Phaseolin Attenuates Lipopolysaccharide-Induced Inflammation in RAW 264.7 Cells and Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
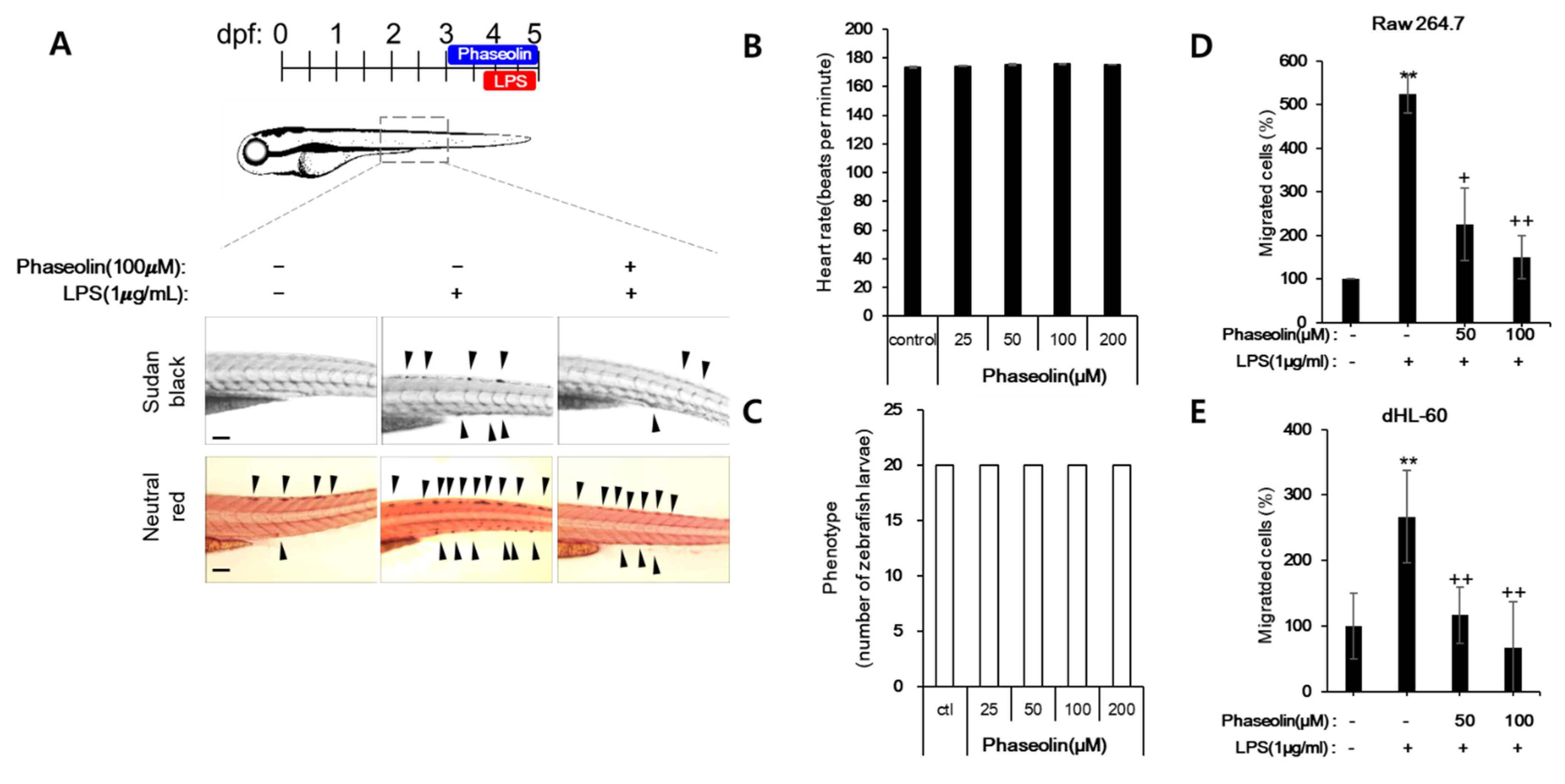
2.2. Zebrafish Maintenance, Toxicity Test, and Induction of Endotoxin Shock
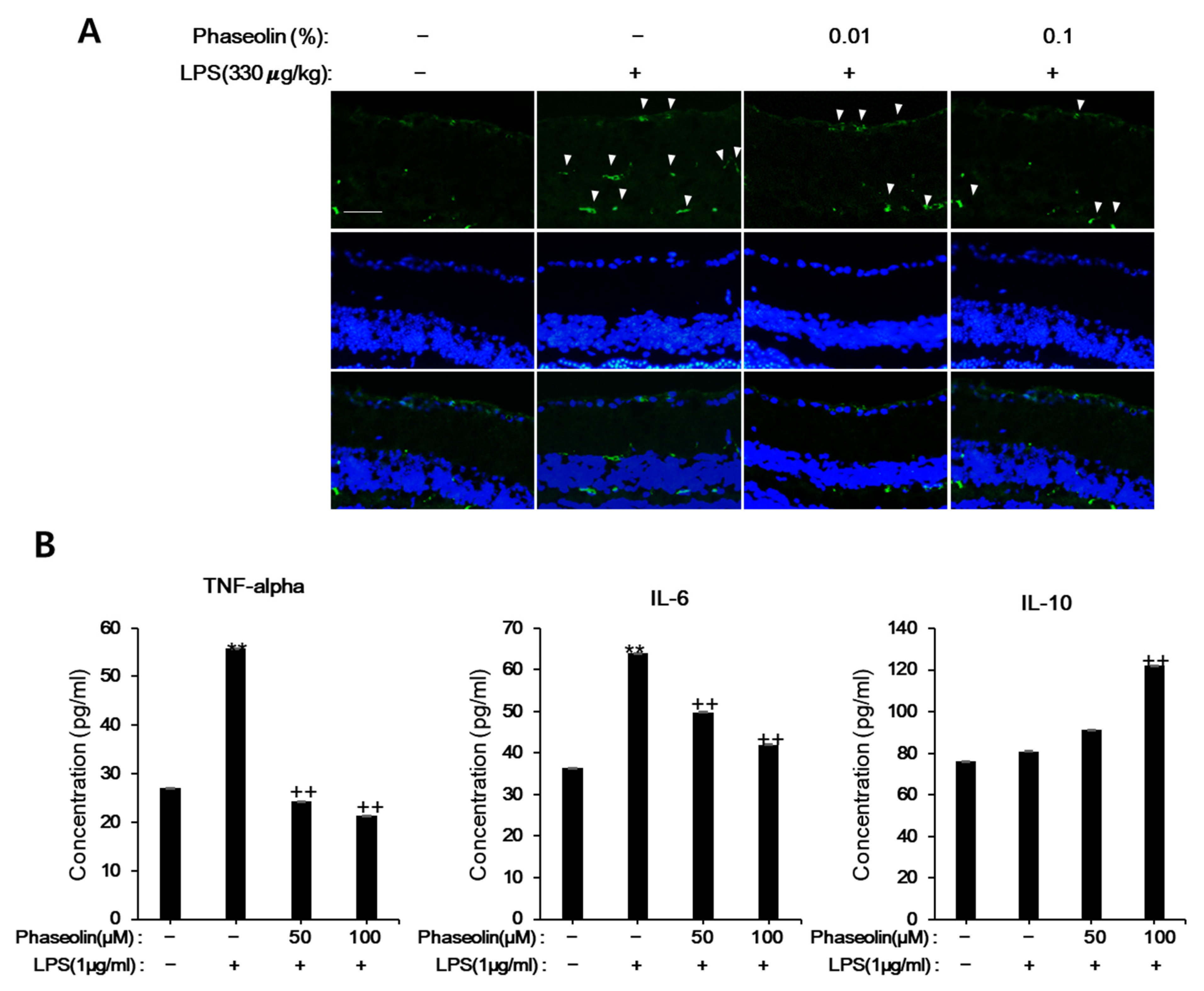
2.3. Endotoxin-Induced Uveitis in Mouse
2.4. Nitric Oxide (NO) Assay
2.5. Cell Viability Test
2.6. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
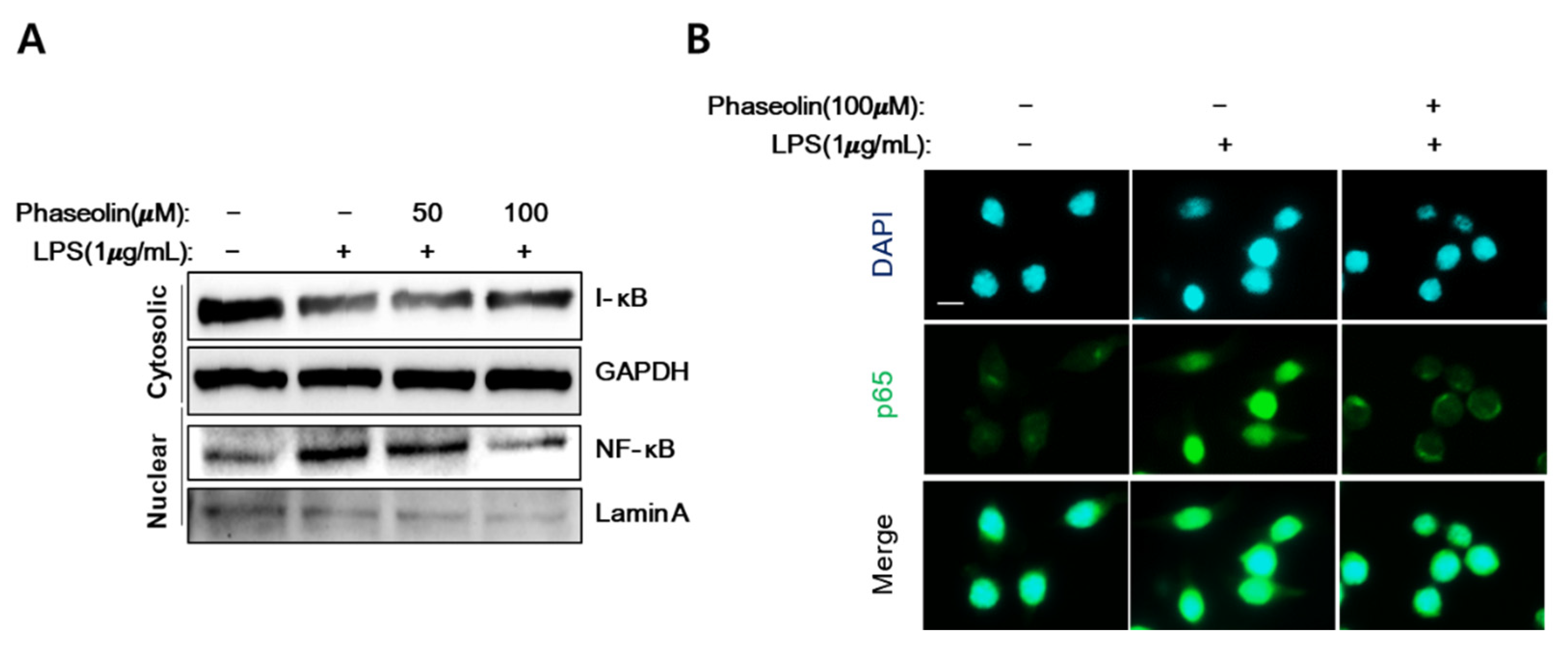
2.7. Protein Extract and Western Blotting
2.8. ELISA
2.9. Cell Adhesion Assay
2.10. Cell Migration Assay
2.11. Immunofluorescence Microscopy and Quantification
2.12. Gelatin Zymography
2.13. Statistical Analysis
3. Results
3.1. Phaseolin Inhibited NO Production and iNOS Expression in LPS-Stimulated RAW 264.7 Macrophages without Affecting Cell Viability
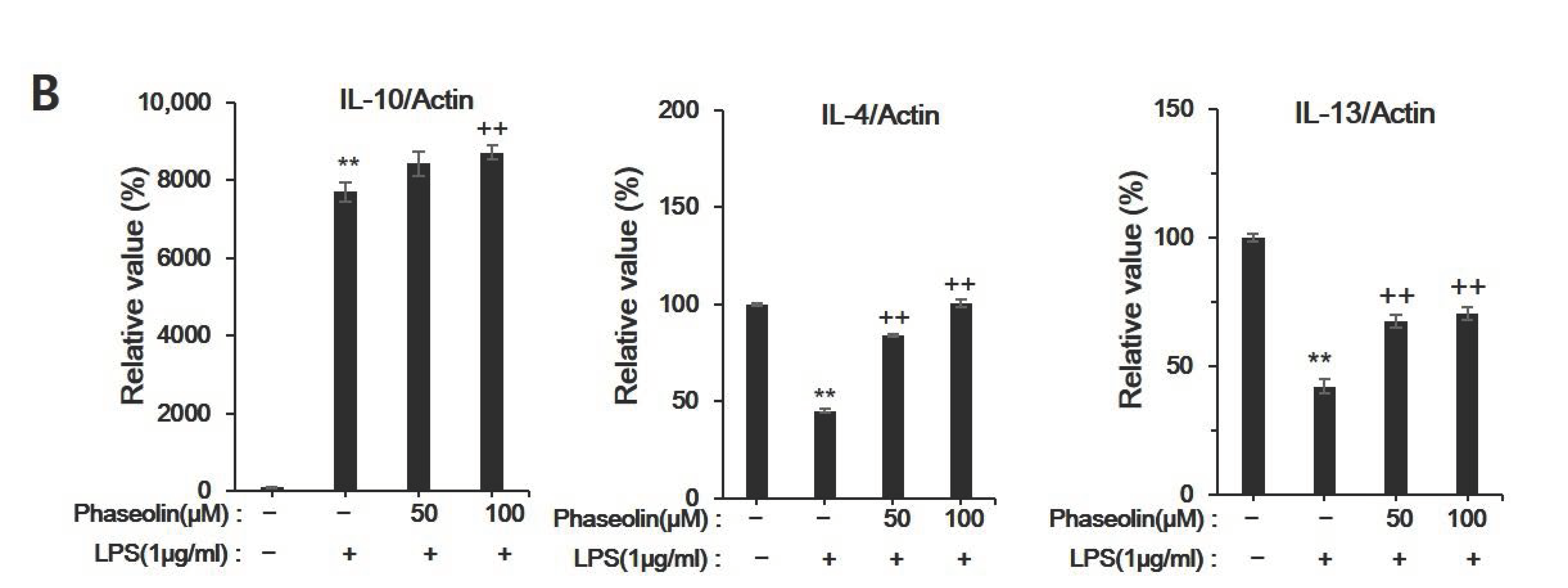
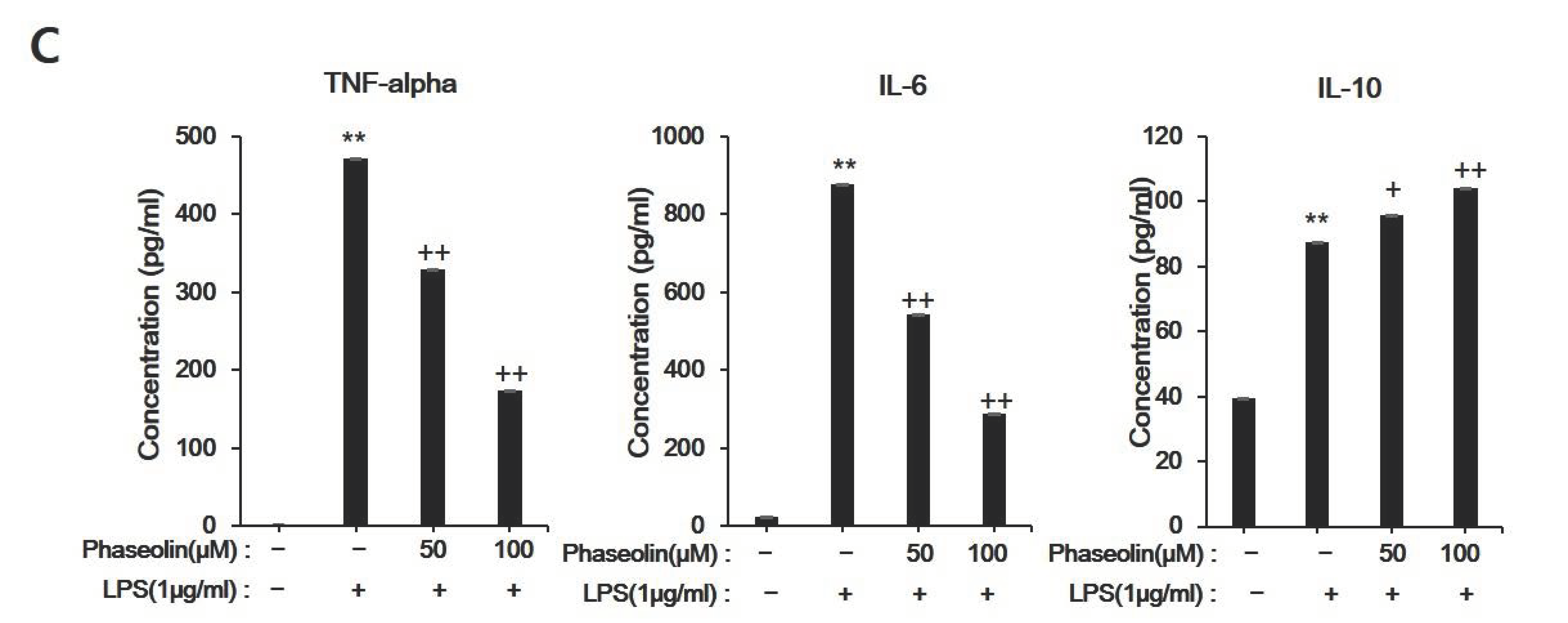
3.2. Phaseolin Suppressed Pro-Inflammatory Cytokines and Other Inflammation-Related Genes in LPS-Stimulated RAW 264.7 Cells
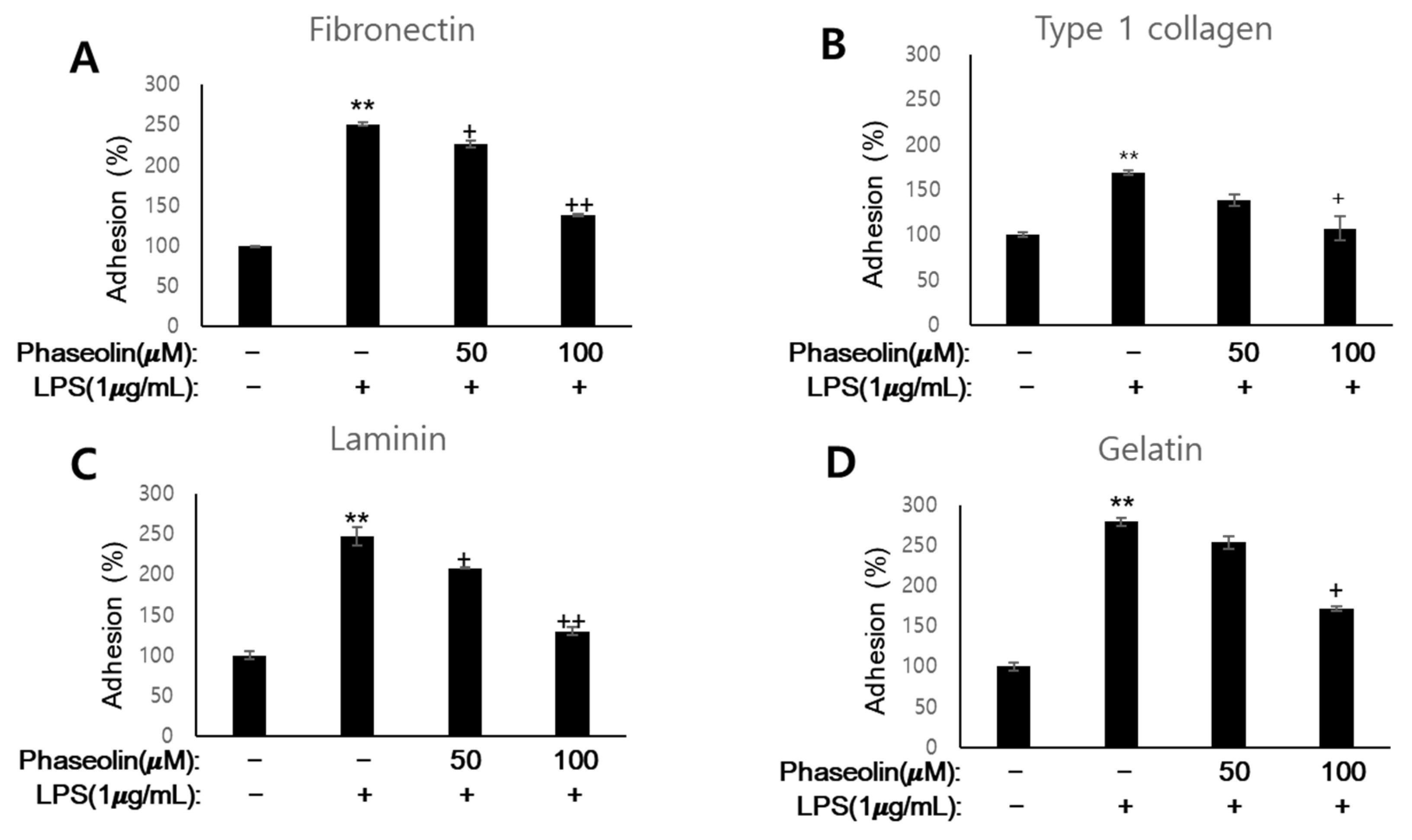
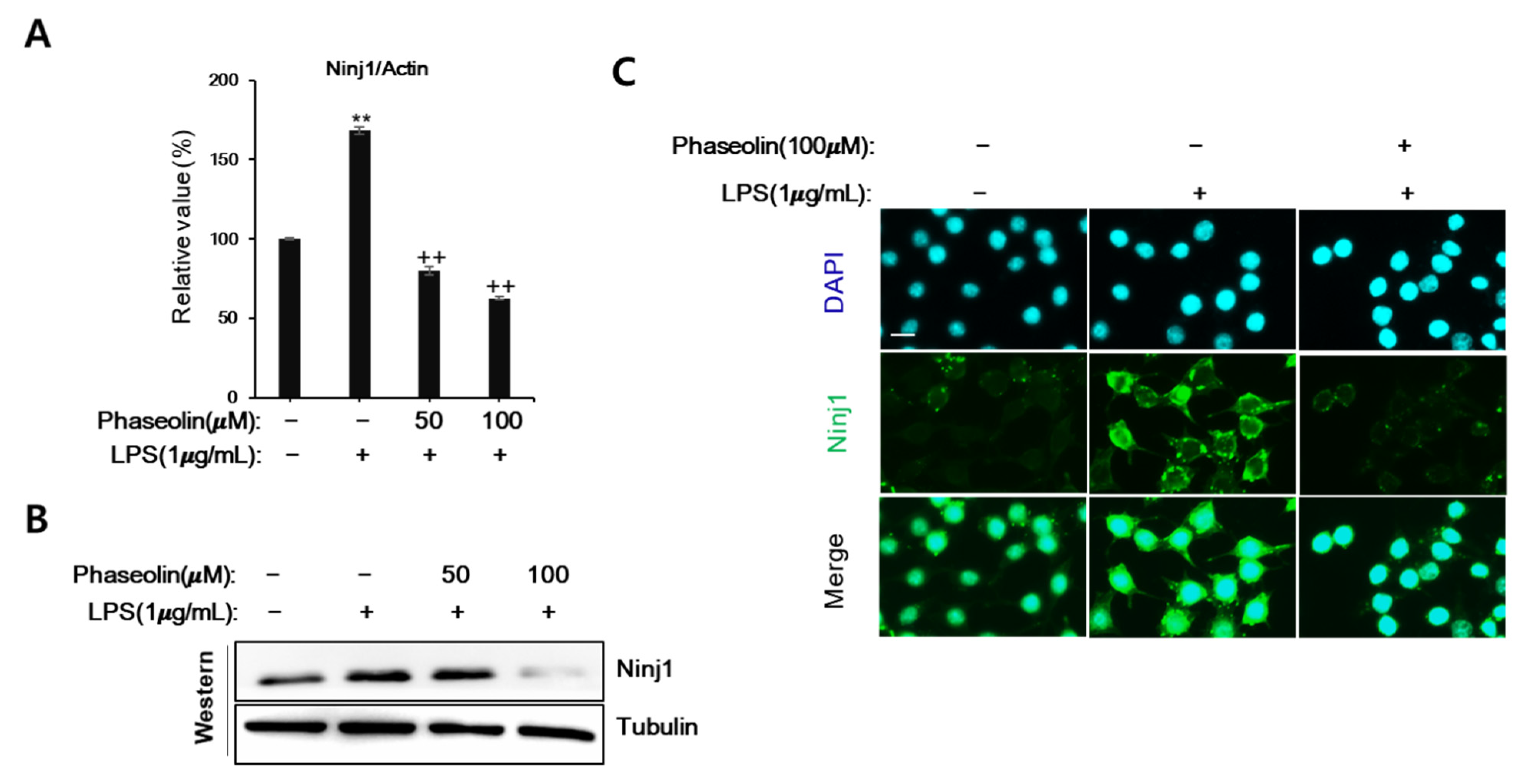
3.3. Phaseolin Suppressed Cell-ECM Adhesion by Downregulating Ninj1
3.4. Phaseolin Inhibited Cell Migration and Matrix Metalloproteinase (MMP)
3.5. Phaseolin Inhibited NF-κB Translocation In Vitro
3.6. Phaseolin Attenuated Endotoxin-Induced Uveitis in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiu, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Matsui, M.; Yano, S.; Matsumoto, N.; Futai, M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem. Biophys. Res. Commun. 2012, 425, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ding, Y.; Tong, Z. Efficacy and Safety of Sophora flavescens (Kushen) Based Traditional Chinese Medicine in the Treatment of Ulcerative Colitis: Clinical Evidence and Potential Mechanisms. Front. Pharmacol. 2020, 11, 603476. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, Z.; Zhou, X.; Xu, J. Synergic Anti-Pruritus Mechanisms of Action for the Radix Sophorae Flavescentis and Fructus Cnidii Herbal Pair. Molecules 2017, 22, 1465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Li, X.; Zhang, D.; Han, Y.; Zhang, X. Comprehensive two-dimensional PC-3 prostate cancer cell membrane chromatography for screening anti-tumor components from Radix Sophorae flavescentis. J. Sep. Sci. 2017, 40, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shin, S.J.; Kim, J.N.; Kwon, M.J.; Lim, E.Y.; Kim, Y.T.; Kim, H.; Kim, B.J. Radix Sophorae Flavescentis inhibits proliferation and induces apoptosis of AGS human gastric cancer cells. Mol. Med. Rep. 2019, 19, 1911–1918. [Google Scholar] [CrossRef]
- Yang, X.; Cai, W.; Yang, Q.; Lu, Z.; Li, J.; Yu, J. Compound Radix Sophorae Flavescentis exerts antitumor effects by inhibiting the proliferation and inducing the apoptosis of esophageal carcinoma TE-8 cells. Oncol. Lett. 2015, 10, 2209–2213. [Google Scholar] [CrossRef]
- Yi, L.; Lu, Y.; Yu, S.; Cheng, Q.; Yi, L. Formononetin inhibits inflammation and promotes gastric mucosal angiogenesis in gastric ulcer rats through regulating NF-kappaB signaling pathway. J. Recept. Signal. Transduct. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Nucera, E.; Pandolfi, F. Luteolin, inflammation and cancer: Special emphasis on gut microbiota. Biofactors 2021. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Med. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M.B.; Perrin, D.R.; Dawn, R.; Whittle, C.P. Phaseollin and phaseollidin relationships in infection-droplets on endocarp of Phaseolus vulgaris. Physiological. Plant Pathol. 1974, 4, 261–276. [Google Scholar] [CrossRef]
- Telikepalli, H.; Gollapudi, S.R.; Keshavarzshokri, A.; Velazquez, L.; Sandmann, R.A.; Veliz, E.A.; Rao, K.V.J.; Madhavi, A.S.; Mitscher, L.A. Isoflavonoids and a Cinnamyl Phenol from Root Extracts of Erythrina-Variegata. Phytochemistry 1990, 29, 2005–2007. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Zhang, D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Kulawik, A.; Suckow-Schnitker, A.K.; Chovolou, Y.; Rohrig, R.; Ruhl, S.; Kampkotter, A.; Addae-Kyereme, J.; Wright, C.W.; Passreiter, C.M. Pterocarpans phaseollin and neorautenol isolated from Erythrina addisoniae induce apoptotic cell death accompanied by inhibition of ERK phosphorylation. Toxicology 2007, 242, 71–79. [Google Scholar] [CrossRef]
- Novoa, B.; Figueras, A. Zebrafish: Model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012, 946, 253–275. [Google Scholar] [CrossRef]
- Henry, K.M.; Loynes, C.A.; Whyte, M.K.; Renshaw, S.A. Zebrafish as a model for the study of neutrophil biology. J. Leukoc. Biol. 2013, 94, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Loynes, C.A.; Martin, J.S.; Robertson, A.; Trushell, D.M.; Ingham, P.W.; Whyte, M.K.; Renshaw, S.A. Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J. Leukoc. Biol. 2010, 87, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rosowski, E.E. Determining macrophage versus neutrophil contributions to innate immunity using larval zebrafish. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef]
- Philip, A.M.; Wang, Y.; Mauro, A.; El-Rass, S.; Marshall, J.C.; Lee, W.L.; Slutsky, A.S.; dosSantos, C.C.; Wen, X.Y. Development of a zebrafish sepsis model for high-throughput drug discovery. Mol. Med. 2017, 23, 134–148. [Google Scholar] [CrossRef]
- Yang, L.L.; Wang, G.Q.; Yang, L.M.; Huang, Z.B.; Zhang, W.Q.; Yu, L.Z. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: A novel screening method for anti-inflammatory drugs. Molecules 2014, 19, 2390–2409. [Google Scholar] [CrossRef]
- Jeong, J.W.; Cha, H.J.; Han, M.H.; Hwang, S.J.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol. Ther. 2018, 26, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Jun, S.H.; Park, Y.; Cha, S.H.; Yoon, M.; Cho, S.; Lee, H.J.; Park, Y. Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation. Nanomedicine 2015, 11, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Lee, H.J.; Ahn, B.J.; Shin, M.W.; Choi, J.H.; Kim, K.W. Ninjurin1: A potential adhesion molecule and its role in inflammation and tissue remodeling. Mol. Cells 2010, 29, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- Zuñiga, A.E.R.a.G. Induced plant secondary metabolites for phytopatogenic fungi control: A review. J. Soil. Sci. Plant Nutr. 2012, 12, 893–911. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Davis, C.D.; Milner, J.A. Phytoalexins in cancer prevention. Front. Biosci. 2012, 17, 2035–2058. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.; Yaya, E.E. Plant chemical defenses: Are all constitutive antimicrobial metabolites phytoanticipins? Nat. Prod. Commun. 2015, 10, 209–218. [Google Scholar] [CrossRef]
- Jeandet, P.; Hebrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Chripkova, M.; Zigo, F.; Mojzis, J. Antiproliferative Effect of Indole Phytoalexins. Molecules 2016, 21, 1626. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Zhu, N.; Hou, J. Molecular mechanism of the anti-inflammatory effects of Sophorae Flavescentis Aiton identified by network pharmacology. Sci. Rep. 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Carmody, R.J. NF-kappaB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.J.; Le, H.; Shin, M.W.; Bae, S.J.; Lee, E.J.; Wee, H.J.; Cha, J.H.; Lee, H.J.; Lee, H.S.; Kim, J.H.; et al. Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice. J. Biol. Chem. 2014, 289, 3328–3338. [Google Scholar] [CrossRef]
- Ifergan, I.; Kebir, H.; Terouz, S.; Alvarez, J.I.; Lecuyer, M.A.; Gendron, S.; Bourbonniere, L.; Dunay, I.R.; Bouthillier, A.; Moumdjian, R.; et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann. Neurol. 2011, 70, 751–763. [Google Scholar] [CrossRef]
- Choi, H.; Bae, S.J.; Choi, G.; Lee, H.; Son, T.; Kim, J.G.; An, S.; Lee, H.S.; Seo, J.H.; Kwon, H.B.; et al. Ninjurin1 deficiency aggravates colitis development by promoting M1 macrophage polarization and inducing microbial imbalance. FASEB J. 2020, 34, 8702–8720. [Google Scholar] [CrossRef]
- Wang, X.; Qin, J.; Zhang, X.; Peng, Z.; Ye, K.; Wu, X.; Yang, X.; Shi, H.; Zhao, Z.; Guo, X.; et al. Functional blocking of Ninjurin1 as a strategy for protecting endothelial cells in diabetes mellitus. Clin. Sci. 2018, 132, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, T.K.; Jeong, S.J.; Jung, I.H.; Kim, N.; Lee, M.N.; Sonn, S.K.; Seo, S.; Jin, J.; Kweon, H.Y.; et al. Anti-Inflammatory Actions of Soluble Ninjurin-1 Ameliorate Atherosclerosis. Circulation 2020, 142, 1736–1751. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-J.; Song, Y.-S.; Lee, H.-J. Phaseolin Attenuates Lipopolysaccharide-Induced Inflammation in RAW 264.7 Cells and Zebrafish. Biomedicines 2021, 9, 420. https://doi.org/10.3390/biomedicines9040420
Hwang S-J, Song Y-S, Lee H-J. Phaseolin Attenuates Lipopolysaccharide-Induced Inflammation in RAW 264.7 Cells and Zebrafish. Biomedicines. 2021; 9(4):420. https://doi.org/10.3390/biomedicines9040420
Chicago/Turabian StyleHwang, Su-Jung, Ye-Seul Song, and Hyo-Jong Lee. 2021. "Phaseolin Attenuates Lipopolysaccharide-Induced Inflammation in RAW 264.7 Cells and Zebrafish" Biomedicines 9, no. 4: 420. https://doi.org/10.3390/biomedicines9040420
APA StyleHwang, S.-J., Song, Y.-S., & Lee, H.-J. (2021). Phaseolin Attenuates Lipopolysaccharide-Induced Inflammation in RAW 264.7 Cells and Zebrafish. Biomedicines, 9(4), 420. https://doi.org/10.3390/biomedicines9040420






