Silencing Antibiotic Resistance with Antisense Oligonucleotides
Abstract
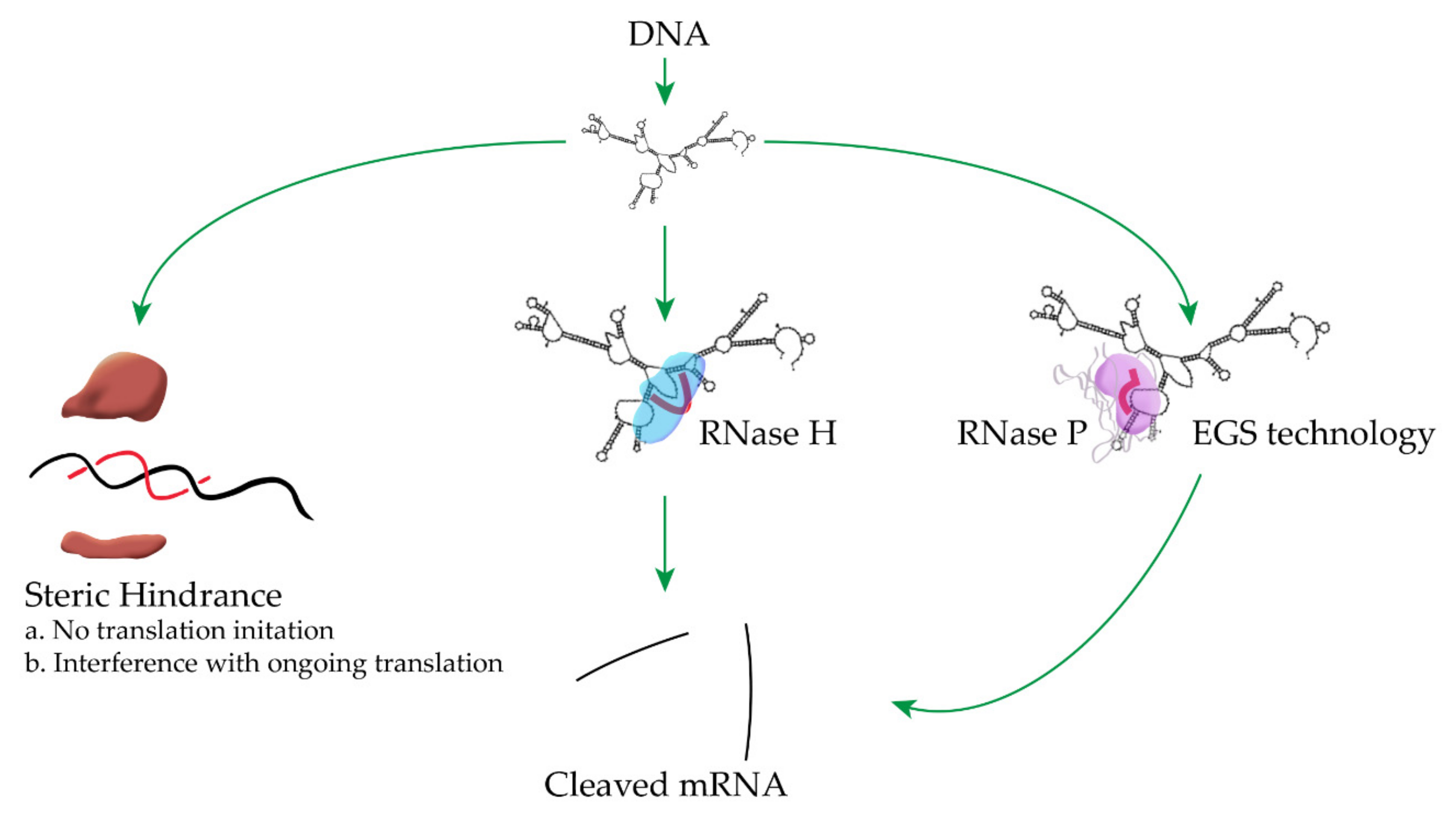
:1. Introduction
2. External Guide Sequence (EGS) Technology
3. RNase H
4. Steric Hindrance
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwick, C. First “Antisense” Drug Will Treat CMV Retinitis. JAMA 1998, 280, 871. [Google Scholar] [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.-H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 1–27. [Google Scholar] [CrossRef]
- Crooke, S.T.; Liang, X.-H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense oligonucleotides: An emerging area in drug discovery and development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Gogtay, N.J. Therapeutic nucleic acids: Current clinical status. Br. J. Clin. Pharmacol. 2016, 82, 659–672. [Google Scholar] [CrossRef] [Green Version]
- Scharner, J.; Aznarez, I. Clinical applications of single-stranded oligonucleotides: Current landscape of approved and in-development therapeutics. Mol. Ther. 2021, 29, 540–554. [Google Scholar] [CrossRef]
- Pifer, R.; Greenberg, D.E. Antisense antibacterial compounds. Transl. Res. 2020, 223, 89–106. [Google Scholar] [CrossRef]
- Rasmussen, L.C.V.; Sperling-Petersen, H.U.; Mortensen, K.K. Hitting bacteria at the heart of the central dogma: Sequence-specific inhibition. Microb. Cell Factories 2007, 6, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Titze-De-Almeida, R.; David, C.; Titze-De-Almeida, S.S. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm. Res. 2017, 34, 1339–1363. [Google Scholar] [CrossRef]
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2019, 30, 114–127. [Google Scholar]
- Drury, R.E.; O’Connor, D.; Pollard, A.J. The clinical application of microRNAs in infectious disease. Front. Immunol. 2017, 8, 1182. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Santos, R.D.; Blom, D.J.; Marais, A.D.; Charng, M.-J.; Cromwell, W.C.; Lachmann, R.H.; Gaudet, D.; Tan, J.L.; Chasan-Taber, S.; et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 998–1006. [Google Scholar] [CrossRef]
- Acsadi, G.; Crawford, T.O.; Müller-Felber, W.; Shieh, P.B.; Richardson, R.; Natarajan, N.; Castro, D.; Ramirez-Schrempp, D.; Gambino, G.; Sun, P.; et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve 2021. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A review of Patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.; Maruyama, R.; Yokota, T. Inotersen for the treatment of hereditary transthyretin amyloidosis. Methods in Molecular Biology 2020, 2176, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Goemans, N.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Shao, J.; Kaye, E.M.; Mercuri, E.; Eteplirsen Study Group; Telethon Foundation DMD Italian Network. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 2016, 79, 257–271. [Google Scholar] [CrossRef]
- Heo, Y.A. Golodirsen: First approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 trial of RNAi therapeutic Givosiran for acute intermittent porphyria. New Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; El Achkar, C.M.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-customized oligonucleotide therapy for a rare genetic disease. New Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef]
- Komaki, H.; Takeshima, Y.; Matsumura, T.; Ozasa, S.; Funato, M.; Takeshita, E.; Iwata, Y.; Yajima, H.; Egawa, Y.; Toramoto, T.; et al. Viltolarsen in Japanese Duchenne muscular dystrophy patients: A phase 1/2 study. Ann. Clin. Transl. Neurol. 2020, 7, 2393–2408. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef]
- Rodrigues, M.; Yokota, T. An overview of recent advances and clinical applications of exon skipping and splice modulation for muscular dystrophy and various genetic diseases. Methods Mol. Biol. 2018, 1828, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Sully, E.K.; Geller, B.L. Antisense antimicrobial therapeutics. Curr. Opin. Microbiol. 2016, 33, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, A.M.; Loftus, B.J. (Non-)translational medicine: Targeting bacterial RNA. Front. Genet. 2013, 4, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goltermann, L.; Nielsen, P.E. PNA Antisense Targeting in Bacteria: Determination of Antibacterial Activity (MIC) of PNA-Peptide Conjugates. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 231–239. [Google Scholar]
- Tolmasky, M.E. Strategies to prolong the useful life of existing antibiotics and help overcoming the antibiotic resistance crisis In Frontiers in Clinical Drug Research-Anti Infectives; Atta-ur-Rhaman, Ed.; Bentham Books: Sharjah, UAE, 2017; Volume 1. [Google Scholar]
- Davies-Sala, C.; Soler-Bistué, A.; Bonomo, R.A.; Zorreguieta, A.; Tolmasky, M.E. External guide sequence technology: A path to development of novel antimicrobial therapeutics. Ann. N. Y. Acad. Sci. 2015, 1354, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, C.D.; Soler-Bistué, A.J.C.; Korprapun, L.; Zorreguieta, A.; Tolmasky, M.E. Inhibition of Cell Division Induced by External Guide Sequences (EGS Technology) Targeting ftsZ. PLoS ONE 2012, 7, e47690. [Google Scholar] [CrossRef] [Green Version]
- Streicher, L.M. Exploring the future of infectious disease treatment in a post-antibiotic era: A comparative review of alternative therapeutics. J. Glob. Antimicrob. Resist. 2021, 24, 285–295. [Google Scholar] [CrossRef]
- Vogel, J. An RNA biology perspective on species-specific programmable RNA antibiotics. Mol. Microbiol. 2020, 113, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Roy, I. Making sense of therapeutics using antisense technology. Expert Opin. Drug Discov. 2011, 6, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef]
- Good, L.; Stach, J.E.M. Synthetic RNA silencing in bacteria? Antimicrobial discovery and resistance breaking. Front. Microbiol. 2011, 2, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Altman, S. A view of RNase P. Mol. BioSyst. 2007, 3, 604–607. [Google Scholar] [CrossRef]
- Mondragón, A. Structural Studies of RNase P. Annu. Rev. Biophys. 2013, 42, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Reiter, N.J.; Osterman, A.; Torres-Larios, A.; Swinger, K.K.; Pan, T.; Mondragón, A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nat. Cell Biol. 2010, 468, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, V.; Vioque, A.; Altman, S. RNase P: Variations and Uses. J. Biol. Chem. 2002, 277, 6759–6762. [Google Scholar] [CrossRef] [Green Version]
- Kirsebom, L.A. RNase P RNA mediated cleavage: Substrate recognition and catalysis. Biochim. 2007, 89, 1183–1194. [Google Scholar] [CrossRef]
- Kirsebom, L.A.; Svärd, S.G. The kinetics and specificity of cleavage by RNase P is mainly dependent on the structure of the amino acid acceptor stem. Nucleic Acids Res. 1992, 20, 425–432. [Google Scholar] [CrossRef]
- Forster, A.C.; Altman, S. External guide sequences for an RNA enzyme. Sci. 1990, 249, 783–786. [Google Scholar] [CrossRef]
- Lundblad, E.W.; Altman, S. Inhibition of gene expression by RNase P. New Biotechnol. 2010, 27, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Guerrier-Takada, C.; Li, Y.; Altman, S. Artificial regulation of gene expression in Escherichia coli by RNase P. Proc. Natl. Acad. Sci. USA 1995, 92, 11115–11119. [Google Scholar] [CrossRef] [Green Version]
- Guerrier-Takada, C.; Salavati, R.; Altman, S. Phenotypic conversion of drug-resistant bacteria to drug sensitivity. Proc. Natl. Acad. Sci. USA 1997, 94, 8468–8472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.-Y.; Xu, C.-R.; Chen, R.; Liu, S.-G.; Feng, J.-N. Chloromycetin resistance of clinically isolated E coli is conversed by using EGS technique to repress the chloromycetin acetyl transferase. World J. Gastroenterol. 2005, 11, 7368–7373. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Bistué, A.J.C.S.; Ha, H.; Sarno, R.; Don, M.; Zorreguieta, A.; Tolmasky, M.E. External guide sequences targeting the aac(6′)-Ib mRNA induce inhibition of amikacin resistance. Antimicrob. Agents Chemother. 2007, 51, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Gait, M.J. CHAPTER 1. History and development of nucleotide analogues in nucleic acids drugs. In Drug Discovery; Royal Society of Chemistry (RSC): Cambridge, UK, 2019; pp. 1–21. [Google Scholar]
- Bistue, A.J.C.S.; Martin, F.A.; Vozza, N.; Ha, H.; Joaquin, J.C.; Zorreguieta, A.; Tolmasky, M.E. Inhibition of aac(6′)-Ib-mediated amikacin resistance by nuclease-resistant external guide sequences in bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 13230–13235. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.; Jani, S.; Davies-Sala, C.; Soler-Bistué, A.J.C.; Zorreguieta, A.; E Tolmasky, M. Assessment of configurations and chemistries of bridged nucleic acids-containing oligomers as external guide sequences: A methodology for inhibition of expression of antibiotic resistance genes. Biol. Methods Protoc. 2016, 1, 1. [Google Scholar] [CrossRef]
- Soler-Bistué, A.; Zorreguieta, A.; Tolmasky, M.E. Bridged Nucleic Acids Reloaded. Molecules 2019, 24, 2297. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, A.; Vitali, A.; Stach, J.E.; Nielsen, P.E. Role of SbmA in the uptake of peptide nucleic acid (PNA)-peptide conjugates in E. coli. ACS Chem. Biol. 2012, 8, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Yavari, N.; Goltermann, L.; Nielsen, P.E. Uptake, stability, and activity of antisense anti-acpP PNA-peptide conjugates in Escherichia coli and the role of SbmA. ACS Chem. Biol. 2021, 16, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Boisguérin, P.; Deshayes, S.; Gait, M.J.; O’Donovan, L.; Godfrey, C.; Betts, C.A.; Wood, M.J.; Lebleu, B. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Adv. Drug Deliv. Rev. 2015, 87, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef] [PubMed]
- Reissmann, S. Cell penetration: Scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. 2014, 20, 760–784. [Google Scholar] [CrossRef] [PubMed]
- Kurrikoff, K.; Vunk, B.; Langel, Ü. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin. Biol. Ther. 2021, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lehto, T.; Ezzat, K.; Wood, M.J.; EL Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef]
- Puckett, S.E.; Reese, K.A.; Mitev, G.M.; Mullen, V.; Johnson, R.C.; Pomraning, K.R.; Mellbye, B.L.; Tilley, L.D.; Iversen, P.L.; Freitag, M.; et al. Bacterial resistance to antisense peptide phosphorodiamidate morpholino oligomers. Antimicrob. Agents Chemother. 2012, 56, 6147–6153. [Google Scholar] [CrossRef] [Green Version]
- Jani, S.; Jackson, A.; Davies-Sala, C.; Chiem, K.; Soler-Bistué, A.; Zorreguieta, A.; Tolmasky, M.E. Assessment of external guide sequences’ (EGS) efficiency as inducers of RNase P-mediated cleavage of mRNA target molecules. Methods Mol. Biol. 2018, 1737, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Xie, G.; Marshall, S.H.; Hujer, K.M.; Chain, P.S.G.; Bonomo, R.A.; Tolmasky, M.E. Multidrug-resistant (MDR) Klebsiella pneumoniae clinical isolates: A zone of high heterogeneity (HHZ) as a tool for epidemiological studies. Clin. Microbiol. Infect. 2012, 18, E254–E258. [Google Scholar] [CrossRef] [Green Version]
- Arivett, B.A.; Fiester, S.E.; Ream, D.C.; Centrón, D.; Ramírez, M.S.; Tolmasky, M.E.; Actis, L.A. Draft genome of the multidrug-resistant Acinetobacter baumannii strain A155 clinical isolate. Genome Announc. 2015, 3, e00212–e00215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarno, R.; Ha, H.; Weinsetel, N.; Tolmasky, M.E. Inhibition of aminoglycoside 6′-N-acetyltransferase type Ib-mediated amikacin resistance by antisense oligodeoxynucleotides. Antimicrob. Agents Chemother. 2003, 47, 3296–3304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, N.; Ko, J.-H.; Xiao, G.; Wesolowski, N.; Shan, G.; Geller, B.; Izadjoo, M.; Altman, S. Inactivation of expression of several genes in a variety of bacterial species by EGS technology. Proc. Natl. Acad. Sci. USA 2009, 106, 8163–8168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesolowski, D.; Alonso, D.; Altman, S. Combined effect of a peptide-morpholino oligonucleotide conjugate and a cell-penetrating peptide as an antibiotic. Proc. Natl. Acad. Sci. USA 2013, 110, 8686–8689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyjek, M.; Figiel, M.; Nowotny, M. RNases H: Structure and mechanism. DNA Repair 2019, 84, 102672. [Google Scholar] [CrossRef]
- White, D.G.; Maneewannakul, K.; Von Hofe, E.; Zillman, M.; Eisenberg, W.; Field, A.K.; Levy, S.B. Inhibition of the multiple antibiotic resistance (mar) operon in Escherichia coli by antisense DNA analogs. Antimicrob. Agents Chemother. 1997, 41, 2699–2704. [Google Scholar] [CrossRef] [Green Version]
- Burnett, J.C.; Rossi, J.J. RNA-based therapeutics: Current progress and future prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Meng, J.; Jia, M.; Ma, X.; He, G.; Yu, J.; Wang, R.; Bai, H.; Hou, Z.; Luo, X. oprMas a new target for reversion of multidrug resistance inPseudomonas aeruginosaby antisense phosphorothioate oligodeoxynucleotides. FEMS Immunol. Med Microbiol. 2010, 60, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Nikaido, H.; Poole, K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1995, 39, 1948–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsumi, K.; Yonehara, R.; Ishizaka-Ikeda, E.; Miyazaki, N.; Maeda, S.; Iwasaki, K.; Nakagawa, A.; Yamashita, E. Structures of the wild-type MexAB–OprM tripartite pump reveal its complex formation and drug efflux mechanism. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Phan, G.; Picard, M.; Broutin, I. Focus on the outer membrane factor OprM, the forgotten player from efflux pumps assemblies. Antibiotics 2015, 4, 544–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillion, P.; Desjardins, A.; Sayasith, K.; Lagacé, J. Encapsulation of DNA in negatively charged liposomes and inhibition of bacterial gene expression with fluid liposome-encapsulated antisense oligonucleotides. Biochim Biophys Acta. 2001, 1515, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Santos, R.S.; Moreira, L.; Guimarães, N.M.; Braeckmans, K.; De Smedt, S.C.; Azevedo, N.F. Delivery of oligonucleotides into bacteria by fusogenic liposomes. Methods Mol. Biol. 2021, 2246, 87–96. [Google Scholar] [PubMed]
- Kauss, T.; Arpin, C.; Bientz, L.; Nguyen, P.V.; Vialet, B.; Benizri, S.; Barthélémy, P. Lipid oligonucleotides as a new strategy for tackling the antibiotic resistance. Sci. Rep. 2020, 10, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Pathania, R.; Sharma, A.; Gupta, V.K. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med Res. 2019, 149, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.Y.; Jamshidi, S.; Sutton, J.M.; Rahman, K.M. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016, 23, 1062–1081. [Google Scholar] [CrossRef]
- Helene, C.; Montenay-Garestier, T.; Saison, T.; Takasugi, M.; Toulme, J.-J.; Asseline, U.; Lancelot, G.; Maurizot, J.; Toulme, F.; Thuong, N. Oligodeoxynucleotides covalently linked to intercalating agents: A new class of gene regulatory substances. Biochimie 1985, 67, 777–783. [Google Scholar] [CrossRef]
- Gasparro, F.; Edelson, R.; O’Malley, M.; Ugent, S.; Wong, H. Photoactivatable antisense DNA: Suppression of ampicillin resistance in normally resistant Escherichia coli. Antisense Res. Dev. 1991, 1, 117–140. [Google Scholar] [CrossRef]
- Good, L.; Nielsen, P.E. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat. Biotechnol. 1998, 16, 355–358. [Google Scholar] [CrossRef] [PubMed]
- E Nielsen, P.; Egholm, M. An introduction to peptide nucleic acid. Curr. Issues Mol. Biol. 1999, 1, 89–104. [Google Scholar]
- Nielsen, P.E. Gene targeting and expression modulation by peptide nucleic acids (PNA). Curr. Pharm. Des. 2010, 16, 3118–3123. [Google Scholar] [CrossRef]
- Lundin, K.E.; Good, L.; Strömberg, R.; Gräslund, A.; Smith, C.I.E. Biological activity and biotechnological aspects of peptide nucleic acid. Nat. Var. Clocks 2006, 56, 1–51. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, S.K.; Yoon, J.W. Antisense peptide nucleic acids as a potential anti-infective agent. J. Microbiol. 2019, 57, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Loeffler, A.; Lloyd, D.H.; Nair, S.P.; Good, L. Oxacillin sensitization of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius by antisense peptide nucleic acids in vitro. BMC Microbiol. 2015, 15, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Sang, G.; You, Y.; Xue, X.; Zhou, Y.; Hou, Z.; Meng, J.; Luo, X. Targeting RNA polymerase primary σ70 as a therapeutic strategy against methicillin-resistant Staphylococcus aureus by antisense peptide nucleic acid. PLoS ONE 2012, 7, e29886. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Boberek, J.M.; Nakashima, N.; Stach, J.; Good, L. Concurrent growth rate and transcript analyses reveal essential gene stringency in Escherichia coli. PLoS ONE 2009, 4, e6061. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of β-lactamase-producing pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Bonomo, R.A.; Tolmasky, M.E. Carbapenemases: Transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules 2020, 10, 720. [Google Scholar] [CrossRef]
- Sully, E.K.; Geller, B.L.; Li, L.; Moody, C.M.; Bailey, S.M.; Moore, A.L.; Wong, M.; Nordmann, P.; Daly, S.M.; Sturge, C.R.; et al. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogensin vitroandin vivo. J. Antimicrob. Chemother. 2016, 72, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Zhang, Q. Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter. J. Antimicrob. Chemother. 2009, 63, 946–948. [Google Scholar] [CrossRef] [Green Version]
- Aunins, T.R.; E Erickson, K.; Chatterjee, A. Transcriptome-based design of antisense inhibitors potentiates carbapenem efficacy in CRE Escherichia coli. Proc. Natl. Acad. Sci. USA 2020, 117, 30699–30709. [Google Scholar] [CrossRef]
- Eller, K.A.; Aunins, T.R.; Courtney, C.M.; Campos, J.K.; Otoupal, P.B.; Erickson, K.E.; Madinger, N.E.; Chatterjee, A. Facile accelerated specific therapeutic (FAST) platform develops antisense therapies to counter multidrug-resistant bacteria. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Ling, Z.; Zhang, C.; Fu, M.; Wang, Y.; Wang, S.; Zhang, S.; Shen, Z. Peptide nucleic acid restores colistin susceptibility through modulation of MCR-1 expression in Escherichia coli. J. Antimicrob. Chemother. 2020, 75, 2059–2065. [Google Scholar] [CrossRef]
- Fauci, A.S. Emerging and reemerging infectious diseases: The perpetual challenge. Acad. Med. 2005, 80, 1079–1085. [Google Scholar] [CrossRef]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E.; et al. Tackling antibiotic resistance. Nat. Rev. Genet. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Sprenger, M.; Fukuda, K. New mechanisms, new worries. Science 2016, 351, 1263–1264. [Google Scholar] [CrossRef]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Harris, P.N.A.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant Gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59, S335–S339. [Google Scholar] [CrossRef]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52, 397–428. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging infectious diseases in 2012: 20 years after the Institute of Medicine report. mBio 2012, 3, 3. [Google Scholar] [CrossRef] [Green Version]
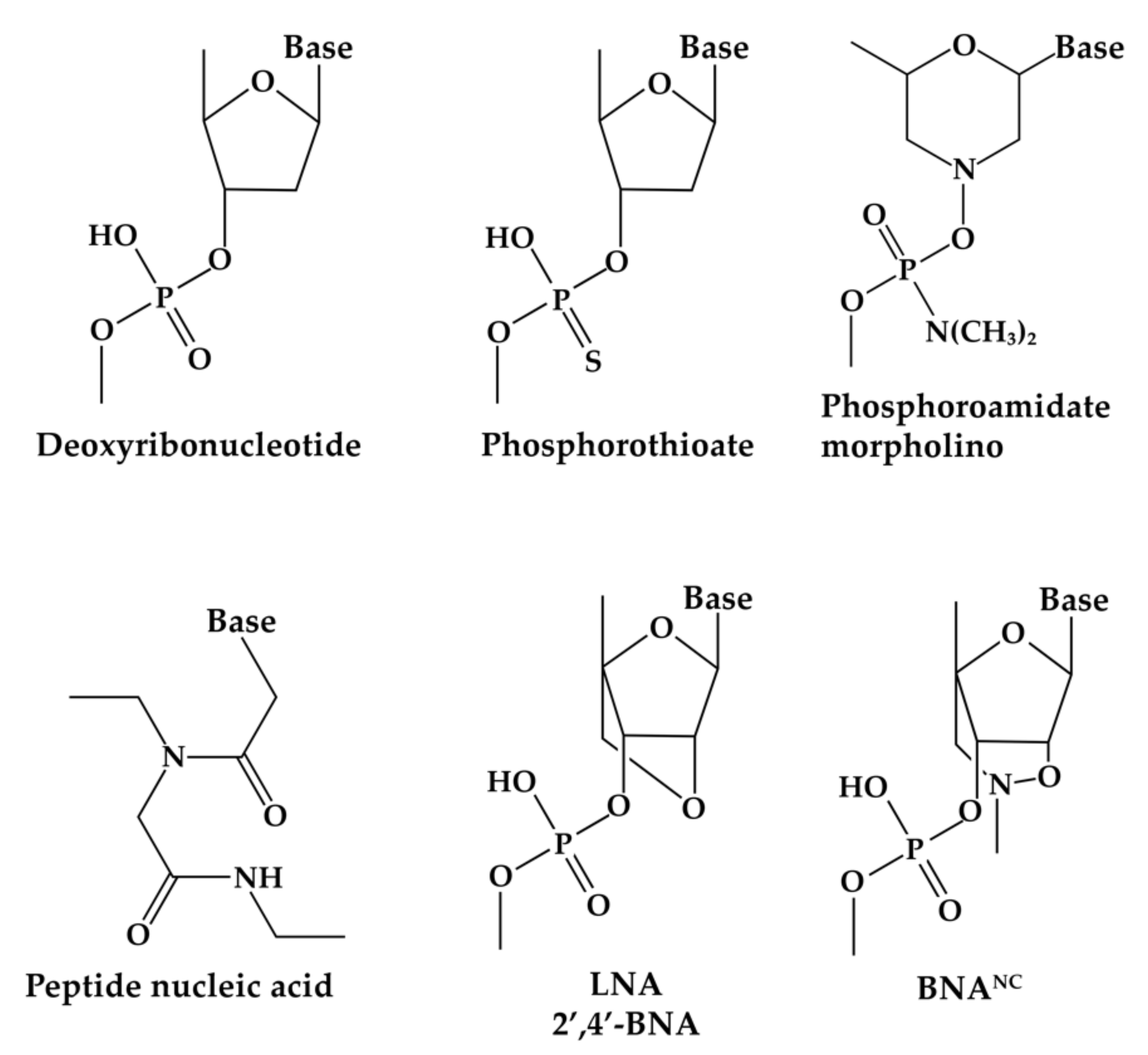
| Drug | Chemistry | Route | Target | Indication | Year (FDA Approval) | Year (EMA Approval) | Company, Reference |
|---|---|---|---|---|---|---|---|
| Fomivirsen (VitraveneTM) | Phosphorothioate | Intravitreal | Cytomegalovirus mRNA | Cytomegalovirus infection | 1998 | - | Ionis Pharmaceuticals [2] |
| Mipomersen (KynamroTM) | 2′-O-Methoxyethyl, Phosphorothioate, 5-methyl cytosine | Subcutaneous | Apo-B-100 mRNA | Homozygous familial hypercholesterolemia | 2013 | - | Genzyme [13] |
| Nusinersen (Spinraza®) | 2′-O-Methoxyethyl, Phosphorothioate, 5-methyl cytosine | Intrathecal | Pre-mRNA | Spinal muscular atrophy | 2016 | 2017 | Biogen [14] |
| Patisiran (Onpattro®) | siRNA | Intravenous | Transthyretin mRNA | hereditary transthyretin-mediated amyloidosis | 2018 | 2018 | Alnylam [15] |
| Inotersen (Tegsedi®) | 2′-O-Methoxyethyl, Phosphorothioate | Subcutaneous | Transthyretin mRNA | hereditary transthyretin-mediated amyloidosis | 2018 | 2018 | Ionis Pharmaceuticals [16] |
| Eteplirsen (Exondys 51®) | Phosphorodiamidate morpholino | Intravenous | Exon 51 | Duchenne muscular dystrophy | 2016 | 2018 | Sarepta [17] |
| Golodirsen (Vyondys 53TM) | Phosphorodiamidate morpholino | Intravenous | Exon 53 | Duchenne muscular dystrophy | 2019 | Sarepta [18] | |
| Givosiran (Givlaari®) | siRNA | Subcutaneous | ALS1 mRNA | Acutehepaticporphyria | 2019 | 2020 | Alnylam [19] |
| Milasen | 2′-O-Methoxyethyl, Phosphorothioate, 5-methyl cytosine | Intrathecal | Intron 6 spice acceptor cryptic site | Neuronal ceroid Lipofuscinosis 7 | * 2018 | Boston Children’s Hospital [20] | |
| Vitolarsen | Phosphorodiamidate morpholino | Intravenous | Exon 53 | Duchenne muscular dystrophy | 2020 | Nippon Shinyaku [21] | |
| Volanesorsen (Waylivra®) | 2′-O-Methoxyethyl, 5-methyl cytosine, 2′-deoxy | Subcutaneous injection | Apolipoprotein C3 | Familial chylomicronaemia syndrome | 2019 | Akcea Therapeutics [22] | |
| Casimersen (Amondys 45 TM) | Phosphorodiamidate morpholino | Intravenous | Exon 45 | Duchenne muscular dystrophy | 2021 | Sarepta Therapeutics, Inc. [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jani, S.; Ramirez, M.S.; Tolmasky, M.E. Silencing Antibiotic Resistance with Antisense Oligonucleotides. Biomedicines 2021, 9, 416. https://doi.org/10.3390/biomedicines9040416
Jani S, Ramirez MS, Tolmasky ME. Silencing Antibiotic Resistance with Antisense Oligonucleotides. Biomedicines. 2021; 9(4):416. https://doi.org/10.3390/biomedicines9040416
Chicago/Turabian StyleJani, Saumya, Maria Soledad Ramirez, and Marcelo E. Tolmasky. 2021. "Silencing Antibiotic Resistance with Antisense Oligonucleotides" Biomedicines 9, no. 4: 416. https://doi.org/10.3390/biomedicines9040416
APA StyleJani, S., Ramirez, M. S., & Tolmasky, M. E. (2021). Silencing Antibiotic Resistance with Antisense Oligonucleotides. Biomedicines, 9(4), 416. https://doi.org/10.3390/biomedicines9040416








