Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target?
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. siRNA Transfection
2.4. Cell Proliferation Assay
2.5. Spheroid Formation Assay
2.6. RNA Extraction and qRT-PCR
2.7. Western Blotting
2.8. Immunocytochemistry
2.9. Scratch Assay
2.10. Patients
2.11. Immunohistochemistry
2.12. Evaluation of NECTIN4 Staining
2.13. Statistical Analysis
3. Results
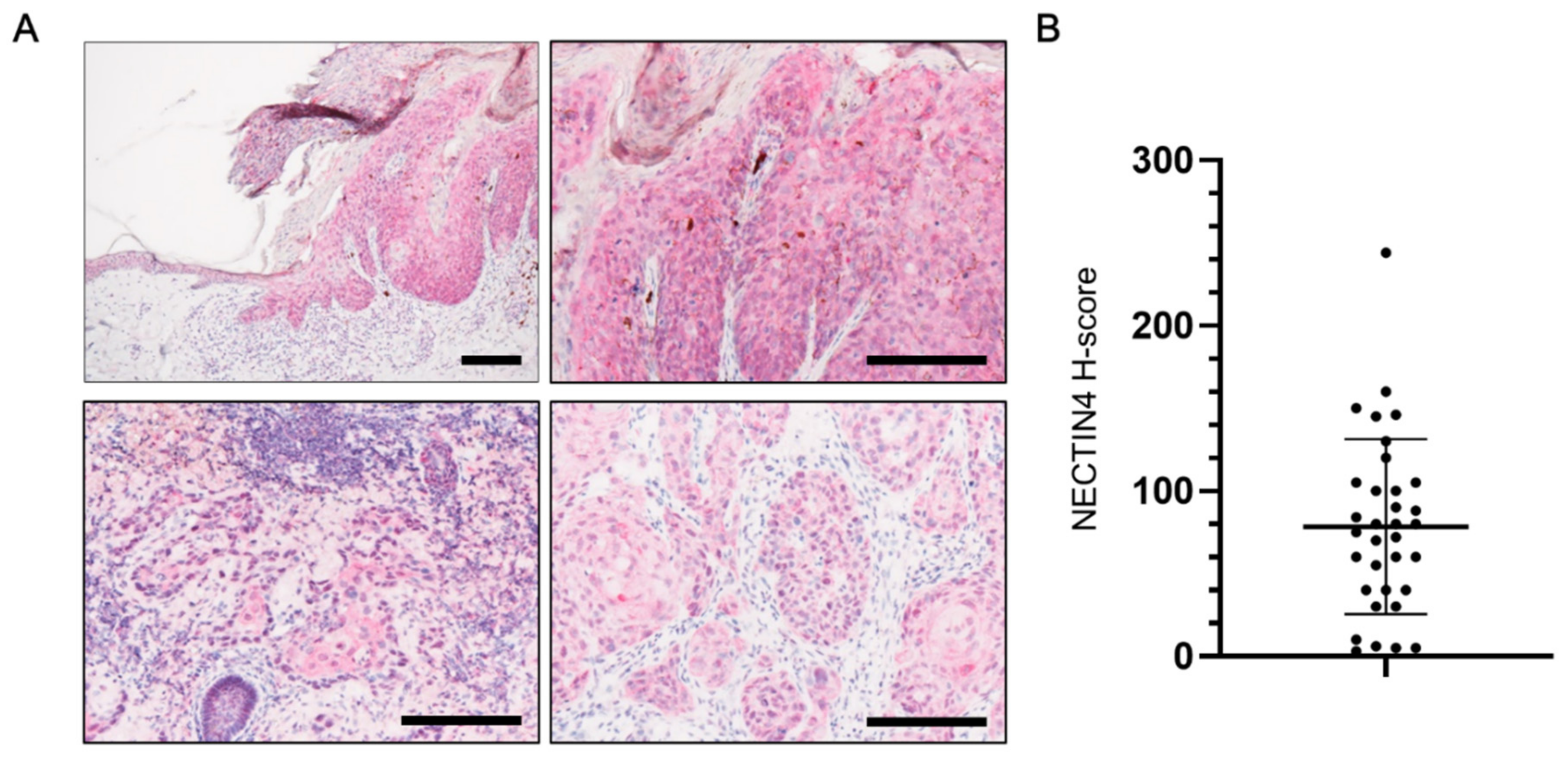
3.1. NECTIN4 Expression in Tissues from cSCC Patients
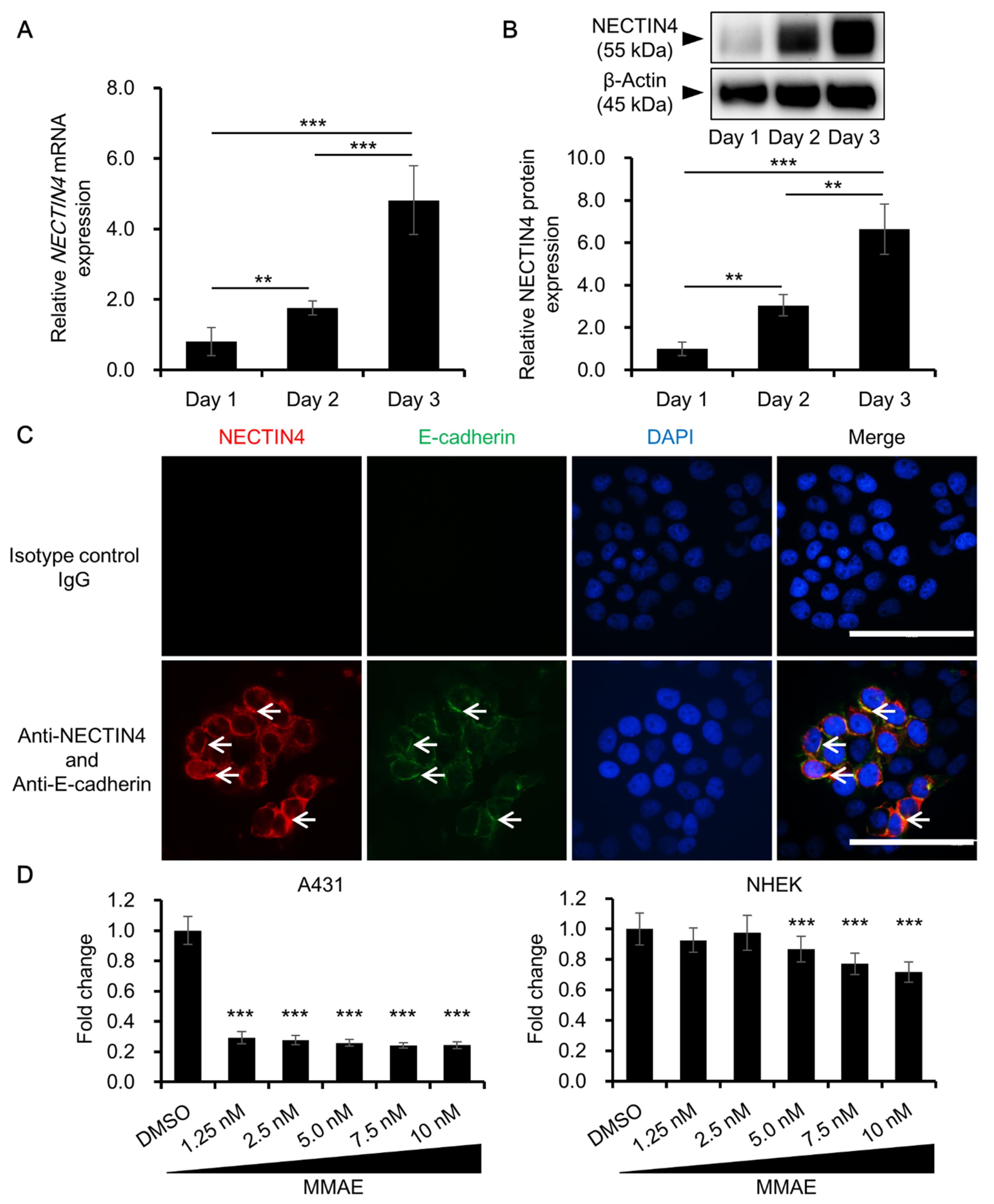
3.2. NECTIN4 Expression in A431 Cells and the Effects of MMAE on Cell Viability
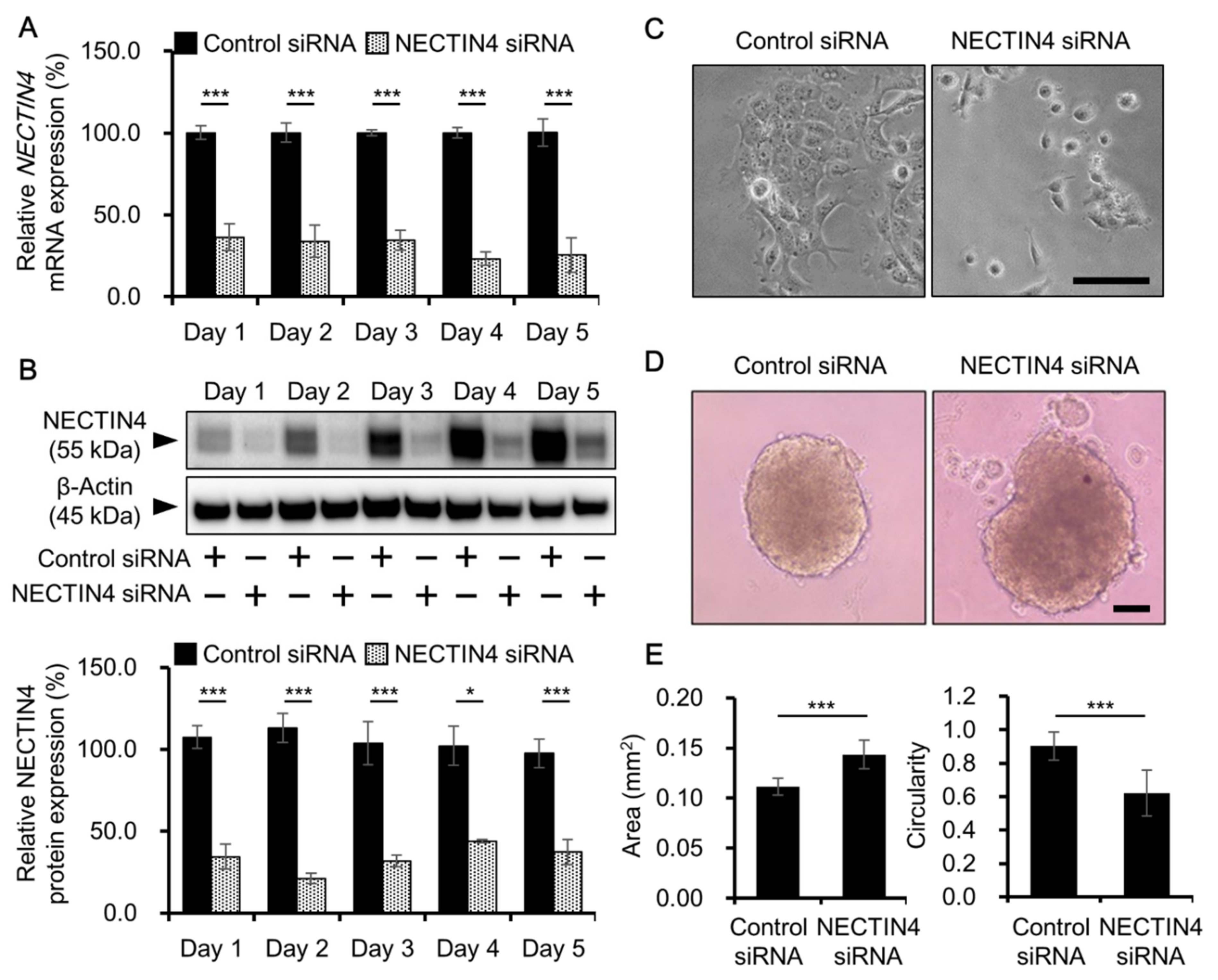
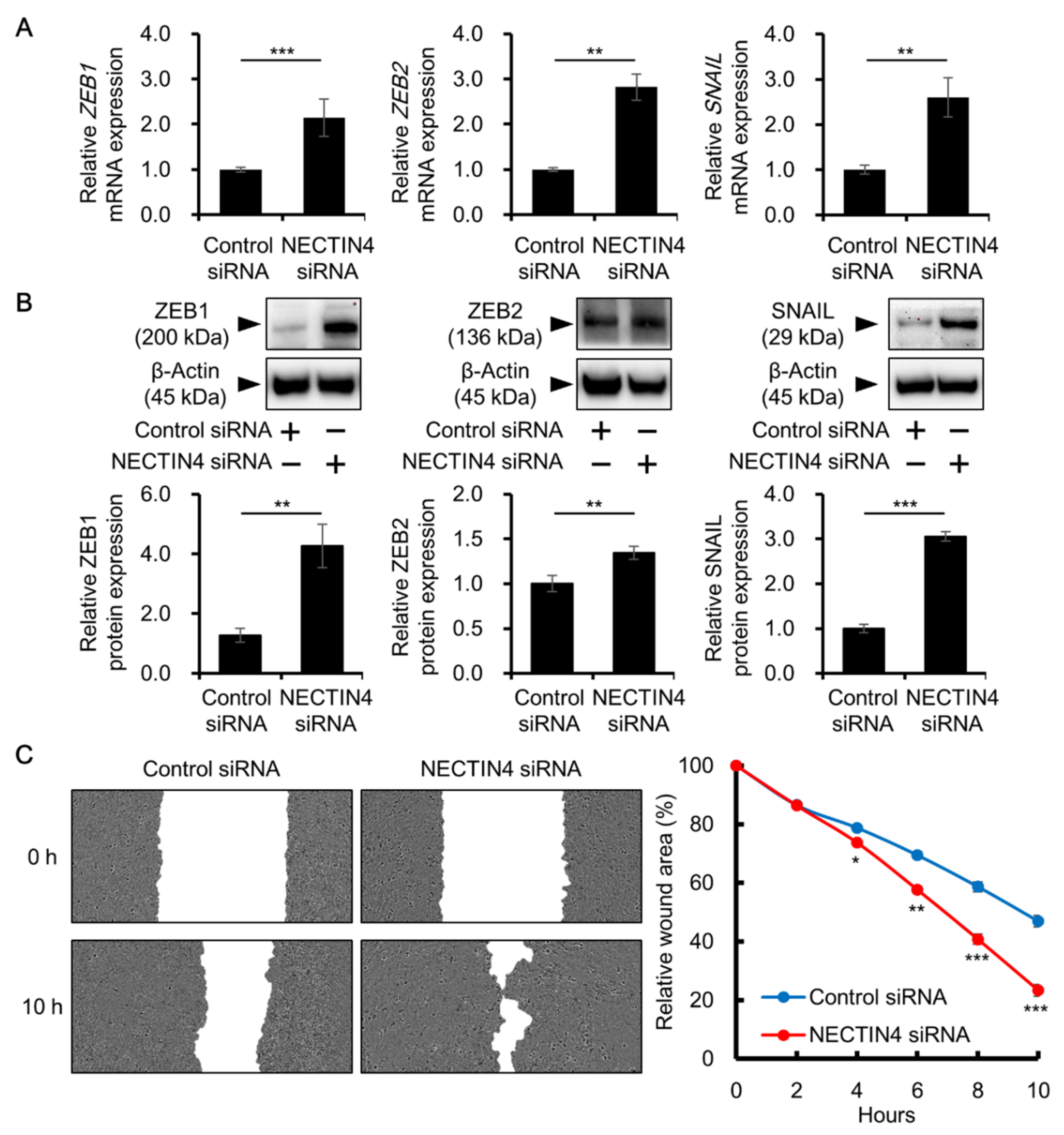
3.3. Effects of NECTIN4 Silencing on Cell–Cell Interaction and Cell Migration
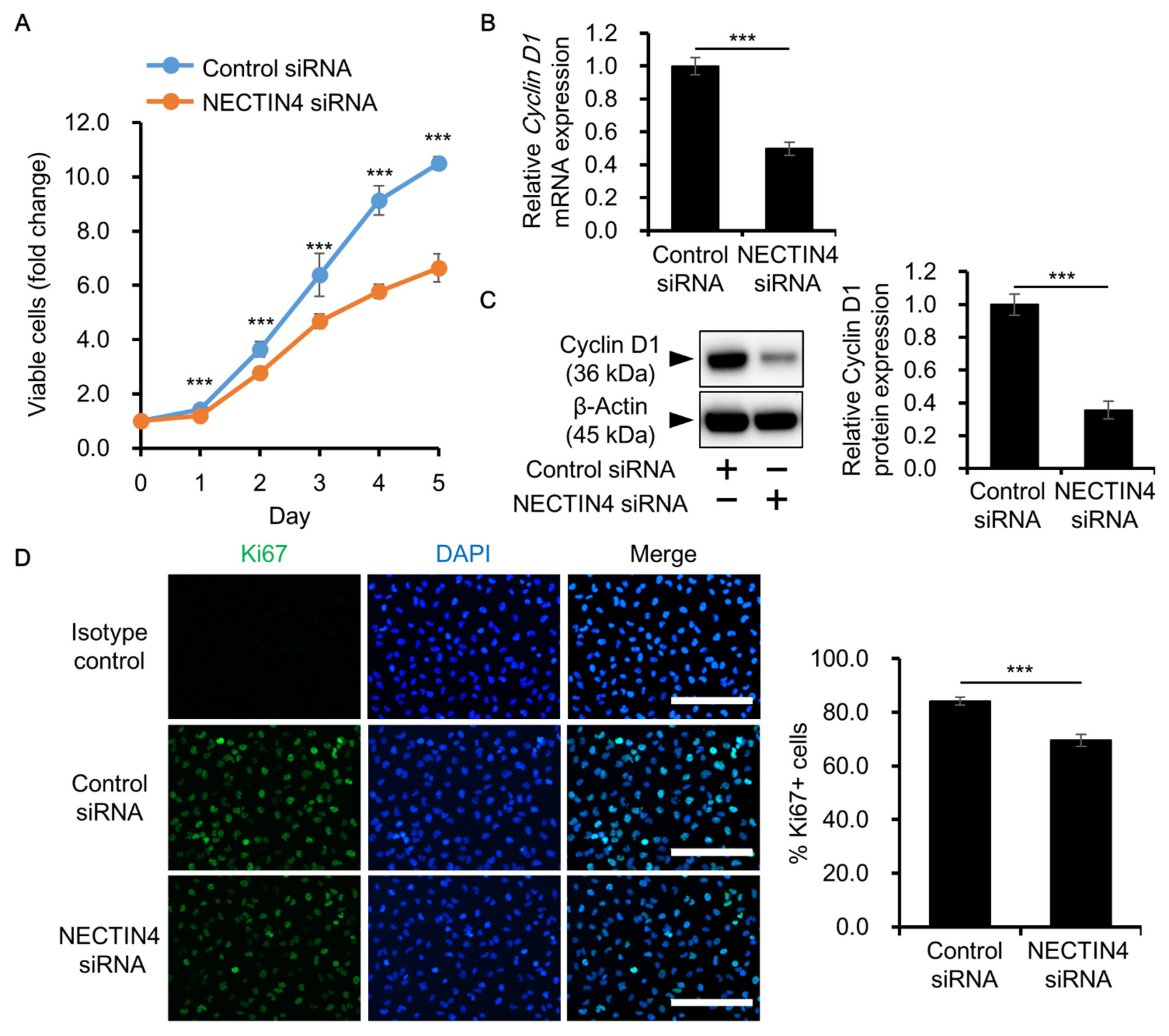
3.4. Effects of NECTIN4 Silencing on the Proliferation of A431 Cells
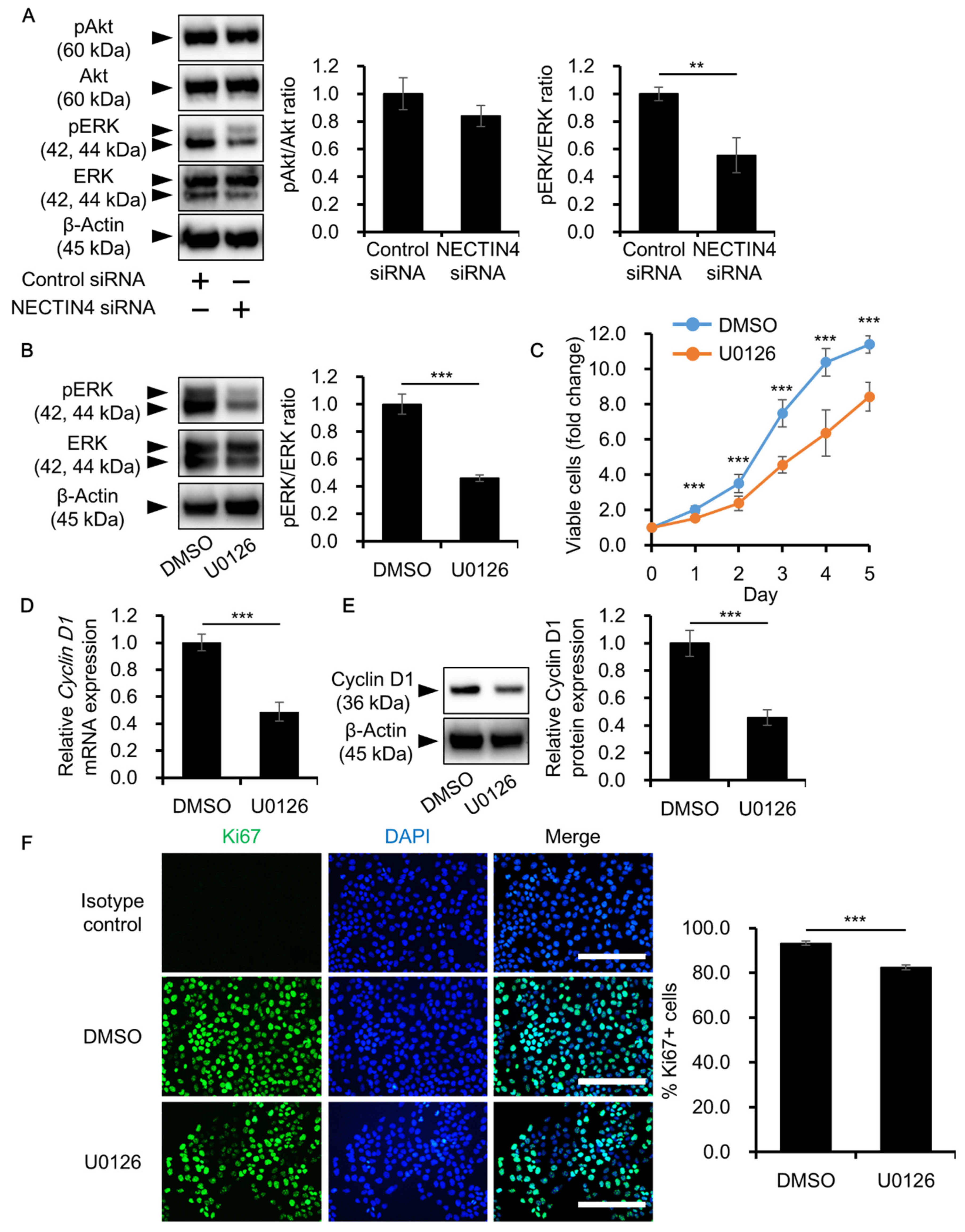
3.5. NECTIN4 Regulation of Cell Proliferation through ERK Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2017, 78, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Lucas, R.; Hales, S.; Neale, R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1918–2012: Empirical relationships. JAMA Dermatol. 2014, 150, 1063–1071. [Google Scholar] [CrossRef]
- Jensen, P.; Hansen, S.; Møller, B.; Leicestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef]
- Lindelöf, B.; Sigurgeirsson, B.; Gäbel, H.; Stern, R.S. Incidence of skin cancr in 5356 patients following organ transplantation. Br. J. Dermatol. 2000, 143, 513–519. [Google Scholar]
- Schipani, G.; Duca, E.D.; Todaro, G.; Scali, E.; Dastoli, S.; Bennardo, L.; Bonacci, S.; Raimondo, C.D.; Pavel, A.B.; Colica, C.; et al. Arsenic and chromium levels in hair correlate with actinic keratosis/non melanoma skin cancer: Results of an observational controlled study. G. Ital. Dermatol. Venereol. 2020, in press. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Hrause-Bergmann, A.; Schulze, H.J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns-result of a non-interventional study of the DeCOG. Eur. J. Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef]
- Amaral, T.; Osewold, M.; Presser, D.; Meiwes, A.; Garve, C.; Leiter, U. Advanced cutaneous squamous cell carcinoma: Real world data of patient profiles and treatment patterns. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 44–51. [Google Scholar] [CrossRef]
- Corchado-Cobos, R.; Garcia-Sancha, N.; Gonzalez-Sarmiento, R.; Perez-Losada, J.; Canueto, J. Cutaneous squamous cell carcinoma: From biology to therapy. Int. J. Med. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef] [PubMed]
- Ribero, S.; Stucci, L.S.; Daniels, G.A.; Borradori, L. Drug therapy of advanced cutaneous squamous cell carcinoma: Is there any evidence? Curr. Opin. Oncol. 2017, 29, 129–135. [Google Scholar] [CrossRef]
- Acevedo-Henao, C.M.; Valette, G.; Miglierini, P.; Lefur, E.; Pradier, O. Radiotherapy combined with cetuximab for locally advanced head and neck cancer: Results and toxicity. Cancer Radiother. 2012, 16, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, M.; Gauler, T.C.; Gruenwald, V.; Matzdorff, A.; Schroeder, M.; Dietz, A.; Jordan, W.O.; Arnold, D.; Hennemann, B.; Hofele, C.; et al. Phase II study of cetuximab in combination with docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck after platinum-containing therapy: A multicenter study of the Arbeitsgemeinschaft Internistische Onkologie. Oncology 2013, 84, 284–289. [Google Scholar] [CrossRef] [PubMed]
- William, W.N., Jr.; Feng, L.; Ferrarotto, R.; Ginsberg, L.; Kies, M.; Lippman, S.; Glisson, B.; Kim, E.S. Gefitinib for patients with incurable cutaneous squamous cell carcinoma: A single-arm phase II clinical trial. J. Am. Acad. Dermatol. 2017, 77, 1110–1113.e2. [Google Scholar] [CrossRef]
- Lewis, C.M.; Glisson, B.S.; Feng, L.; Wan, F.; Tang, X.; Wistuva, I.I.; El-Naggar, A.K.; Rosenthal, D.I.; Chambers, M.S.; Lustig, R.A.; et al. A phase II study of Gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2012, 18, 1435–1446. [Google Scholar] [CrossRef]
- Gold, K.A.; Kies, M.S.; William, W.N., Jr.; Johnson, F.M.; Lee, J.J.; Glisson, B.S. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma. A single-arm phase 2 clinical trial. Cancer 2018, 124, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Huang, S.; Kruser, T.J.; Nechrebecki, M.M.; Armstrong, E.A.; Benaventa, S.; Gondi, V.; Hsu, F.T.; Harari, P.M. Mechanisms of acquired resistance to cetuximab: Role of HER (ErbB) family members. Oncogene 2008, 27, 3944–3956. [Google Scholar] [CrossRef]
- Samanta, D.; Almo, S.C. Nectin family of cell-adhesion molecules: Structural and molecular aspects of function and specificity. Cell. Mol. Life Sci. 2015, 72, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Ogita, H.; Ikeda, W.; Takai, Y. Roles of cell adhesion molecules nectin and nectin-like molecule-5 in the regulation of cell movement and proliferation. J. Microsc. 2008, 231, 455–465. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Nectin and nectin-like molecules: Biology and pathology. Am. J. Nephrol. 2007, 27, 590–604. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Tamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Monville, F.; Garrido-Urbani, S.; Berruyer-Pouyet, C.; Ginestier, C.; Reymond, N.; Finetti, R.; Saucan, R.; Adelaide, J.; Geneix, J.; et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 2007, 7, 73. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2019. MAbs 2019, 11, 219–238. [Google Scholar] [CrossRef]
- Takahashi, S.; Uemura, M.; Kimura, T.; Kawasaki, Y.; Takamoto, A.; Yamaguchi, A.; Melhem-Bertrandt, A.; Gartner, E.M.; Inoue, T.; Akazawa, R.; et al. A phase I study of enfortumab vedotin in Japanese patients with locally advanced or metastatic urothelial carcinoma. Investig. New Drugs 2020, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, R.M.; Pinelli, J.M.; et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04225117 (accessed on 23 February 2021).
- Mollo, M.R.; Antonini, D.; Mitchell, K.; Fortugno, P.; Costanzo, A.; Dixon, J.; Brancati, F.; Missero, C. P63-dependent and independent mechanisms of nectin-1 and nectin-4 regulation in the epidermis. Exp. Dermatol. 2015, 24, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Brancati, F.; Fortugno, P.; Bottillo, I.; Lopez, M.; Josselin, E.; Boudghene-Stambouli, O.; Agolini, E.; Bernardini, L.; Bellacchio, E.; Iannicelli, M.; et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am. J. Hum. Genet. 2010, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Ito, T.; Tanaka, Y.; Kaku-Ito, Y.; Furue, M. NECTIN4 expression in extramammary Paget’s disease: Implication of a new therapeutic target. Int. J. Mol. Sci. 2020, 21, 5891. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Murata, M.; Shen, C.H.; Furue, M.; Ito, T. NECTIN4: A novel therapeutic target for melanoma. Int. J. Mol. Sci. 2021, 22, 976. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef]
- Ito, T.; Kaku-Ito, Y.; Murata, M.; Furue, K.; Shen, C.H.; Oda, Y.; Furue, M. Immunohistochemical BRAF V600E expression and intratumor BRAF V600E heterogeneity in acral melanoma: Implication in melanoma-specific survival. J. Clin. Med. 2020, 9, 690. [Google Scholar] [CrossRef]
- Ito, T.; Kaku-Ito, Y.; Murata, M.; Ichiki, T.; Kuma, Y.; Tanaka, Y.; Ide, T.; Ohno, F.; Wada-Ohno, M.; Yamada, Y.; et al. Intra- and inter-tumor BRAF heterogeneity in acral melanoma: An immunohistochemical analysis. Int. J. Mol. Sci. 2019, 20, 6191. [Google Scholar] [CrossRef]
- McClelland, R.A.; Finlay, P.; Walker, K.J.; Nicholson, D.; Robertson, J.F.; Blamey, R.W.; Nicholson, R.I. Automated quantification of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990, 50, 3545–3550. [Google Scholar] [PubMed]
- Siddharth, S.; Goutam, K.; Das, S.; Nayak, A.; Nayak, D.; Sethy, C.; Wyatt, M.S.; Kundu, C.N. Nectin-4 is a breast cancer stem cell marker that induces WNT/β-catenin signaling via Pi3k/Akt axis. Int. J. Biochem. Cell Biol. 2017, 89, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, P.; Yim, W.; Ji, Y.; Shen, Q.; Ni, Q. Nectin-4 promotes gastric cancer progression via the PI3K/AKT signaling pathway. Hum. Pathol. 2018, 72, 107–116. [Google Scholar] [CrossRef]
- Hao, R.T.; Zheng, C.; Wu, C.Y.; Xia, E.J.; Zhou, X.F.; Quan, R.D.; Zhang, X.H. NECTIN4 promotes papillary thyroid cancer cell proliferation, migration, and invasion and triggers EMT by activating AKT. Cancer Manag. Res. 2019, 11, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 1999, 11, 732–736. [Google Scholar] [CrossRef]
- Chambard, J.C.; Lefloch, R.; Pouyssegur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Boylan, K.L.M.; Buchanan, P.C.; Manion, R.D.; Shukla, D.M.; Braumberger, K.; Bruggemeyer, C.; Skubitz, A.P.N. The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget 2017, 8, 9717–9738. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Pallasch, C.; Elia, A.E.; Braun, C.J.; Westbrook, T.F.; Hemann, M.; Elledge, S.J. A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2013, 2, e00358. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Shtherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef]
- Kuzmanov, A.; Johansen, P.; Holbauer, G. FBXO25 promotes cutaneous squamous cell carcinoma growth and metastasis through cyclin D1. J. Investig. Dermatol. 2020, 140, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Hao, F.; Hizli, A.A.; Lum, M.A.; Swetzig, W.M.; Black, A.R.; Black, J. Differential regulation of cyclin D1 expression by protein kinase C α and ε signaling in intestinal epithelial cells. J. Biol. Chem. 2014, 289, 22268–22283. [Google Scholar] [CrossRef]
- Li, T.; Song, T.; Ni, L.; Yang, G.; Song, X.; Wu, L.; Liu, B.; Liu, C. The p-ERK–pc-Jun–cyclinD1 pathway is involved in proliferation of smooth muscle cells after exposure to cigarette smoke extract. Biochem. Biophys. Res. Commun. 2014, 453, 316–320. [Google Scholar] [CrossRef]
- Arumugam, A.; Weng, Z.; Talwelkar, S.S.; Chaudhary, S.C.; Kopelovich, L.; Elmets, C.A.; Afaq, F.; Athar, M. Inhibiting cyclooxygenase and ornithine decarboxylase by diclofenac and alpha-difluoromethylornithine blocks cutaneous SCCs by targeting Akt-ERK axis. PLoS ONE 2013, 8, e80076. [Google Scholar] [CrossRef]
- Zhang, M.L.; Tao, Y.; Zhou, W.Q.; Ma, P.C.; Cao, Y.P.; He, C.D.; Wei, J.; Li, L.J. All-trans retinoic acid induces cell-cycle arrest in human cutaneous squamous carcinoma cells by inhibiting the mitogen-activated protein kinase-activated protein 1 pathway. Clin. Exp. Dermatol. 2014, 39, 354–360. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Nilsson, M.; Sundfeldt, K. TGF-β isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014, 14, 72. [Google Scholar] [CrossRef]
- Fardi, M.; Alivand, M.; Baradaran, B.; Hagh, M.F.; Solali, S. The crucial role of ZEB2: From development to epithelial-to-mesenchymal transition and cancer complexity. J. Cell. Physiol. 2019, 234, 14783–14799. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Murata, M.; Oda, Y.; Furue, M.; Ito, T. Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target? Biomedicines 2021, 9, 355. https://doi.org/10.3390/biomedicines9040355
Tanaka Y, Murata M, Oda Y, Furue M, Ito T. Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target? Biomedicines. 2021; 9(4):355. https://doi.org/10.3390/biomedicines9040355
Chicago/Turabian StyleTanaka, Yuka, Maho Murata, Yoshinao Oda, Masutaka Furue, and Takamichi Ito. 2021. "Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target?" Biomedicines 9, no. 4: 355. https://doi.org/10.3390/biomedicines9040355
APA StyleTanaka, Y., Murata, M., Oda, Y., Furue, M., & Ito, T. (2021). Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target? Biomedicines, 9(4), 355. https://doi.org/10.3390/biomedicines9040355





