.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.A.; et al. Response Prediction of 177Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J. Nucl. Med. 2020, 61, 689–695. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Basch, E.; Heller, G. End Points and Outcomes in Castration-Resistant Prostate Cancer: From Clinical Trials to Clinical Practice. J. Clin. Oncol. 2011, 29, 3695–3704. [Google Scholar] [CrossRef]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Sweeney, C.; Chen, Y.-H.; Liu, G.; Carducci, M.; Jarrard, D.; Eisenberger, M.; Wong, Y.-N.; Patrick-Miller, L.; Hahn, N.; Kohli, M.; et al. Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. Ann. Oncol. 2016, 27, vi244. [Google Scholar] [CrossRef]
- Murga, J.D.; Moorji, S.M.; Han, A.Q.; Magargal, W.W.; DiPippo, V.A.; Olson, W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 2014, 75, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Banerjee, S. The rationality of combining second-generation antiandrogens with 177Lu-PSMA or its alpha-emitting congeners for better and durable results: Will this dominate the therapeutic landscape and be an upfront consideration in metastatic castration-resistant prostate cancer in the coming years? Nucl. Med. Commun. 2018, 39, 1061–1063. [Google Scholar]
- Crumbaker, M.; Joshua, A.M.; Epstein, R.; Emmett, L. A phase I study of 177Lu-DKFZ PSMA 617 combined with the radiosensitizer idronoxil in men with metastatic castrate-resistant prostate cancer (mCRPC) (LuPin trial). J. Clin. Oncol. 2018, 36, TPS5088. [Google Scholar] [CrossRef]

| Patient selection | Patients with mCRPC who are ineligible or finalized the approved alternative options and with adequate uptake of PSMA ligands on the basis of a pre-therapy imaging study can be considered for treatment [13]. |
| Uptake of tumors > liver uptake (at least 1.5 times the SUVmean) [16]. Liver metastases negative on PSMA-ligand PET should be ruled out, even if the remainder of the disease demonstrates intense PSMA expression [13]. | |
| Life expectancy > 6 months ECOG performance status > 2 Unless the main objective is alleviating suffering from disease-related symptoms [13]. | |
| Patient preparation and cautionary considerations | Complete blood tests need to be performed within the two weeks before the 177Lu-PSMA therapy |
| White blood cells > 2500/L Platelet > 75,000/L Hemoglobulin > 8 mg/dL If blood cell counts were below the suggested thresholds, blood cell transfusion can be considered to avoid adverse effects [13]. | |
| Myelosuppressive therapies should be discontinued for protecting bone marrow reserves [14]. | |
| Patients with obstructive urinary disorders which might be evaluated with 99m Tc-MAG3 or 99m Tc-DTPA scintigraphy should be resolved before the therapy to reduce the radiation exposure to the kidneys [15]. | |
| Creatinine level should <2× upper limit of normal GFR > 30 mL/min [13]. | |
| Liver transaminase levels should be <5× upper limit of normal [13]. |
| Reference Study | Patient Number | Molecule | Imaging Method | Dose Convolution | Kidneys | Salivary Glands | Lacrimal Glands | Bone Marrow | Liver | Spleen | Tumors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delker [28] | 5 | 617 | Whole body planar + SPECT-CT | MIRD * | 0.6 Gy/GBq | 1.4 Gy/GBq | - | 0.012 Gy/GBq | 0.1 Gy/GBq | 0.1 Gy/GBq | 13.1 Gy/GBq |
| Hohberg [29] | 9 | 617 | Whole body planar | MIRD | 0.53 Gy/GBq | 0.72 Gy/GBq | 2.82 Gy/GBq | - | - | - | - |
| Okamato [30] | 18 | I&T | Whole body planar | MIRD | 0.72 Gy/Gbq | 0.55–0.64 Gy/GBq | 3.8 Gy/GBq | - | 0.12 Gy/GBq | - | 3.2 Gy/GBq |
| Fendler [18] | 15 | 617 | SPECT | MIRD | 0.5–0.6 Gy/GBq | 1.0 Gy/GBq | - | 0.002 Gy/Gbq | 0.1 Gy/Gbq | 0.1 Gy/Gbq | 6.1 Gy/GBq |
| Yadav [31] | 26 | 617 | Whole body planar | MIRD | 0.99 Gy/GBq | 1.24 Gy/GBq | - | 0.048 Gy/GBq | 0.36 Gy/GBq | - | 10.94 Gy/GBq |
| Violet [20] | 30 | 617 | SPECT-CT | Voxel based and MIRD | 0.39 Gy/GBq | 0.44–0.58 Gy/GBq | - | 0.11 Gy/GBq | 0.1 Gy/GBq | 0.06 Gy/GBq | 11.5 Gy/GBq |
| Reference Study | Patient Number | Response % (RECIST) | Response % (Symptom) | PSA Decline > 50% | PFS | OS |
|---|---|---|---|---|---|---|
| Hofman [16] | 30 | CR: 40% PR: 37% SD: 37% | 37% | 57% | 7.6 months | 13.5 months |
| Baum [19] | 56 | CR: 20% PR: 52% SD: 28% | 33% | 59% | 13.7 months | - |
| Rahbar [21] | 145 | PR: 45% * SD: 28% * | - | 45% | - | - |
| Yadav [38] | 90 | PR: 23% SD: 54% PD: 23% | 54% | 45.5% | 11.8 months | 14 months |
| Tagawa [40] | 47 | 10.6% | 3 months | - | ||
| Kim [42] | 455 | - | - | 34.45% | - | - |
| Calopedos [43] | 369 | - | - | 37% | - | - |
| Von Eyben [45] | 669 | - | - | 43% | - | - |
| Bräuer [46] | 59 | - | - | 53% | 4.5 months | 8 months |
| Ahmadzadehfar [47] | 52 | - | - | 44.2% | - | 15 months |
| Kulkarni [48] | 119 | - | - | 57.5% | 10.7 months | - |
| Ahmadzadehfar [49] | 100 | - | - | 69% | - | 15 months |
| Rahbar [50] | 104 | - | - | 49% | - | 14 months |
| Rahbar [51] | 71 | - | - | 56% | - | - |
| Rahbar [52] | 28 | - | - | 50% | - | 7.3 months |
| Barber [53] | 167 | - | - | 40% ** 57% *** | 6 months ** 8.8 months *** | 10.7 months ** 27.1 months *** |
| Ferdinandius [56] | 40 | - | - | 32.5% | - | - |
| Overall | 28–669 | CR: 20–40% PR: 23–52% SD: 28–54% PD: 23% | 33–54% | 10.6–69% | 3–13.7 months | 7.3–27.1 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanli, Y.; Simsek, D.H.; Sanli, O.; Subramaniam, R.M.; Kendi, A.T. 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines 2021, 9, 430. https://doi.org/10.3390/biomedicines9040430
Sanli Y, Simsek DH, Sanli O, Subramaniam RM, Kendi AT. 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines. 2021; 9(4):430. https://doi.org/10.3390/biomedicines9040430
Chicago/Turabian StyleSanli, Yasemin, Duygu Has Simsek, Oner Sanli, Rathan M. Subramaniam, and Ayse Tuba Kendi. 2021. "177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer" Biomedicines 9, no. 4: 430. https://doi.org/10.3390/biomedicines9040430
APA StyleSanli, Y., Simsek, D. H., Sanli, O., Subramaniam, R. M., & Kendi, A. T. (2021). 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines, 9(4), 430. https://doi.org/10.3390/biomedicines9040430







