Polymerizable Matrix Metalloproteinases’ Inhibitors with Potential Application for Dental Restorations
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Docking Studies
2.2. Synthesis of A, B and C Compounds
2.3. Cytotoxicity Study Using MTT Assay
2.4. Statistical Analysis
3. Results and Discussion
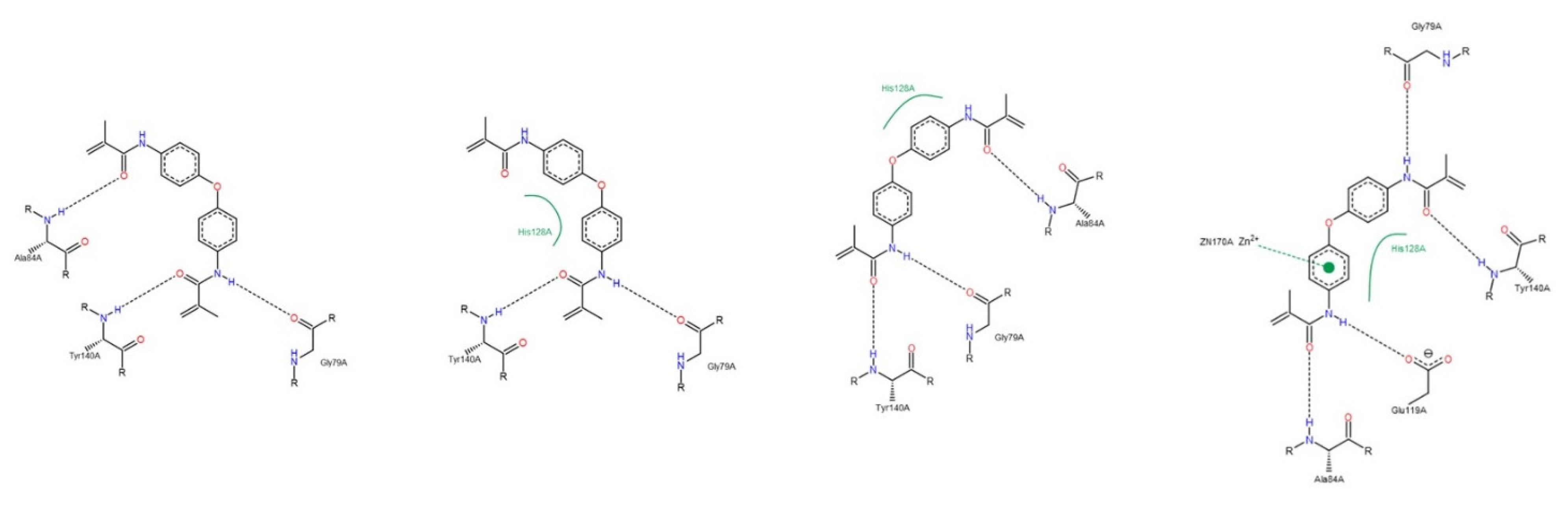
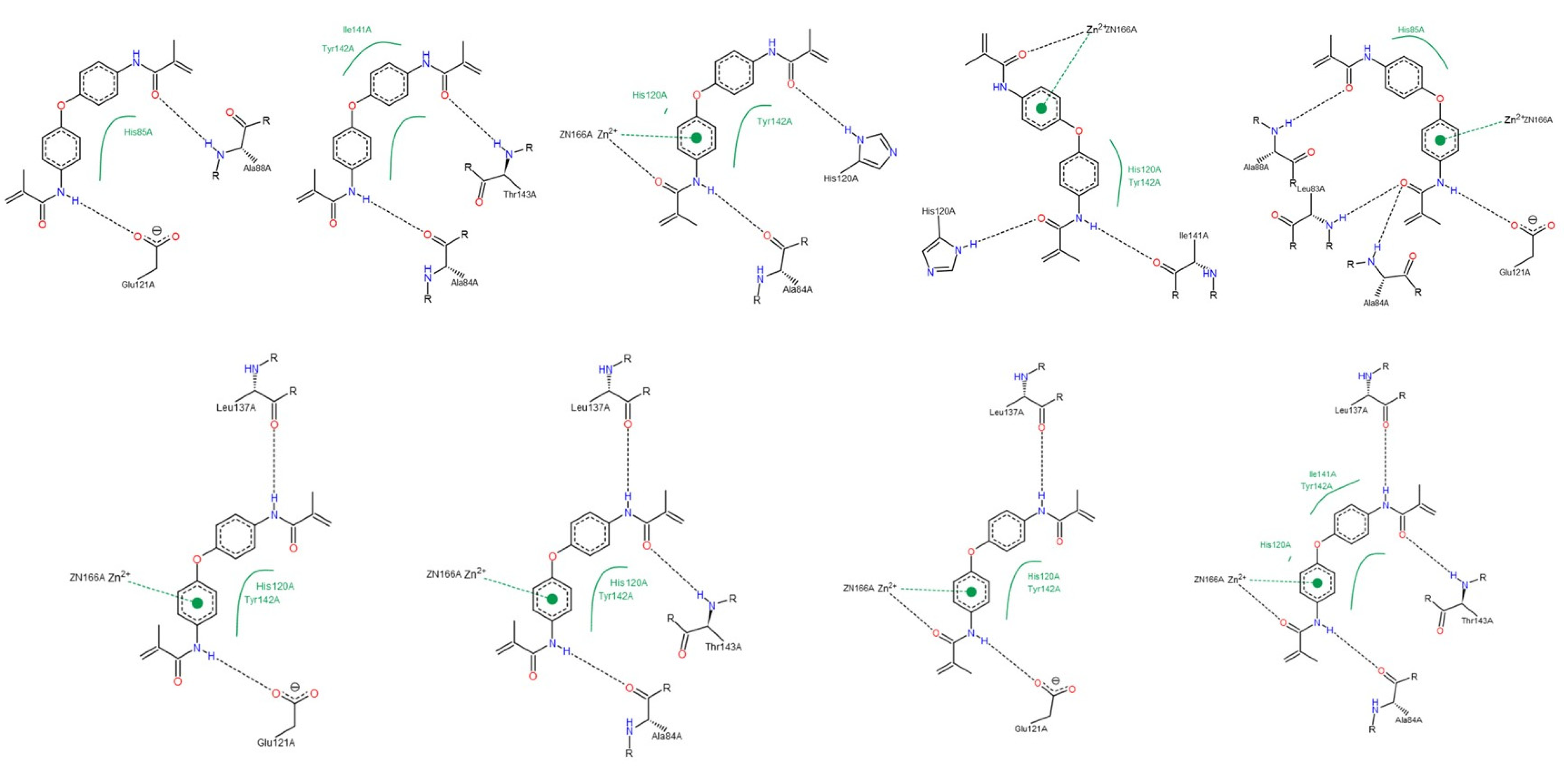
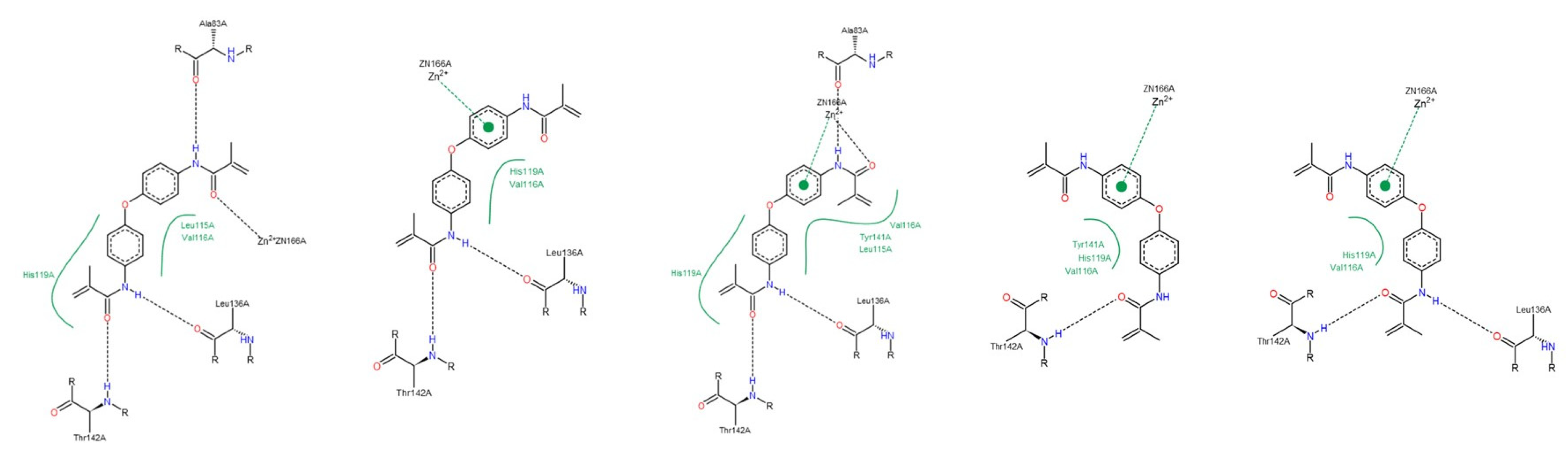
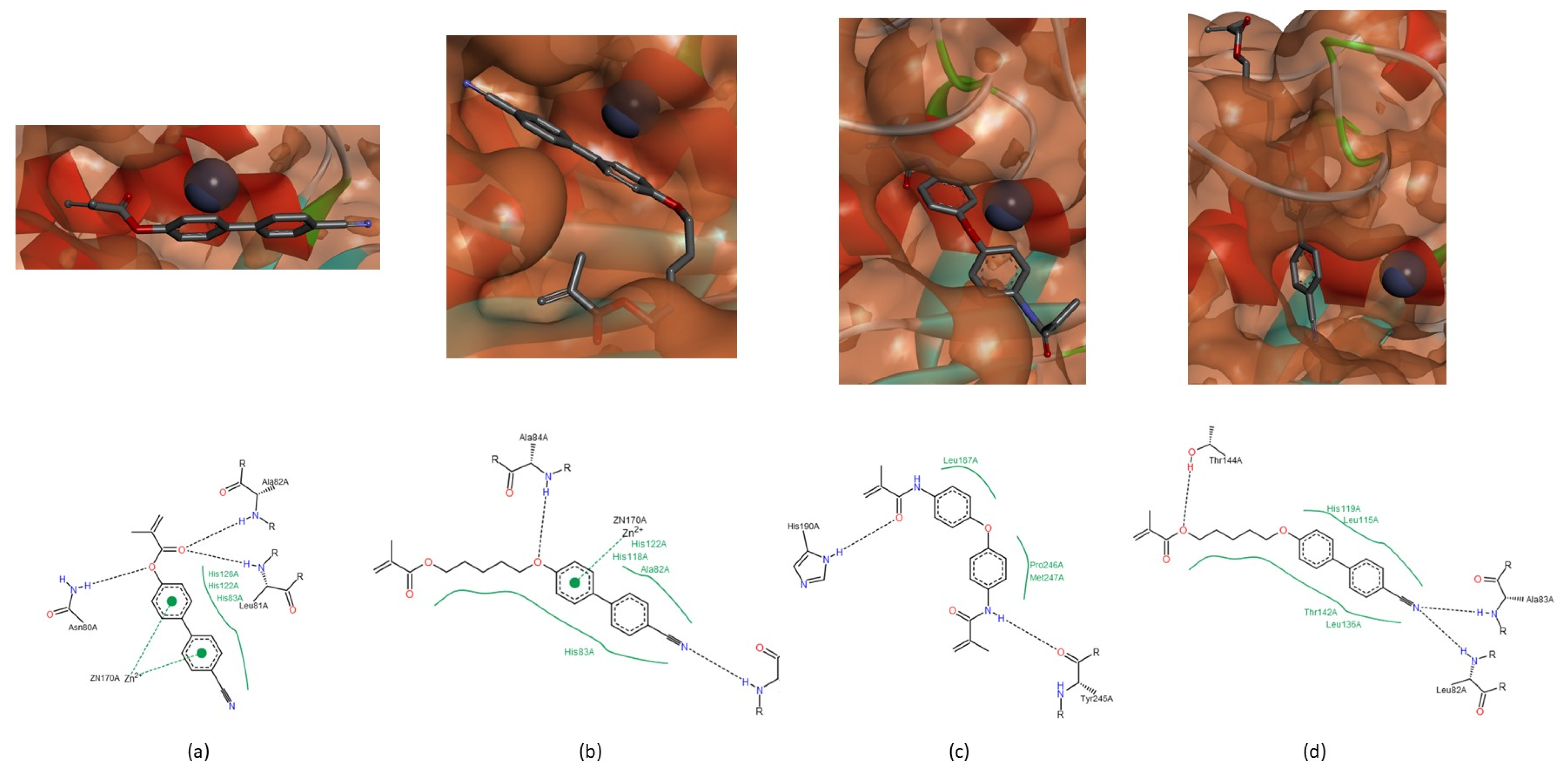
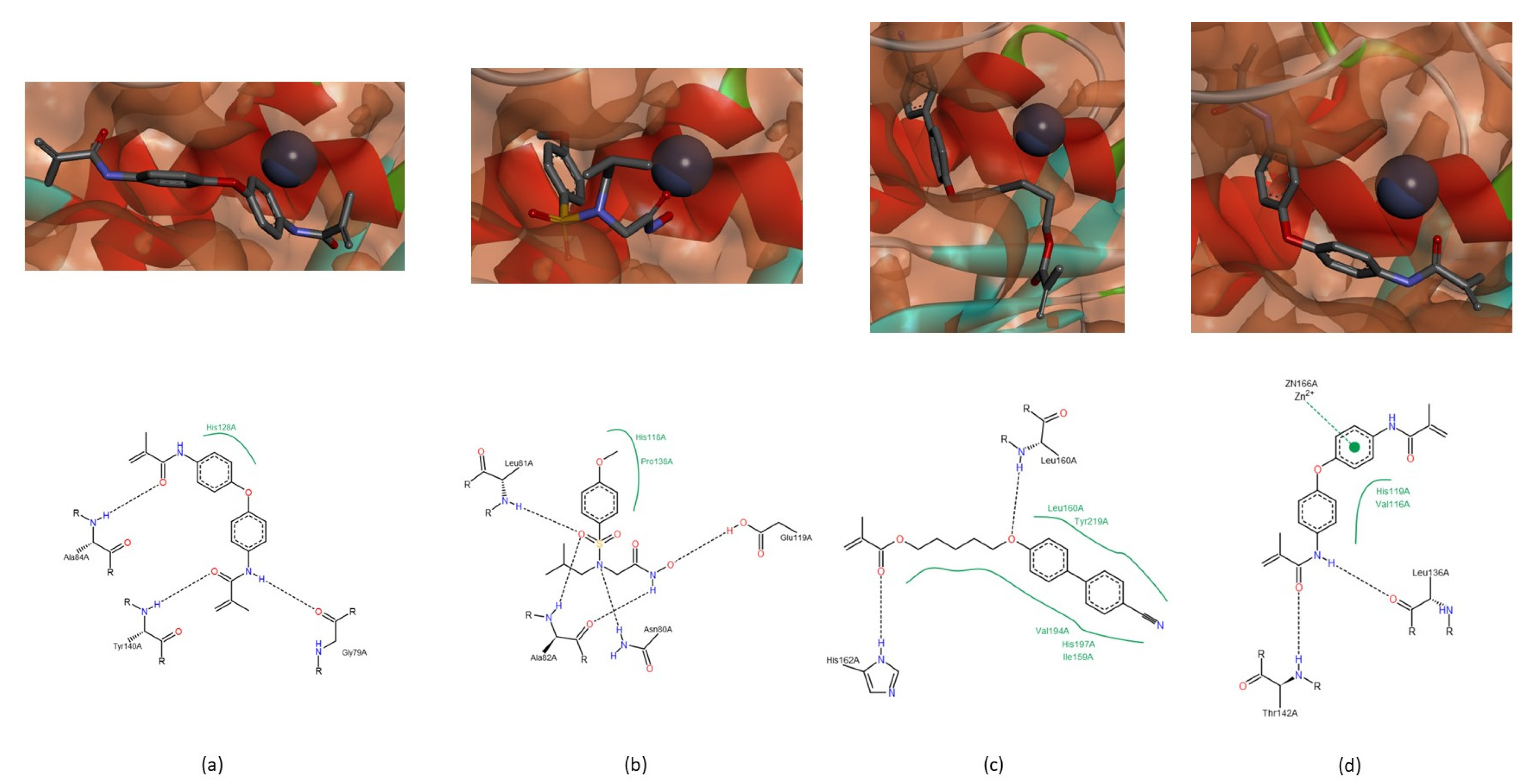
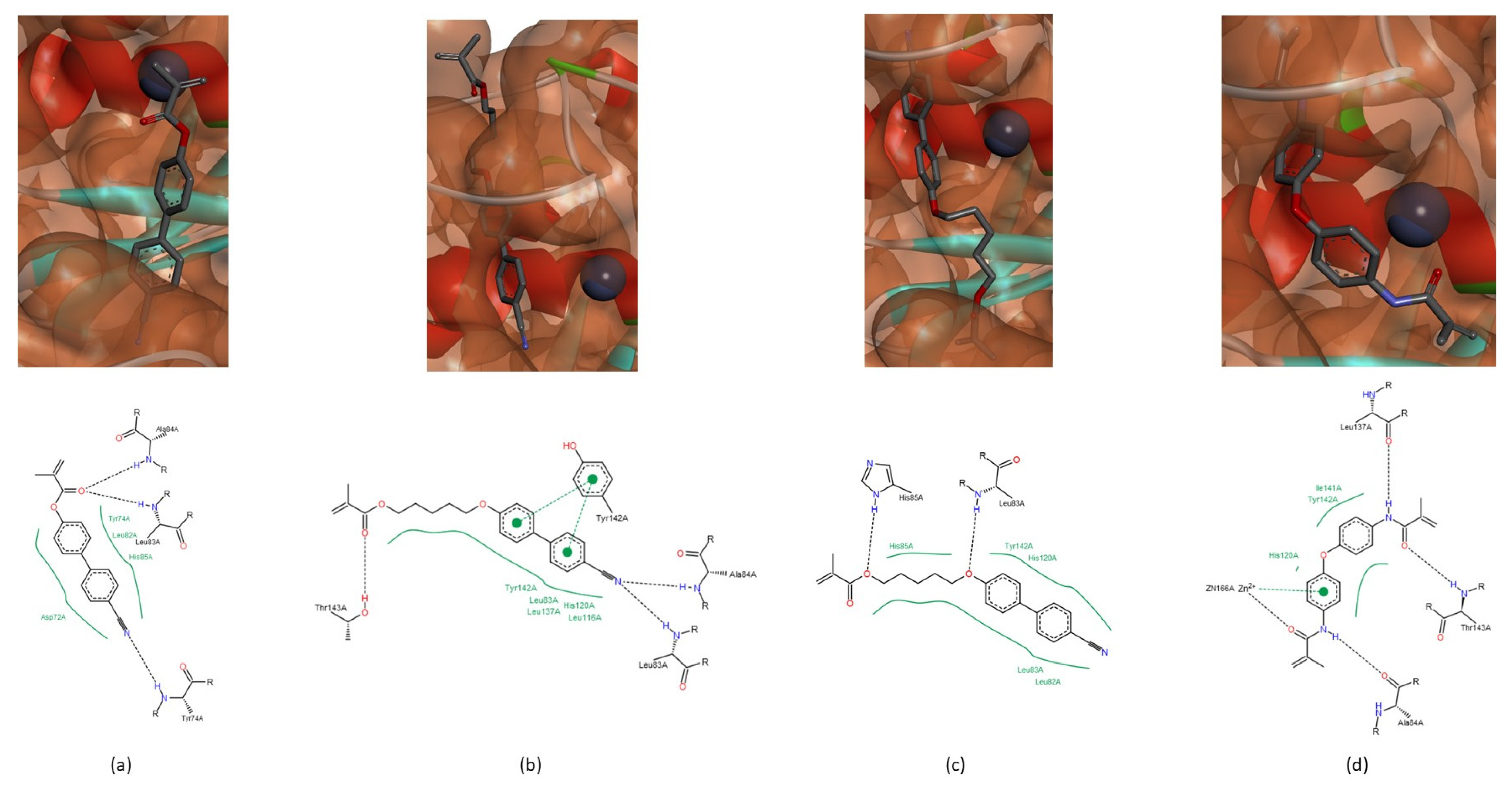
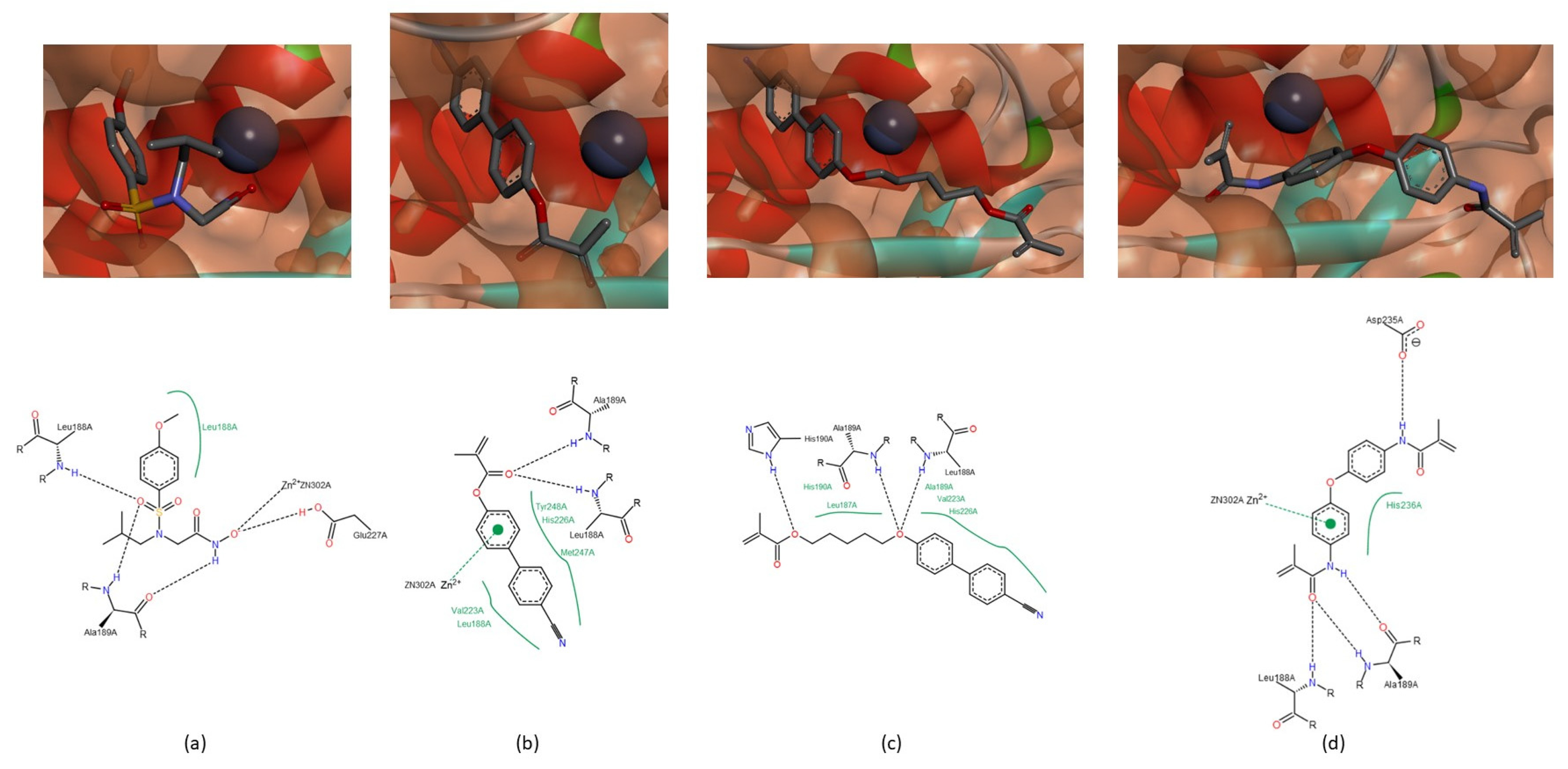
3.1. Docking Studies
3.1.1. Physicochemical and ADMET Properties of NNGH Commercial Inhibitor, Compounds A, B and C
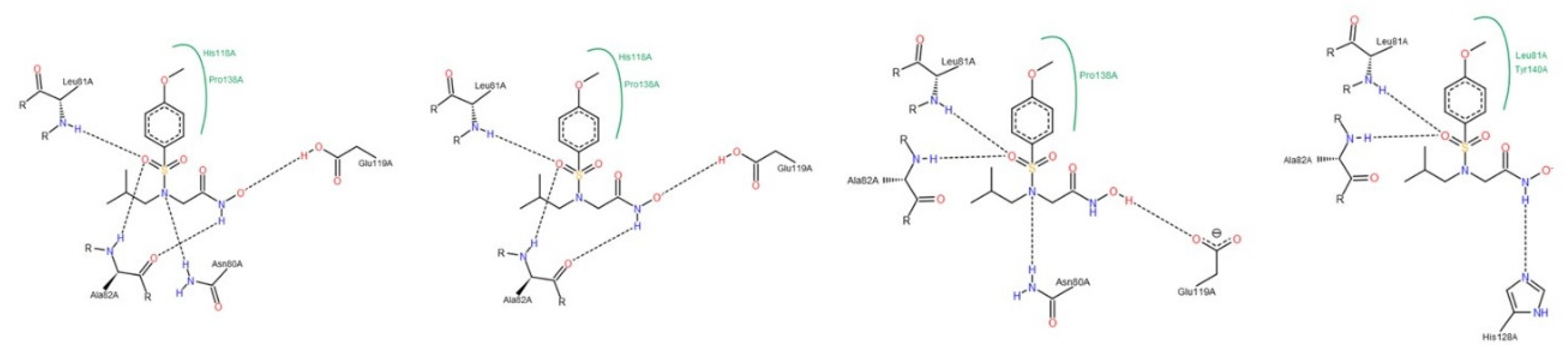
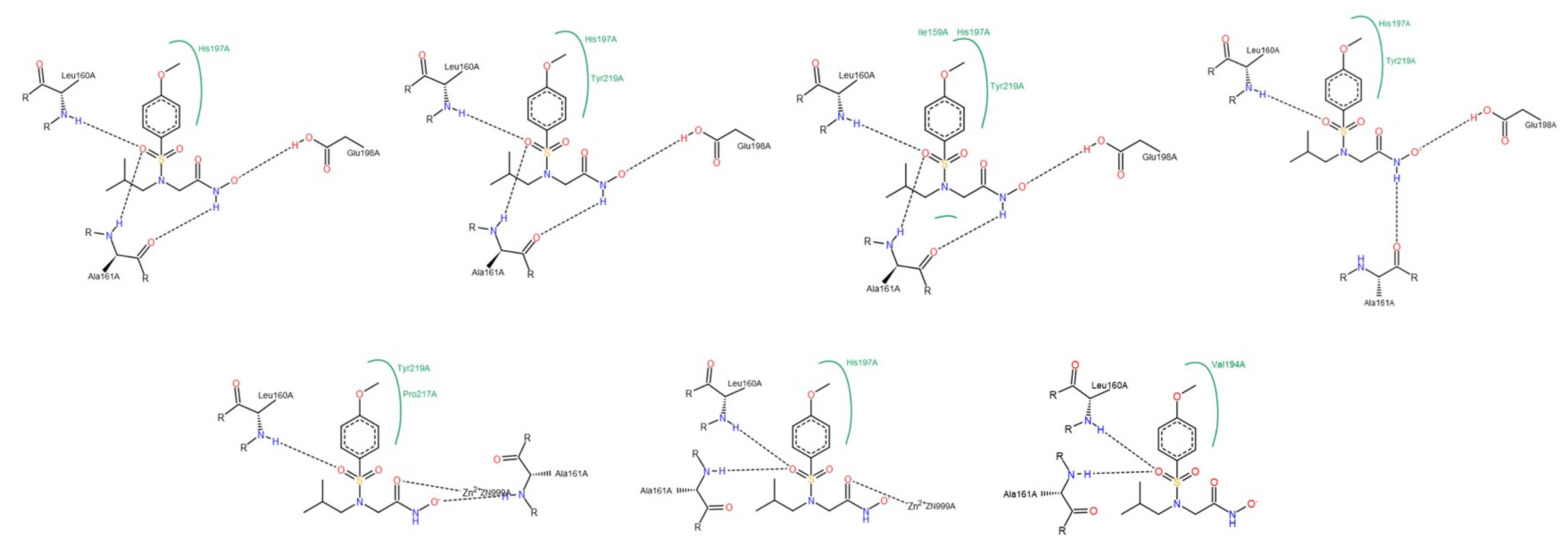
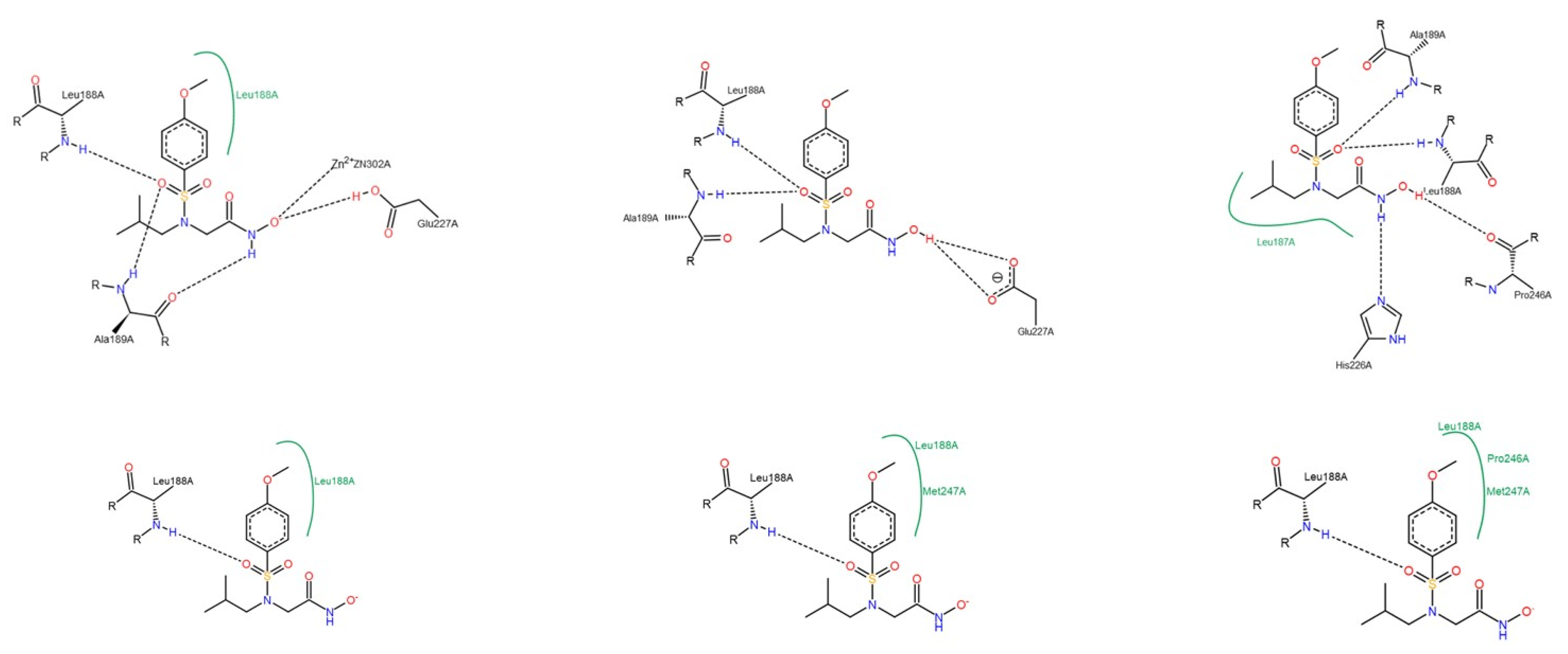
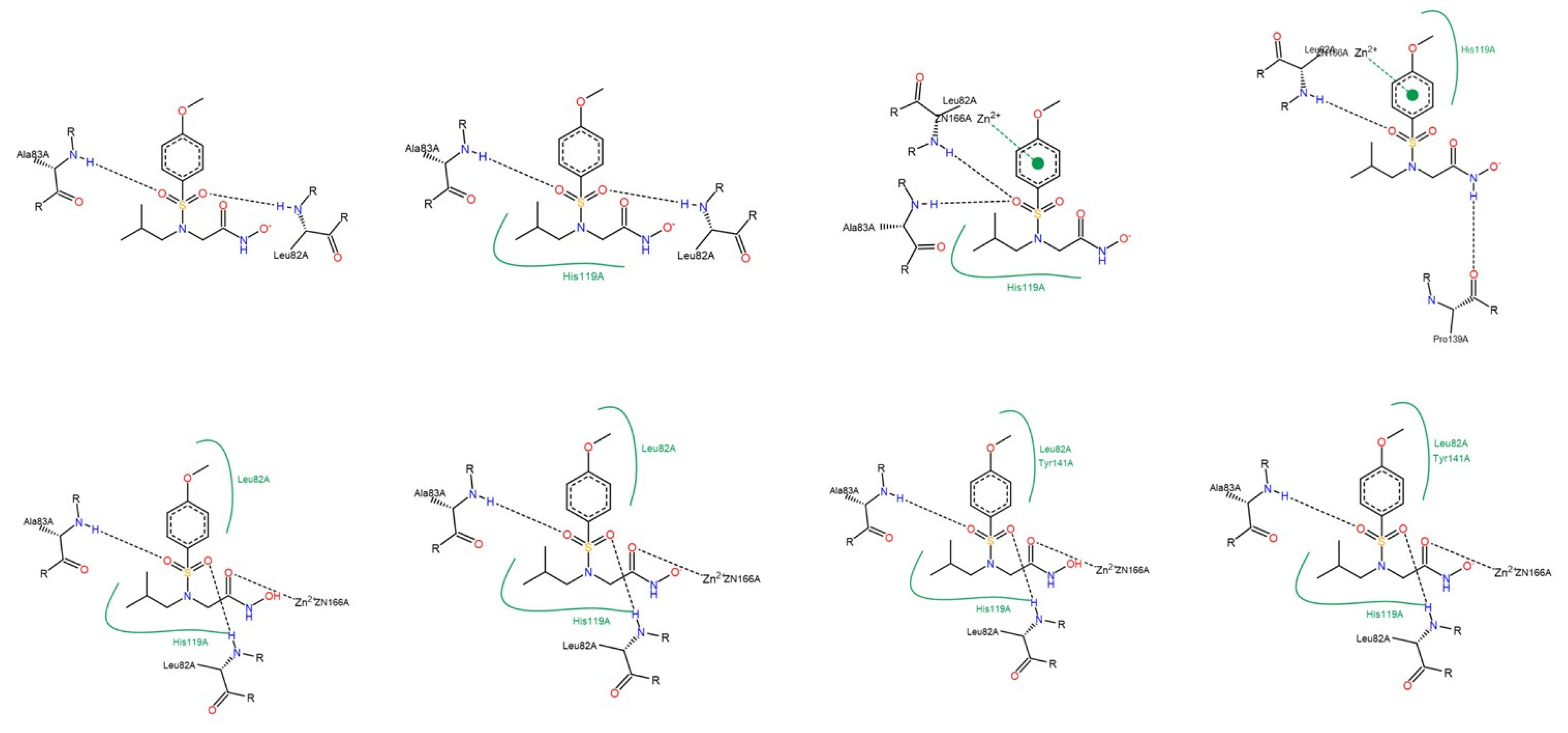
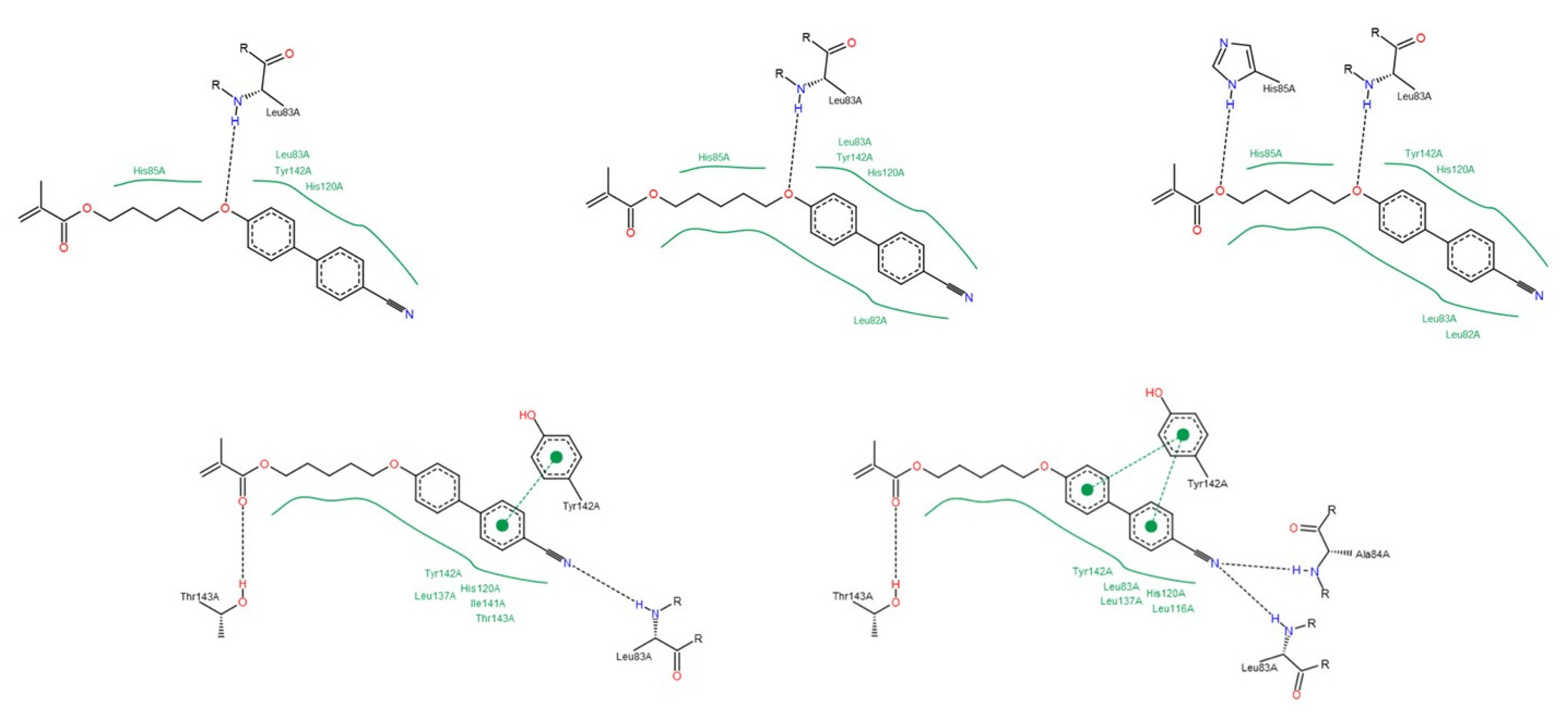
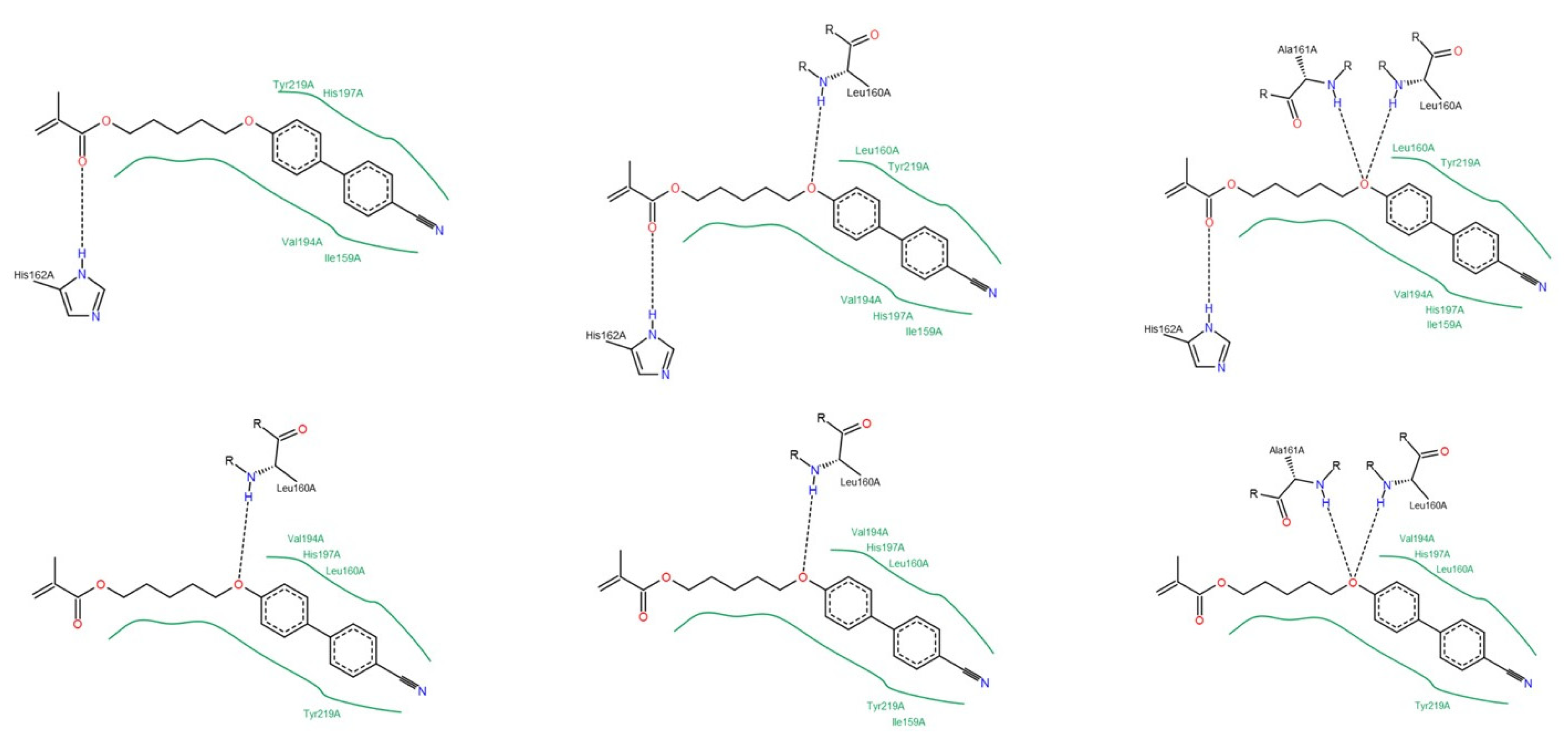
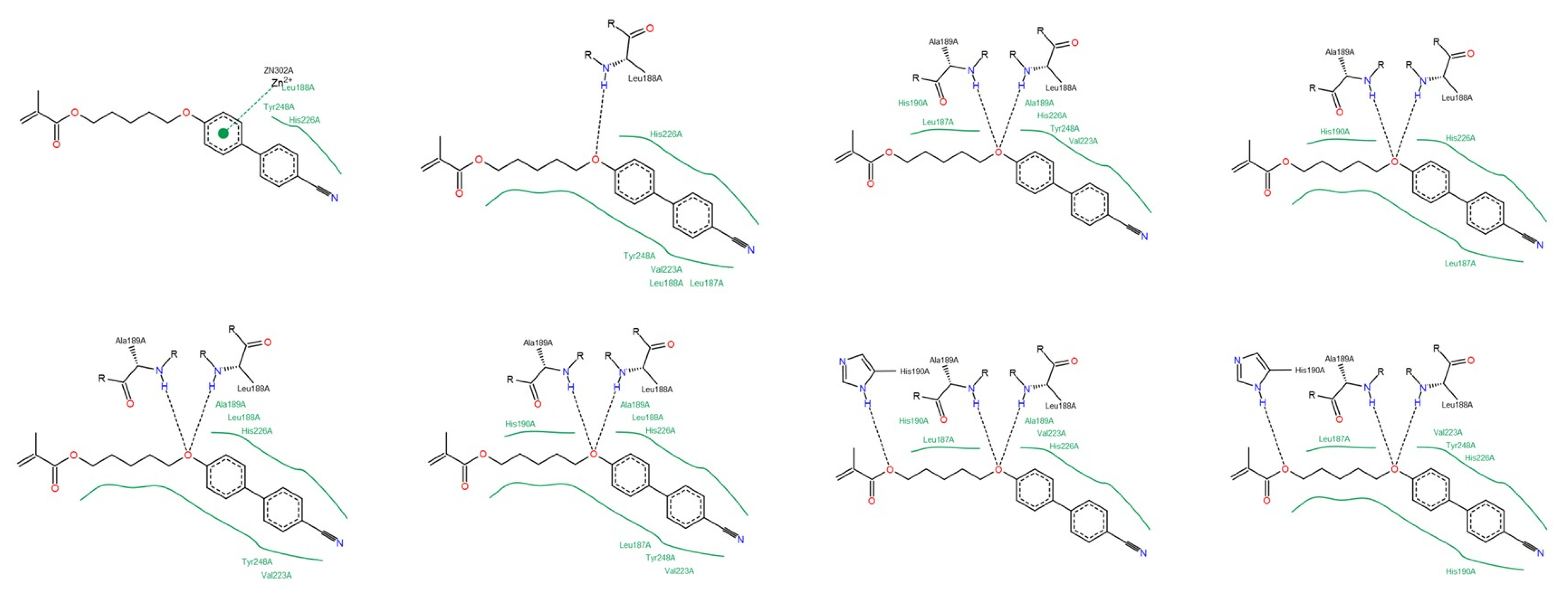
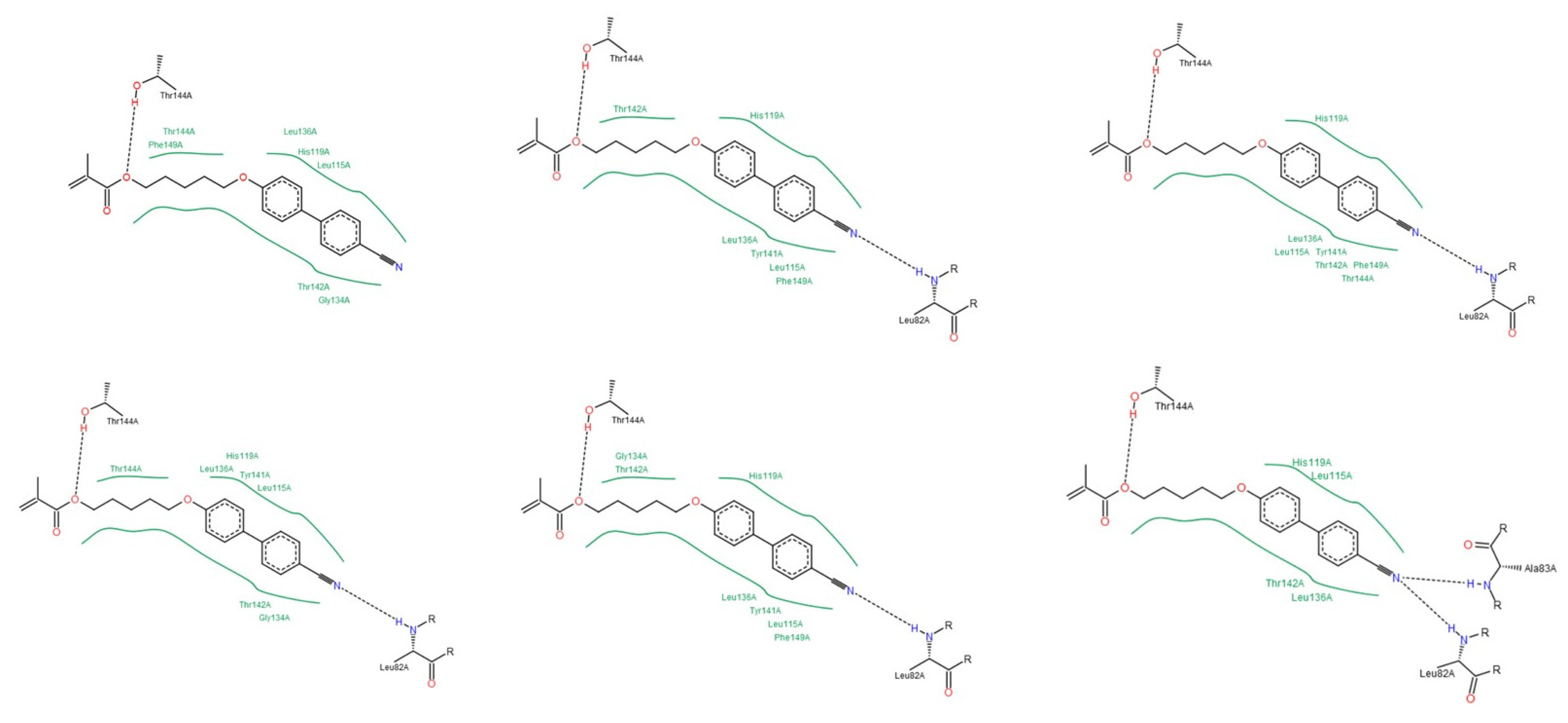
3.1.2. Interactions between Ligand and Protein
Collagenases (MMP-1, -8 and -13)
Gelatinases (MMP-2 and -9)
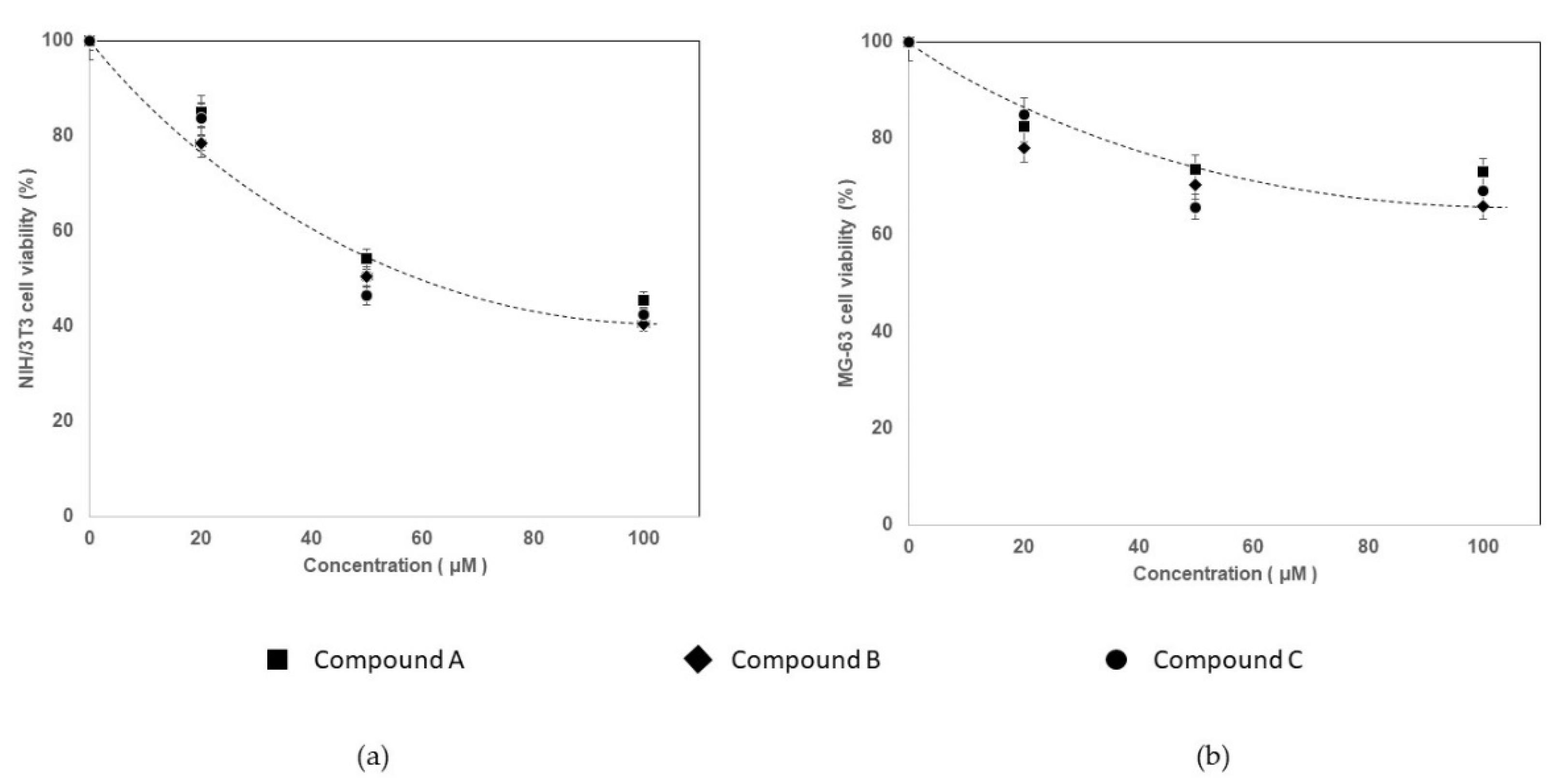
3.2. Cytotoxicity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Parameter | NNGH | Compound A | Compound B | Compound C |
|---|---|---|---|---|
| MW | 316.375 | 263.295 | 349.428 | 336.389 |
| H-bonds acceptors | 6 | 3 | 4 | 2 |
| H-bonds donors | 2 | 0 | 0 | 2 |
| Aromatic rings | 1 | 2 | 2 | 2 |
| Stereo centers | 0 | 0 | 0 | 0 |
| Rotatable bonds | 5 | 0 | 6 | 0 |
| Total charge | 0 (OH)/ −1 (O−) | 0 | 0 | 0 |
| Log P | 1.472 | 3.828 | 5.089 | 2.943 |
| Log D | −0.075 | 3.828 | 5.089 | 2.943 |
| TPSA | 95.94 | 50.09 | 59.32 | 67.43 |
| BBB category | − | + | + | − |
| BBB log ([brain]:[blood]) | 0.703 | −0.158 | −0.150 | −0.492 |
| P-gp category | No | No | No | Yes |
| 2C9 pki | 4.377 | 4.931 | 5.040 | 5.125 |
| 2D6 affinity | Low | Medium | High | Medium |
| HIA category | + | + | + | + |
| PPB90 category | Low | High | High | High |
| hERG pIC50 | 4.319 | 5.073 | 5.749 | 4.894 |
| Log S (pH = 7.4) | 3.264 | 0.653 | -0.217 | 2.336 |
Appendix B

Appendix C
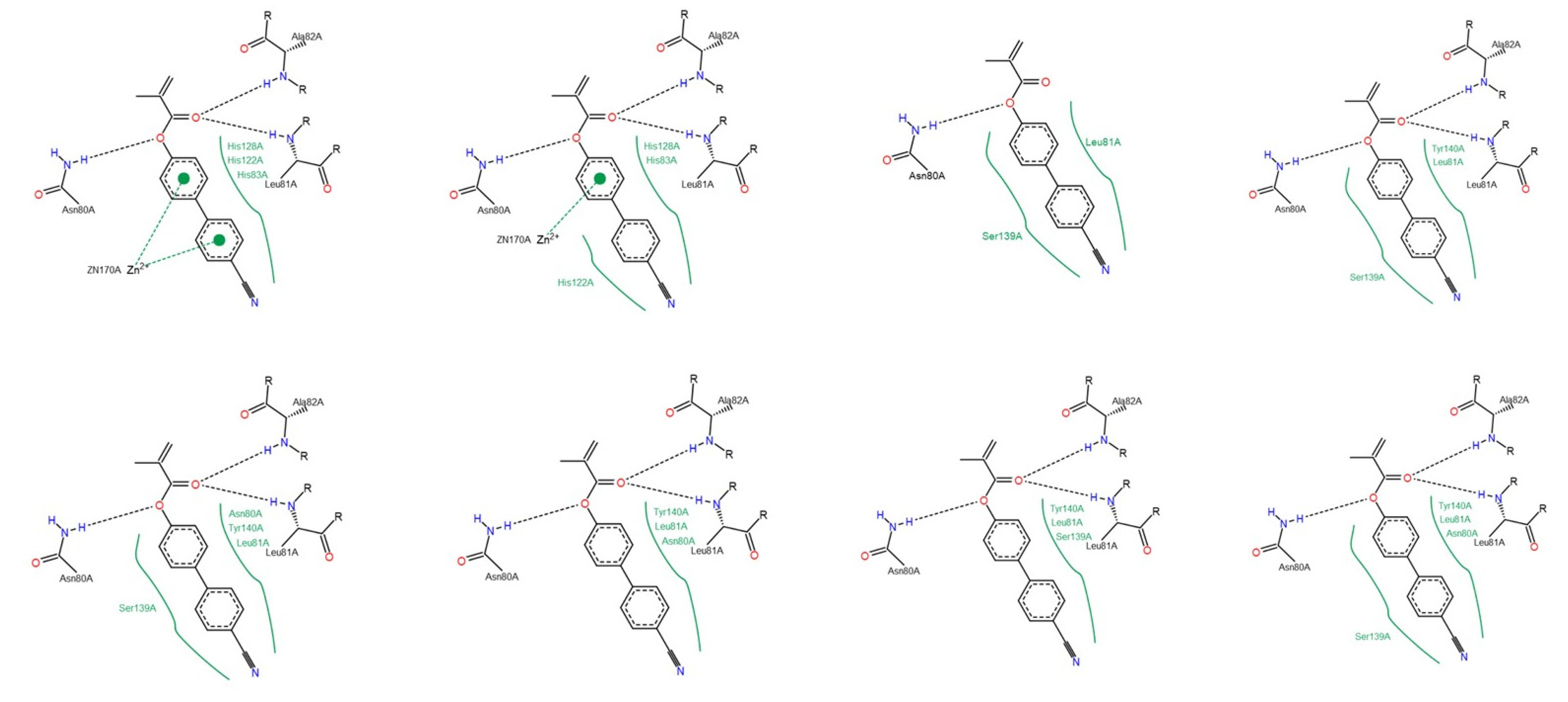
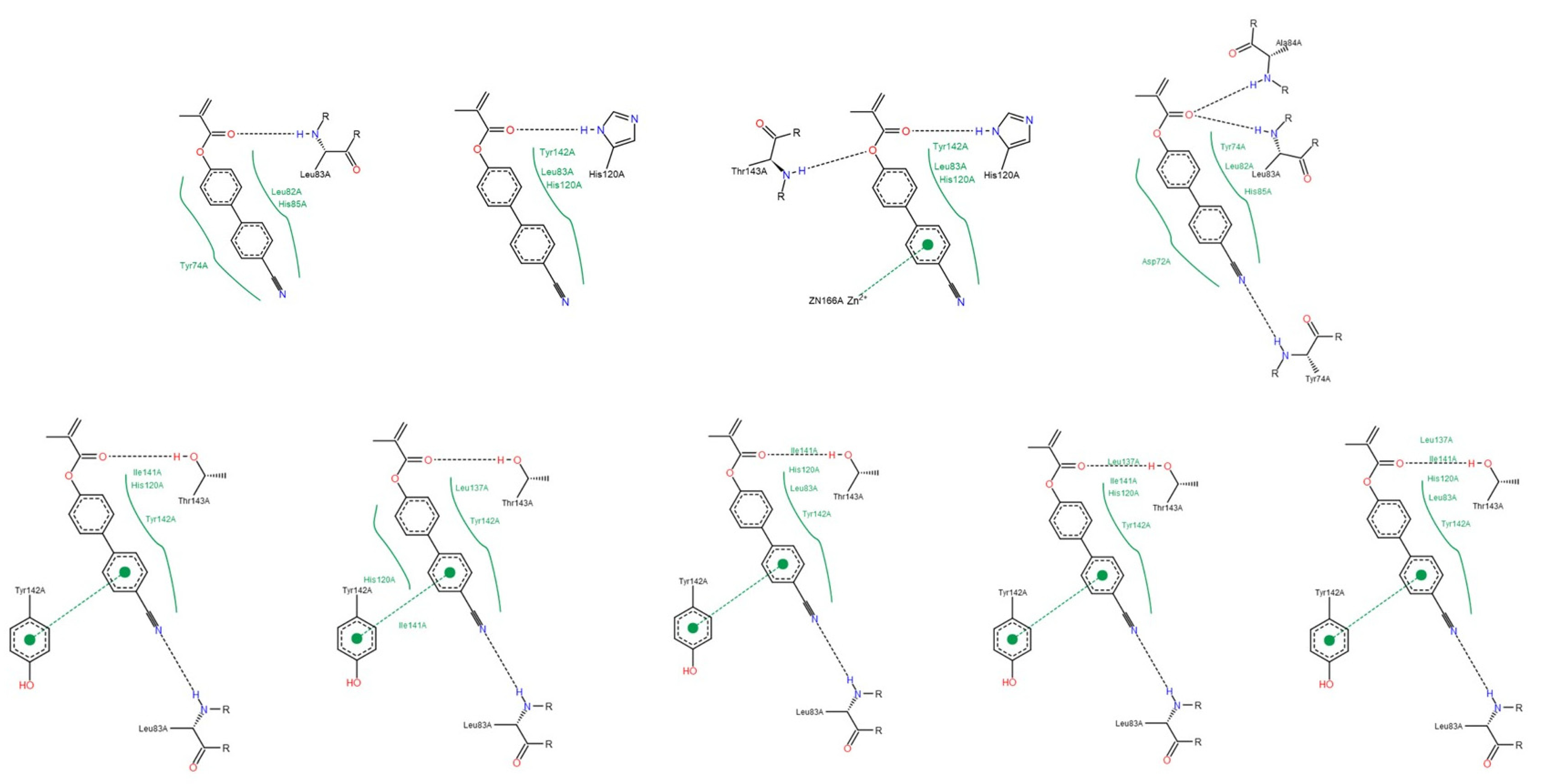
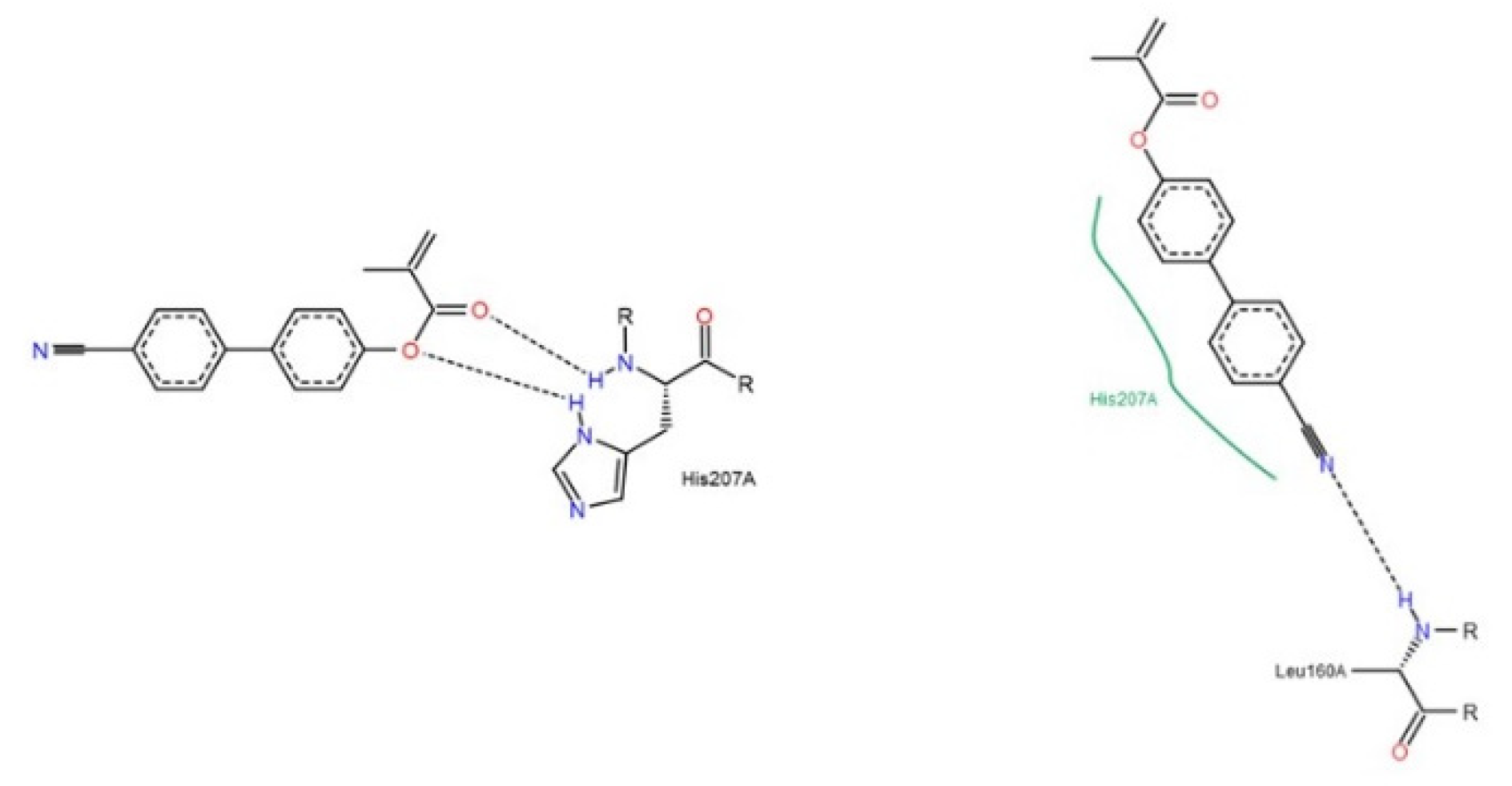

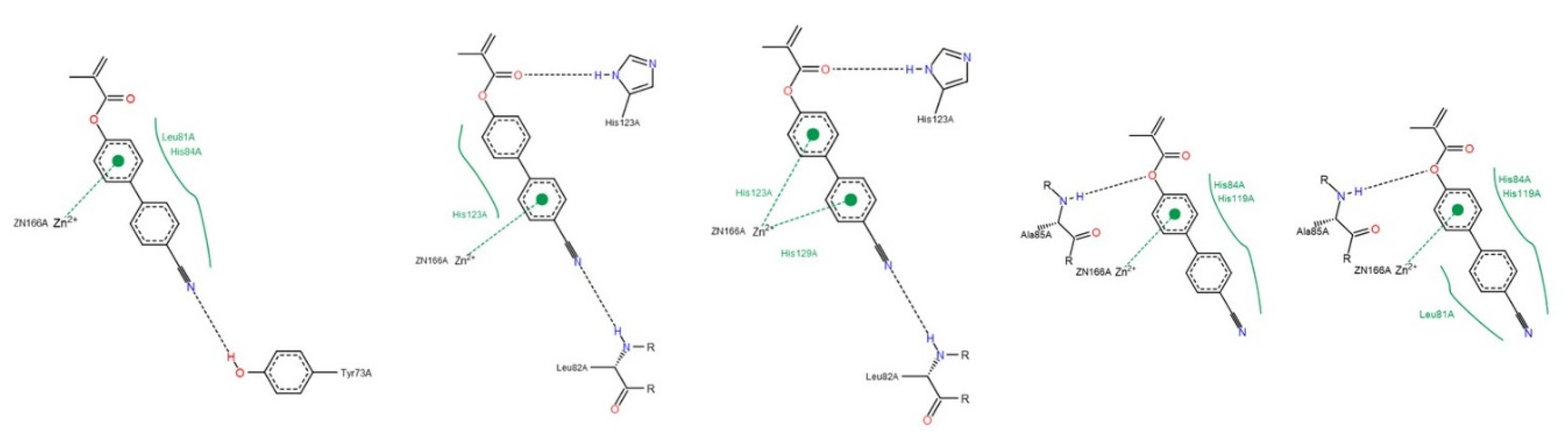
Appendix D

Appendix E


References
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, L. A Closer Look At Metalloproteinases; Nova Science Publishers, Inc.: New York, NY, USA, 2019; p. 310. [Google Scholar]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Maskos, K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie 2005, 87, 249–263. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Oberholzer, A.E.; Bumann, M.; Hege, T.; Russo, S.; Baumann, U. Metzincin’s canonical methionine is responsible for the structural integrity of the zinc-binding site. Biol. Chem. 2009, 390, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.A.; Major Jourden, J.L.; Miller, M.T.; Cohen, S.M. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim. Biophys. Acta 2010, 1803, 72–94. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Mannello, F.; Medda, V. Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 2012, 47, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, L.; Geronimo, B.D.; Ortín, I.; Coderch, C.; Zapico, J.M.; Ramos, A.; de Pascual-Teresa, B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs). Molecules 2019, 24, 2982. [Google Scholar] [CrossRef] [PubMed]
- Amălinei, C.; Căruntu, I.D.; Bălan, R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334. [Google Scholar]
- Liu, J.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [CrossRef]
- Cerofolini, L.; Fragai, M.; Luchinat, C. Mechanism and Inhibition of Matrix Metalloproteinases. Curr. Med. Chem. 2019, 26, 2609–2633. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Dejonckheere, E.; Libert, C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur. Respir. J. 2011, 38, 1200–1214. [Google Scholar] [CrossRef]
- Sekhon, B.S. Matrix metalloproteinases—An overview. Dovepress 2010, 1, 1–20. [Google Scholar]
- Henn, S.; De Carvalho, R.; Ogliari, F.; De Souza, A.; Line, S.; Da Silva, A.; Demarco, F.; Etges, A.; Piva, E. Addition of zinc methacrylate in dental polymers: MMP-2 inhibition and ultimate tensile strength evaluation. Clin. Oral Investig. 2012, 16, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Eckhardt, S.G. Development of matrix metalloproteinase inhibitors in cancer therapy. J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N.; Ventura, T.M.D.S.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J. Appl. Oral Sci. 2019, 27, e20180596. [Google Scholar] [CrossRef]
- Young, D.; Das, N.; Anowai, A.; Dufour, A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int. J. Mol. Sci. 2019, 20, 3847. [Google Scholar] [CrossRef] [PubMed]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjäderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef]
- Lührs, A.K.; De Munck, J.; Geurtsen, W.; Van Meerbeek, B. Does inhibition of proteolytic activity improve adhesive luting? Eur. J. Oral Sci. 2013, 121, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Nuti, E.; Tuccinardi, T.; Rossello, A. Matrix metalloproteinase inhibitors: New challenges in the era of post broad-spectrum inhibitors. Curr. Pharm. Des. 2007, 13, 2087–2100. [Google Scholar] [CrossRef]
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Carpinteiro, I.; Portugal, J.; Azul, A.; Polido, M.; Petrova, K.T.; Salema-Oom, M.; Caldeira, J. Challenges in Matrix Metalloproteinases Inhibition. Biomolecules 2020, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, D.; Yiotakis, A. Specific targeting of metzincin family members with small-molecules inhibitors: Progress toward a multifarious challenge. Bioorg. Med. Chem. 2008, 8781–8794. [Google Scholar] [CrossRef]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Cuniasse, P.; Devel, L.; Makaritis, A.; Beau, F.; Georgiadis, D.; Matziari, M.; Yiotakis, A.; Dive, V. Future challenges facing the development of specific active-site-directed synthetic inhibitors of MMPs. Biochimie 2005, 87, 393–402. [Google Scholar] [CrossRef]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Folgueras, A.R.; Pendás, A.M.; Sánchez, L.M.; López-Otín, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Rossello, A.; Nuti, E.; Orlandini, E.; Carelli, P.; Rapposelli, S.; Macchia, M.; Minutolo, F.; Carbonaro, L.; Albini, A.; Benelli, R.; et al. New N-arylsulfonyl-N-alkoxyaminoacetohydroxamic acids as selective inhibitors of gelatinase A (MMP-2). Bioorg. Med. Chem. 2004, 12, 2441–2450. [Google Scholar] [CrossRef]
- Tamura, Y.; Watanabe, F.; Nakatani, T.; Yasui, K.; Fuji, M.; Komurasaki, T.; Tsuzuki, H.; Maekawa, R.; Yoshioka, T.; Kawada, K.; et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem. 1998, 41, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Terp, G.E.; Cruciani, G.; Christensen, I.T.; Jørgensen, F.S. Structural differences of matrix metalloproteinases with potential implications for inhibitor selectivity examined by the GRID/CPCA approach. J. Med. Chem. 2002, 45, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Tuccinardi, T.; Martinelli, A.; Nuti, E.; Carelli, P.; Balzano, F.; Uccello-Barretta, G.; Murphy, G.; Rossello, A. Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxybiphenyl-4-ylsulfonamido)-3-methylbutanamides. Bioorg. Med. Chem. 2006, 14, 4260–4276. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.M.; Ortwine, D.F.; Pavlovsky, A.G.; Picard, J.A.; Sliskovic, D.R.; Roth, B.D.; Dyer, R.D.; Johnson, L.L.; Man, C.F.; Hallak, H. Structure-activity relationships and pharmacokinetic analysis for a series of potent, systemically available biphenylsulfonamide matrix metalloproteinase inhibitors. J. Med. Chem. 2000, 43, 156–166. [Google Scholar] [CrossRef]
- Gooyit, M.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Mobashery, S.; Chang, M. Selective gelatinase inhibitor neuroprotective agents cross the blood-brain barrier. ACS Chem. Neurosci. 2012, 3, 730–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barros, M.T.; Mouquinho, A.I.; Petrova, K.T.; Saavedra, M.D.; Sotomayor, J.C. Fast synthesis employing a microwave assisted neat protocol of new monomers potentially useful for the preparation of PDLC films. Cent. Eur. J. Chem. 2011, 9. [Google Scholar] [CrossRef]
- Bandarra, S.; Mascarenhas, P.; Luís, A.R.; Catrau, M.; Bekman, E.; Ribeiro, A.C.; Félix, S.; Caldeira, J.; Barahona, I. In vitro and in silico evaluations of resin-based dental restorative material toxicity. Clin. Oral Investig. 2020, 24, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Rankovic, Z. The physicochemical challenges of designing multiple ligands. J. Med. Chem. 2006, 49, 4961–4970. [Google Scholar] [CrossRef]
- Leeson, P.D. Molecular inflation, attrition and the rule of five. Adv. Drug Deliv. Rev. 2016, 101, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Improving drug candidates by design: A focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Hannongbua, S. In-silico ADME models: A general assessment of their utility in drug discovery applications. Curr. Top. Med. Chem. 2011, 11, 358–381. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef]
- Lagorce, D.; Douguet, D.; Miteva, M.A.; Villoutreix, B.O. Computational analysis of calculated physicochemical and ADMET properties of protein-protein interaction inhibitors. Sci. Rep. 2017, 7, 46277. [Google Scholar] [CrossRef] [PubMed]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef]
- Baell, J.; Congreve, M.; Leeson, P.; Abad-Zapatero, C. Ask the experts: Past, present and future of the rule of five. Future Med. Chem. 2013, 5, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Palm, K.; Stenberg, P.; Luthman, K.; Artursson, P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 1997, 14, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.; Summerfield, S. Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol. Dis. 2010, 37, 33–37. [Google Scholar] [CrossRef]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Yu, H.; Zhang, L.; Hou, T. Computational models for predicting substrates or inhibitors of P-glycoprotein. Drug Discov. Today 2012, 17, 343–351. [Google Scholar] [CrossRef]
- Lovejoy, B.; Cleasby, A.; Hassell, A.M.; Longley, K.; Luther, M.A.; Weigl, D.; McGeehan, G.; McElroy, A.B.; Drewry, D.; Lambert, M.H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science 1994, 263, 375–377. [Google Scholar] [CrossRef]
- Gimeno, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Understanding the variability of the S1’ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov. Today 2019. [Google Scholar] [CrossRef]
- Xia, T.; Li, N.; Nel, A.E. Potential health impact of nanoparticles. Annu. Rev. Public Health 2009, 30, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Advances in the management of macular degeneration. F1000Prime Rep. 2014, 6, 29. [Google Scholar] [CrossRef] [PubMed]


| MMP | PDB ID | Total Structure Weight (kDa) | Method | Resolution (Å) | R-Factor (%) |
|---|---|---|---|---|---|
| 1 | 2tcl | 19.63 | X-ray diffraction | 2.20 | 16.2 |
| 2 | 1HOV | 19.28 | Solution NMR | − | − |
| 8 | 1BZS | 19.22 | X-ray diffraction | 1.70 | 19.2 |
| 9 | 4XCT | 18.97 | X-ray diffraction | 1.30 | 17.0 |
| 13 | 1FM1 | 19.21 | Solution NMR | − | − |
| Property | NNGH | Compound A | Compound B | Compound C |
|---|---|---|---|---|
| Molecular weight (MW) | 316.375 | 263.295 | 349.428 | 336.389 |
| H-bonds acceptor | 6 | 3 | 4 | 2 |
| H-bonds donor | 2 | 0 | 0 | 2 |
| Log P | 1.472 | 3.828 | 5.089 | 2.943 |
| Log D | −0.075 | 3.828 | 5.089 | 2.943 |
| Topological polar surface area (TPSA) | 95.94 | 50.09 | 59.32 | 67.43 |
| BBB category | − | + | + | − |
| BBB log[(brain): (blood)] | 0.703 | −0.158 | −0.15 | −0.492 |
| P-gp category | No | No | No | Yes |
| Residue | NNGH | Compound A | Compound B | Compound C |
|---|---|---|---|---|
| Leu81 (MMP-1); Leu160 (MMP-8); Leu82 (MMP-13) | All collagenases | All collagenases | MMP-8 MMP-13 | MMP-8 |
| Ala82 (MMP-1); Ala161 (MMP-8); Ala83 (MMP-13) | All collagenases | MMP-1 | MMP-8 MMP-13 | MMP-8 MMP-13 |
| Ala84 (MMP-1); Ala163 (MMP-8); Ala85 (MMP-13) | No interact | MMP-13 | MMP-1 | All collagenases |
| Groups | Cell Viability (%) | ||||
|---|---|---|---|---|---|
| Inhibitor Compound | Inhibitor Concentration | NIH/3T3 | MG-63 | ||
| M | SD | M | SD | ||
| A | 20 μM | 85.1 | 0.71 | 82.6 | 2.28 |
| 50 µM | 54.2 | 4.47 | 73.6 | 0.78 | |
| 100 µM | 45.5 | 5.30 | 73.0 | 5.40 | |
| B | 20 µM | 78.6 | 1.41 | 78.1 | 1.41 |
| 50 µM | 50.5 | 4.42 | 70.3 | 2.03 | |
| 100 µM | 40.5 | 1.60 | 65.9 | 0.82 | |
| C | 20 µM | 83.7 | 1.41 | 85.0 | 0.36 |
| 50 µM | 46.4 | 4.22 | 65.8 | 2.94 | |
| 100 µM | 42.4 | 8.28 | 69.2 | 9.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laronha, H.; Carpinteiro, I.; Portugal, J.; Azul, A.; Polido, M.; Petrova, K.T.; Salema-Oom, M.; Barahona, I.; Caldeira, J. Polymerizable Matrix Metalloproteinases’ Inhibitors with Potential Application for Dental Restorations. Biomedicines 2021, 9, 366. https://doi.org/10.3390/biomedicines9040366
Laronha H, Carpinteiro I, Portugal J, Azul A, Polido M, Petrova KT, Salema-Oom M, Barahona I, Caldeira J. Polymerizable Matrix Metalloproteinases’ Inhibitors with Potential Application for Dental Restorations. Biomedicines. 2021; 9(4):366. https://doi.org/10.3390/biomedicines9040366
Chicago/Turabian StyleLaronha, Helena, Inês Carpinteiro, Jaime Portugal, Ana Azul, Mário Polido, Krasimira T. Petrova, Madalena Salema-Oom, Isabel Barahona, and Jorge Caldeira. 2021. "Polymerizable Matrix Metalloproteinases’ Inhibitors with Potential Application for Dental Restorations" Biomedicines 9, no. 4: 366. https://doi.org/10.3390/biomedicines9040366
APA StyleLaronha, H., Carpinteiro, I., Portugal, J., Azul, A., Polido, M., Petrova, K. T., Salema-Oom, M., Barahona, I., & Caldeira, J. (2021). Polymerizable Matrix Metalloproteinases’ Inhibitors with Potential Application for Dental Restorations. Biomedicines, 9(4), 366. https://doi.org/10.3390/biomedicines9040366









