The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target
Abstract
1. Introduction
2. The PKA-p38MAPK-NFAT5- Pathway as a Therapeutic Target in Duchenne Muscular Dystrophy
2.1. Protein Kinase A
2.2. P38 Mitogen-Activated Protein Kinase
2.3. Glycogen Synthase Kinase 3 Beta
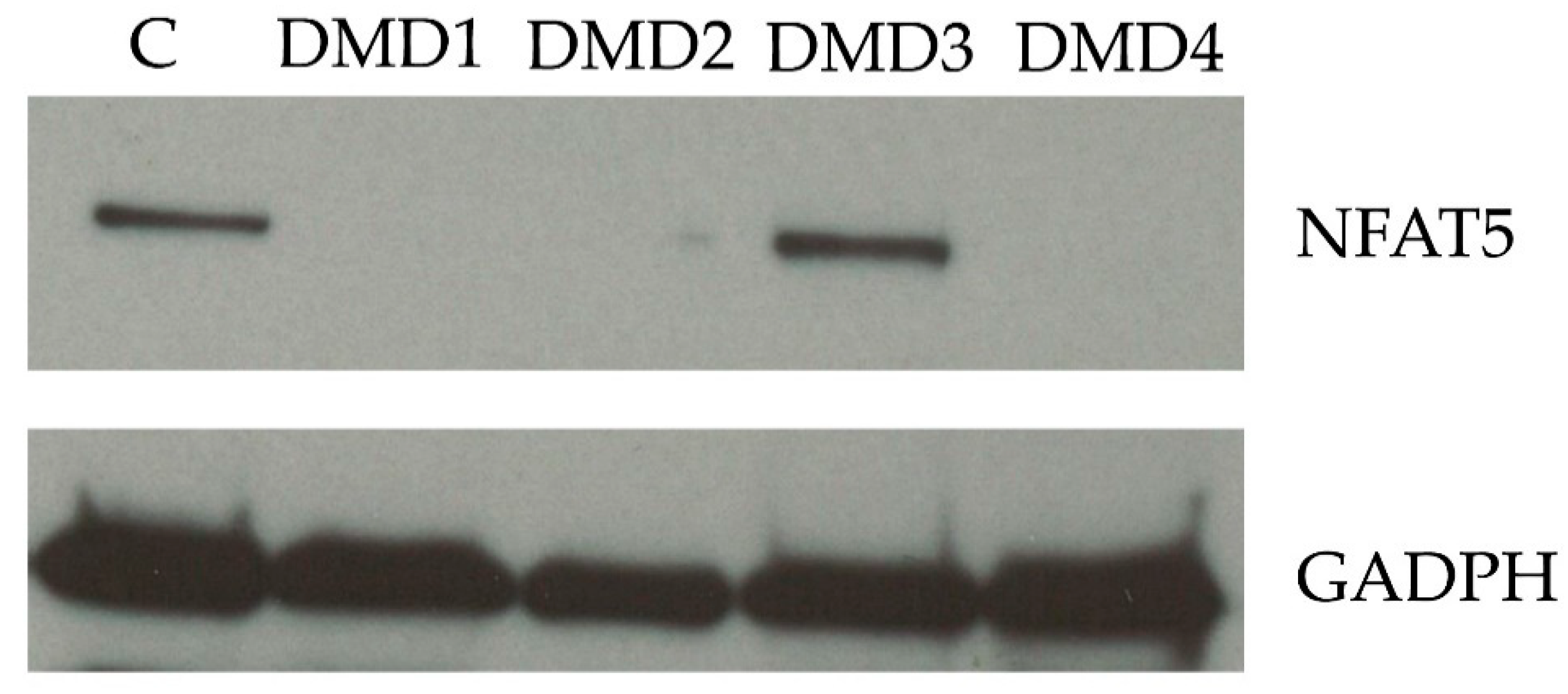
2.4. Nuclear Factor of Activated T-Cells 5
2.5. Factors Downstream of Nuclear Factor of Activated T-Cells 5
2.5.1. Taurine Transporter
2.5.2. Betaine Gamma Aminobutyric Acid Transporter
2.5.3. Sodium/Myo-Inositol Co-transporter
2.5.4. Aldose Reductase
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
|---|---|
| AKAP | A-kinase anchor protein |
| AKAP13 | A-kinase anchor protein 13 |
| ALA | α-lipoic acid |
| ATP | adenosine triphosphate |
| BGT | betaine gamma aminobutyric acid transporter |
| BMD | Becker muscular dystrophy |
| CBP | CREB binding protein |
| COX | cyclooxygenase |
| CRF | corticotropin-releasing factor |
| CXCL | chemokine ligand |
| DAG | diacylglycerol |
| DAPC | dystrophin-associated protein complex |
| DMD | Duchenne muscular dystrophy |
| FDA | Food and Drug Administration |
| GABA | gamma aminobutyric acid |
| GCs | glucocorticoids |
| GLUT4 | glucose transporter type 4 |
| GR | glucocorticoid receptor |
| GSK-3 | glycogen synthase kinase 3 |
| GSK-3β | glycogen synthase kinase 3 beta |
| GTP | guanosinetrifosfaat |
| HNE | 4-hydroxynonenal |
| Hsp70 | heat shock protein 70 |
| ICAM-1 | intracellular adhesion molecule 1 |
| IL | interleukine |
| IP3 | inositol triphosphate |
| L-CAR | L-carnitine |
| MAPK | mitogen-activated protein kinase |
| MI | myo-inositol |
| NFAT | nuclear factor of activated T- cells |
| NFAT5 | nuclear factor of activated T- cells 5 |
| NFAT5c | nuclear factor of activated T- cells c |
| NF-ƘB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| nNOS | neuronal nitric oxide synthase |
| PKA | protein kinase A |
| PKC | protein kinase C |
| p-p38 | phosphorylated p38 |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| Rac 1 | Ras-related C3 botulinum toxin substrate |
| s.c. | subcutaneous |
| SCID | severe combined immunodeficiency |
| SMIT | sodium myo-inositol transporter |
| SRC-1 | steroid receptor coactivator 1 |
| TauT | Taurine transporter |
| TBP | TATA box binding protein |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor α |
| TonEBP | Tonicity-responsive enhancer binding protein |
| Ucns | urocortins |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VSMC | vascular smooth muscle cells |
| xIL-6R | anti-interleukin-6 receptor antibody |
References
- Koenig, M.; Hoffman, E.; Bertelson, C.; Monaco, A.; Feener, C.; Kunkel, L. Complete cloning of the duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Ehmsen, J.; Poon, E.; Davies, K. The dystrophin-associated protein complex. J. Cell Sci. 2002, 115, 2801–2803. [Google Scholar] [PubMed]
- Rahimov, F.; Kunkel, L.M. Cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 2013, 201, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Constantin, B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 635–642. [Google Scholar] [CrossRef]
- Johnson, E.K.; Li, B.; Yoon, J.H.; Flanigan, K.M.; Martin, P.T.; Ervasti, J.; Montanaro, F. Identification of New Dystroglycan Complexes in Skeletal Muscle. PLoS ONE 2013, 8, e73224. [Google Scholar] [CrossRef]
- Li, D.; Long, C.; Yue, Y.; Duan, D. Sub-physiological sarcoglycan expression contributes to compensatory muscle protection in mdx mice. Hum. Mol. Genet. 2009, 18, 1209–1220. [Google Scholar] [CrossRef]
- Gibbs, E.M.; McCourt, J.L.; Shin, K.M.; Hammond, K.G.; Marshall, J.L.; Crosbie, R.H. Loss of sarcospan exacerbates pathology in mdx mice, but does not affect utrophin amelioration of disease. Hum. Mol. Genet. 2021, 264. [Google Scholar] [CrossRef]
- Peter, A.K.; Marshall, J.L.; Crosbie, R.H. Sarcospan reduces dystrophic pathology: Stabilization of the utrophin–glycoprotein complex. J. Cell Biol. 2008, 183, 419–427. [Google Scholar] [CrossRef]
- Marshall, J.L.; Crosbie-Watson, R.H. Sarcospan: A small protein with large potential for Duchenne muscular dystrophy. Skelet. Muscle 2013, 3, 1. [Google Scholar] [CrossRef]
- Koo, T.; Malerba, A.; Athanasopoulos, T.; Trollet, C.; Boldrin, L.; Ferry, A.; Popplewell, L.; Foster, H.; Foster, K.; Dickson, G. Delivery of AAV2/9-Microdystrophin Genes Incorporating Helix 1 of the Coiled-Coil Motif in the C-Terminal Domain of Dystrophin Improves Muscle Pathology and Restores the Level of α1-Syntrophin and α-Dystrobrevin in Skeletal Muscles of mdx Mice. Hum. Gene Ther. 2011, 22, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- De Arcangelis, V.; Serra, F.; Cogoni, C.; Vivarelli, E.; Monaco, L.; Naro, F. β1-Syntrophin Modulation by miR-222 in mdx Mice. PLoS ONE 2010, 5, e12098. [Google Scholar] [CrossRef]
- Yoon, J.H.; Johnson, E.; Xu, R.; Martin, L.T.; Martin, P.T.; Montanaro, F. Comparative Proteomic Profiling of Dystroglycan-Associated Proteins in Wild Type, mdx, and Galgt2 Transgenic Mouse Skeletal Muscle. J. Proteome Res. 2012, 11, 4413–4424. [Google Scholar] [CrossRef]
- Peters, M.F.; Sadoulet-Puccio, H.M.; Grady, R.M.; Kramarcy, N.R.; Kunkel, L.M.; Sanes, J.R.; Sealock, R.; Froehner, S.C. Differential Membrane Localization and Intermolecular Associations of α-Dystrobrevin Isoforms in Skeletal Muscle. J. Cell Biol. 1998, 142, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.C.D.S.; Kiyomoto, B.H.; Cardoso, R.; Oliveira, A.S.B. Duchenne muscular dystrophy: Alpha-dystroglycan immunoexpression in skeletal muscle and cognitive performance. Arq. Neuro-Psiquiatria 2005, 63, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, Y.; Inoue, M.; Kojima, H.; Jimi, T.; Yamashita, S.; Kumagai, T.; Shibuya, S.; Hara, H.; Oniki, H. Altered alpha1-syntrophin expression in myofibers with Duchenne and Fukuyama muscular dystrophies. Histol. Histopathol. 2006, 21, 23–34. [Google Scholar]
- Cirak, S.; Arechavala-Gomeza, V.; Guglieri, M.; Feng, L.; Torelli, S.; Anthony, K.; Abbs, S.; Garralda, M.E.; Bourke, J.; Wells, D.J.; et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet 2011, 378, 595–605. [Google Scholar] [CrossRef]
- Compton, A.G.; Cooper, S.T.; Hill, P.M.; Yang, N.; Froehner, S.C.; North, K.N. The Syntrophin-Dystrobrevin Subcomplex in Human Neuromuscular Disorders. J. Neuropathol. Exp. Neurol. 2005, 64, 350–361. [Google Scholar] [CrossRef]
- Metzinger, L.; Blake, D.J.; Squier, M.V.; Anderson, L.V.B.; Deconinck, A.E.; Nawrotzki, R.; Hilton-Jones, D.; Davies, K.E. Dystrobrevin deficiency at the sarcolemma of patients with muscular dystrophy. Hum. Mol. Genet. 1997, 6, 1185–1191. [Google Scholar] [CrossRef]
- Dombernowsky, N.W.; Ölmestig, J.N.; Witting, N.; Kruuse, C. Role of neuronal nitric oxide synthase (nNOS) in Duchenne and Becker muscular dystrophies—Still a possible treatment modality? Neuromuscul. Disord. 2018, 28, 914–926. [Google Scholar] [CrossRef]
- E Brenman, J.; Chao, D.S.; Xia, H.; Aldape, K.; Bredt, D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 1995, 82, 743–752. [Google Scholar] [CrossRef]
- Chang, W.J.; Iannaccone, S.T.; Lau, K.S.; Masters, B.S.; McCabe, T.J.; McMillan, K.; Padre, R.C.; Spencer, M.J.; Tidball, J.G.; Stull, J.T. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc. Natl. Acad. Sci. USA 1996, 93, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, R.H.; Straub, V.; Yun, H.-Y.; Lee, J.C.; Rafael, J.A.; Chamberlain, J.S.; Dawson, V.L.; Dawson, T.M.; Campbell, K.P. mdx muscle pathology is independent of nNOS perturbation. Hum. Mol. Genet. 1998, 7, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Wehling, M.; Spencer, M.J.; Tidball, J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001, 155, 123–132. [Google Scholar] [CrossRef]
- Chao, D.S.; Silvagno, F.; Bredt, D.S. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J. Neurochem. 2002, 71, 784–789. [Google Scholar] [CrossRef]
- Rando, T.A. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 2001, 24, 1575–1594. [Google Scholar] [CrossRef]
- Sun, B.; Vaughan, D.M.; Tikunova, S.B.; Creamer, T.P.; Davis, J.P.; Kekenes-Huskey, P.M. Calmodulin–Calcineurin Interaction beyond the Calmodulin-Binding Region Contributes to Calcineurin Activation. Biochemistry 2019, 58, 4070–4085. [Google Scholar] [CrossRef]
- Herbelet, S.; Rodenbach, A.; De Paepe, B.; De Bleecker, J.L. Anti-Inflammatory and General Glucocorticoid Physiology in Skeletal Muscles Affected by Duchenne Muscular Dystrophy: Exploration of Steroid-Sparing Agents. Int. J. Mol. Sci. 2020, 21, 4596. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumar, A. Therapeutic targeting of signaling pathways in muscular dystrophy. J. Mol. Med. 2010, 88, 155–166. [Google Scholar] [CrossRef]
- Adcock, I. Glucocorticoid-regulated Transcription Factors. Pulm. Pharmacol. Ther. 2001, 14, 211–219. [Google Scholar] [CrossRef]
- Angelini, C.; Peterle, E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 9–15. [Google Scholar]
- López-Rodríguez, C.; Aramburu, J.; Jin, L.; Rakeman, A.S.; Michino, M.; Rao, A. Bridging the NFAT and NF-κB Families. Immunity 2001, 15, 47–58. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee-Kwon, W.; Kwon, H.M. The evolving role of TonEBP as an immunometabolic stress protein. Nat. Rev. Nephrol. 2020, 16, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Herbelet, S.; De Paepe, B.; De Bleecker, J.L. Abnormal NFAT5 Physiology in Duchenne Muscular Dystrophy Fibroblasts as a Putative Explanation for the Permanent Fibrosis Formation in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2020, 21, 7888. [Google Scholar] [CrossRef]
- Herbelet, S.; De Paepe, B.; De Bleecker, J.L. Description of a Novel Mechanism Possibly Explaining the Antiproliferative Properties of Glucocorticoids in Duchenne Muscular Dystrophy Fibroblasts Based on Glucocorticoid Receptor GR and NFAT5. Int. J. Mol. Sci. 2020, 21, 9225. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Mills, S.T.; Jones, K.A.; Ho, S.N.; Pavlath, G.K. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J. Cell Sci. 2006, 120, 149–159. [Google Scholar] [CrossRef]
- Herbelet, S.; De Vlieghere, E.; Gonçalves, A.; De Paepe, B.; Schmidt, K.; Nys, E.; Weynants, L.; Weis, J.; Van Peer, G.; Vandesompele, J.; et al. Localization and Expression of Nuclear Factor of Activated T-Cells 5 in Myoblasts Exposed to Pro-inflammatory Cytokines or Hyperosmolar Stress and in Biopsies from Myositis Patients. Front. Physiol. 2018, 9, 126. [Google Scholar] [CrossRef]
- Kino, T.; Takatori, H.; Manoli, I.; Wang, Y.; Tiulpakov, A.; Blackman, M.R.; Su, Y.A.; Chrousos, G.P.; DeCherney, A.H.; Segars, J.H. Brx Mediates the Response of Lymphocytes to Osmotic Stress Through the Activation of NFAT5. Sci. Signal. 2009, 2, ra5. [Google Scholar] [CrossRef]
- Aramburu, J.; López-Rodríguez, C. Regulation of Inflammatory Functions of Macrophages and T Lymphocytes by NFAT5. Front. Immunol. 2019, 10, 535. [Google Scholar] [CrossRef]
- Ceccarini, M.; Grasso, M.; Veroni, C.; Gambara, G.; Artegiani, B.; Macchia, G.; Ramoni, C.; Torreri, P.; Mallozzi, C.; Petrucci, T.C.; et al. Association of Dystrobrevin and Regulatory Subunit of Protein Kinase A: A New Role for Dystrobrevin as a Scaffold for Signaling Proteins. J. Mol. Biol. 2007, 371, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.G.; McCalmon, S.A.; Donaghey, J.A.; Naya, F.J. Deregulated Protein Kinase A Signaling and Myospryn Expression in Muscular Dystrophy. J. Biol. Chem. 2008, 283, 8070–8074. [Google Scholar] [CrossRef]
- Fekete, Éva M.; Zorrilla, E.P. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocr. 2007, 28, 1–27. [Google Scholar] [CrossRef]
- Reutenauer-Patte, J.; Boittin, F.-X.; Patthey-Vuadens, O.; Ruegg, U.T.; Dorchies, O.M. Urocortins Improve Dystrophic Skeletal Muscle Structure and Function through Both PKA- and Epac-Dependent Pathways. Am. J. Pathol. 2012, 180, 749–762. [Google Scholar] [CrossRef]
- Manning, J.; Buckley, M.M.; O’Halloran, K.D.; O’Malley, D.; Bsc, J.M. Combined XIL-6R and urocortin-2 treatment restores MDX diaphragm muscle force. Muscle Nerve 2017, 56, E134–E140. [Google Scholar] [CrossRef]
- Burns, D.P.; Canavan, L.; Rowland, J.; O’Flaherty, R.; Brannock, M.; Drummond, S.E.; O’Malley, D.; Edge, D.; O’Halloran, K.D. Recovery of respiratory function in mdx mice co-treated with neutralizing interleukin-6 receptor antibodies and urocortin-2. J. Physiol. 2018, 596, 5175–5197. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.P.; Rowland, J.; Canavan, L.; Murphy, K.H.; Brannock, M.; O’Malley, D.; O’Halloran, K.D.; Edge, D. Restoration of pharyngeal dilator muscle force in dystrophin-deficient (mdx) mice following co-treatment with neutralizing interleukin-6 receptor antibodies and urocortin 2. Exp. Physiol. 2017, 102, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014, 20, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Perdiguero, E.; Muñoz-Cánoves, P. Regulation of Muscle Stem Cell Functions: A Focus on the p38 MAPK Signaling Pathway. Front. Cell Dev. Biol. 2016, 4, 91. [Google Scholar] [CrossRef]
- Tomida, T.; Adachi-Akahane, S. Roles of p38 MAPK signaling in the skeletal muscle formation, regeneration, and pathology. Folia Pharmacol. Jpn. 2020, 155, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Verma, M.; Zhang, L.; Dong, C.; Flavell, R.A.; Bennett, A.M. Improved regenerative myogenesis and muscular dystrophy in mice lacking Mkp5. J. Clin. Investig. 2013, 123, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Hnia, K.; Hugon, G.; Rivier, F.; Masmoudi, A.; Mercier, J.; Mornet, D. Modulation of p38 Mitogen-Activated Protein Kinase Cascade and Metalloproteinase Activity in Diaphragm Muscle in Response to Free Radical Scavenger Administration in Dystrophin-Deficient Mdx Mice. Am. J. Pathol. 2007, 170, 633–643. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, K.; Ueda, H.; Takeda, S.; Ikeda, S.-I. Up-regulation of mitogen activated protein kinases in mdx skeletal muscle following chronic treadmill exercise. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1740, 326–331. [Google Scholar] [CrossRef]
- Wissing, E.R.; Boyer, J.G.; Kwong, J.Q.; Sargent, M.A.; Karch, J.; McNally, E.M.; Otsu, K.; Molkentin, J.D. P38α MAPK underlies muscular dystrophy and myofiber death through a Bax-dependent mechanism. Hum. Mol. Genet. 2014, 23, 5452–5463. [Google Scholar] [CrossRef]
- Lang, J.M.; Esser, K.A.; Dupont-Versteegden, E.E. Altered Activity of Signaling Pathways in Diaphragm and Tibialis Anterior Muscle of Dystrophic Mice. Exp. Biol. Med. 2004, 229, 503–511. [Google Scholar] [CrossRef]
- De Paepe, B.; Martin, J.-J.; Herbelet, S.; Jimenez-Mallebrera, C.; Iglesias, E.; Jou, C.; Weis, J.; De Bleecker, J.L. Activation of osmolyte pathways in inflammatory myopathy and Duchenne muscular dystrophy points to osmoregulation as a contributing pathogenic mechanism. Lab. Investig. 2016, 96, 872–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Péladeau, C.; Adam, N.J.; Jasmin, B.J. Celecoxib treatment improves muscle function in mdx mice and increases utrophin A expression. FASEB J. 2018, 32, 5090–5103. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Villadiego, A.; Brooke, M.H. Increased long chain acyl CoA in Duchenne muscular dystrophy. Neurology 1983, 33, 1507. [Google Scholar] [CrossRef] [PubMed]
- Berthillier, G.; Eichenberger, D.; Carrier, H.; Guibaud, P.; Got, R. Carnitine metabolism in early stages of Duchenne muscular dystrophy. Clin. Chim. Acta 1982, 122, 369–375. [Google Scholar] [CrossRef]
- Escobar-Cedillo, R.E.; Tintos-Hernandez, J.A.; Martinez-Castro, G.; Montes de Oca-Sanchez, B.; Rodriguez-Jurado, R.; Miranda-Duarte, A. L-carnitine supplementation in duchenne muscular dystrophy steroid-naive patients: A pilot study. Curr. Top. Nutraceut. Res. 2013, 3, 97–102. [Google Scholar]
- Angelini, C.; Marozzo, R.; Pegoraro, V. Current and emerging therapies in Becker muscular dystrophy (BMD). Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2019, 38, 172–179. [Google Scholar]
- Angelini, C.; Tasca, E. Drugs in development and dietary approach for Duchenne muscular dystrophy. Orphan Drugs Res. Rev. 2015, 5, 51–60. [Google Scholar] [CrossRef][Green Version]
- Smythe, G.M.; Forwood, J.K. Altered mitogen-activated protein kinase signaling in dystrophic (mdx) muscle. Muscle Nerve 2012, 46, 374–383. [Google Scholar] [CrossRef]
- E Plyte, S. Glycogen synthase kinase-3: Functions in oncogenesis and development. Biochim. Biophys. Acta (BBA) Bioenerg. 1992, 1114, 147–162. [Google Scholar] [CrossRef]
- Duda, P.; Wiśniewski, J.; Wójtowicz, T.; Wójcicka, O.; Jaśkiewicz, M.; Drulis-Fajdasz, D.; Rakus, D.; McCubrey, J.A.; Gizak, A. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 2018, 22, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Pansters, N.A.; Schols, A.M.; Verhees, K.J.; De Theije, C.C.; Snepvangers, F.J.; Kelders, M.C.; Ubags, N.D.; Haegens, A.; Langen, R.C. Muscle-specific GSK-3β ablation accelerates regeneration of disuse-atrophied skeletal muscle. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Villa-Moruzzi, E.; Puntoni, F.; Marin, O. Activation of protein phosphatase-1 isoforms and glycogen synthase kinase-3β in muscle from mdx mice. Int. J. Biochem. Cell Biol. 1996, 28, 13–22. [Google Scholar] [CrossRef]
- Feron, M.; Guevel, L.; Rouger, K.; Dubreil, L.; Arnaud, M.-C.; Ledevin, M.; Megeney, L.A.; Cherel, Y.; Sakanyan, V. PTEN Contributes to Profound PI3K/Akt Signaling Pathway Deregulation in Dystrophin-Deficient Dog Muscle. Am. J. Pathol. 2009, 174, 1459–1470. [Google Scholar] [CrossRef]
- Whitley, K.C.; Hamstra, S.I.; Baranowski, R.W.; Watson, C.J.F.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Vandenboom, R.; Fajardo, V.A. GSK3 inhibition with low dose lithium supplementation augments murine muscle fatigue resistance and specific force production. Physiol. Rep. 2020, 8, e14517. [Google Scholar] [CrossRef]
- Ma, Z.; Zhong, Z.; Zheng, Z.; Shi, X.-M.; Zhang, W. Inhibition of Glycogen Synthase Kinase-3β Attenuates Glucocorticoid-Induced Suppression of Myogenic Differentiation In Vitro. PLoS ONE 2014, 9, e105528. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Eldar-Finkelman, H. Long-Term Treatment with Novel Glycogen Synthase Kinase-3 Inhibitor Improves Glucose Homeostasis in ob/ob Mice: Molecular Characterization in Liver and Muscle. J. Pharmacol. Exp. Ther. 2005, 316, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rodriguezcruz, M.; Sanchez, R.; E Escobar, R.; Cruzguzman, O.D.R.; Lopezalarcon, M.; Garcia, M.B.; Coralvazquez, R.M.; Matute, G.; Wong, A.C.V. Evidence of Insulin Resistance and Other Metabolic Alterations in Boys with Duchenne or Becker Muscular Dystrophy. Int. J. Endocrinol. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Pfisterer, L.; Wagner, A.H.; Hödebeck, M.; Cattaruzza, M.; Hecker, M.; Korff, T. Arterial Wall Stress Controls NFAT5 Activity in Vascular Smooth Muscle Cells. J. Am. Hear. Assoc. 2014, 3, e000626. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W. Role of NFAT5 in Inflammatory Disorders Associated with Osmotic Stress. Curr. Genom. 2010, 11, 584–590. [Google Scholar] [CrossRef][Green Version]
- Woo, S.K.; Lee, S.D.; Na, K.Y.; Park, W.K.; Kwon, H.M. TonEBP/NFAT5 Stimulates Transcription of HSP70 in Response to Hypertonicity. Mol. Cell. Biol. 2002, 22, 5753–5760. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Danielson, K.G.; Guttapalli, A.; Oguz, E.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. TonEBP/OREBP Is a Regulator of Nucleus Pulposus Cell Function and Survival in the Intervertebral Disc. J. Biol. Chem. 2006, 281, 25416–25424. [Google Scholar] [CrossRef]
- Ito, T.; Fujio, Y.; Hirata, M.; Takatani, T.; Matsuda, T.; Muraoka, S.; Takahashi, K.; Azuma, J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem. J. 2004, 382, 177–182. [Google Scholar] [CrossRef]
- Yang, Y.J.; Han, Y.Y.; Chen, K.; Zhang, Y.; Liu, X.; Li, S.; Wang, K.Q.; Ge, J.B.; Liu, W.; Zuo, J. TonEBP modulates the protective effect of taurine in ischemia-induced cytotoxicity in cardiomyocytes. Cell Death Dis. 2015, 6, e2025. [Google Scholar] [CrossRef][Green Version]
- Kempson, S.A.; Zhou, Y.; Danbolt, N.C. The betaine/GABA transporter and betaine: Roles in brain, kidney, and liver. Front. Physiol. 2014, 5, 159. [Google Scholar] [CrossRef]
- Kempson, S.A.; Montrose, M.H. Osmotic regulation of renal betaine transport: Transcription and beyond. Pflüger’s Arch. Gesammte Phys. Menschen Tiere 2004, 449, 227–234. [Google Scholar] [CrossRef]
- Kempson, S.A.; Parikh, V.; Xi, L.; Chu, S.; Montrose, M.H. Subcellular redistribution of the renal betaine transporter during hypertonic stress. Am. J. Physiol. Physiol. 2003, 285, C1091–C1100. [Google Scholar] [CrossRef]
- Kempson, S.A.; Beck, J.A.; Lammers, P.E.; Gens, J.S.; Montrose, M.H. Membrane insertion of betaine/GABA transporter during hypertonic stress correlates with nuclear accumulation of TonEBP. Biochim. Biophys. Acta (BBA) Biomembr. 2005, 1712, 71–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanishi, T.; Turner, R.J.; Burg, M.B. Osmoregulatory changes in myo-inositol transport by renal cells. Proc. Natl. Acad. Sci. USA 1989, 86, 6002–6006. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Yamauchi, A.; Uchida, S.; Robey, R.B.; Garcia-Perez, A.; Burg, M.B.; Handler, J.S. Renal Na-myo-inositol cotransporter mRNA expression in Xenopus oocytes: Regulation by hypertonicity. Am. J. Physiol. Physiol. 1991, 260, F258–F263. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Choi, S.Y.; Lim, S.W.; Lamitina, S.T.; Ho, S.N.; Go, W.Y.; Kwon, H.M. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: Organic osmolyte-dependent and -independent pathways. Am. J. Physiol. Physiol. 2011, 300, F707–F715. [Google Scholar] [CrossRef] [PubMed]
- Rim, J.S.; Atta, M.G.; Dahl, S.C.; Berry, G.T.; Handler, J.S.; Kwon, H.M. Transcription of the Sodium/myo-Inositol Cotransporter Gene Is Regulated by Multiple Tonicity-responsive Enhancers Spread over 50 Kilobase Pairs in the 5′-Flanking Region. J. Biol. Chem. 1998, 273, 20615–20621. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, C.; Antos, C.L.; Shelton, J.M.; Richardson, J.A.; Lin, F.; Novobrantseva, T.I.; Bronson, R.T.; Igarashi, P.; Rao, A.; Olson, E.N. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 2392–2397. [Google Scholar] [CrossRef]
- Mak, M.C.; Lam, K.M.; Chan, P.K.; Lau, Y.B.; Tang, W.H.; Yeung, P.K.K.; Ko, B.C.B.; Chung, S.M.S.; Chung, S.K. Embryonic Lethality in Mice Lacking the Nuclear Factor of Activated T Cells 5 Protein Due to Impaired Cardiac Development and Function. PLoS ONE 2011, 6, e19186. [Google Scholar] [CrossRef][Green Version]
- Buccafusca, R.; Venditti, C.P.; Kenyon, L.C.; Johanson, R.A.; Van Bockstaele, E.; Ren, J.; Pagliardini, S.; Minarcik, J.; Golden, J.A.; Coady, M.J.; et al. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: Myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol. Genet. Metab. 2008, 95, 81–95. [Google Scholar] [CrossRef]
- Ko, B.C.B.; Ruepp, B.; Bohren, K.M.; Gabbay, K.H.; Chung, S.S.M. Identification and Characterization of Multiple Osmotic Response Sequences in the Human Aldose Reductase Gene. J. Biol. Chem. 1997, 272, 16431–16437. [Google Scholar] [CrossRef]
- Jhiang, S.M.; Fithian, L.; Smanik, P.; McGill, J.; Tong, Q.; Mazzaferri, E.L. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 1993, 318, 139–144. [Google Scholar] [CrossRef]
- De Paepe, B. Osmolytes as Mediators of the Muscle Tissue’s Responses to Inflammation: Emerging Regulators of Myositis with Therapeutic Potential. Eur. Med. J. Rheumatol. 2017, 4, 83–89. [Google Scholar]
- Tappaz, M.L. Taurine Biosynthetic Enzymes and Taurine Transporter: Molecular Identification and Regulations. Neurochem. Res. 2004, 29, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Taurine deficiency, synthesis and transport in the mdx mouse model for Duchenne Muscular Dystrophy. Int. J. Biochem. Cell Biol. 2015, 66, 141–148. [Google Scholar] [CrossRef]
- Bank, W.J.; Rowland, L.P.; Ipsen, J. Amino Acids of Plasma and Urine in Diseases of Muscle. Arch. Neurol. 1971, 24, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, F.; Zhang, L.; Duan, Y.; Guo, Q.; Wang, W.; He, S.; Li, J.; Yin, Y. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019, 63, e1800536. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Häussinger, D. Phenotype of the Taurine Transporter Knockout Mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Borsch, E.; Reinehr, R.; Heller-Stilb, B.; Roth, C.; Witt, M.; Häussinger, D. Taurine deficiency and apoptosis: Findings from the taurine transporter knockout mouse. Arch. Biochem. Biophys. 2007, 462, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kimura, Y.; Uozumi, Y.; Takai, M.; Muraoka, S.; Matsuda, T.; Ueki, K.; Yoshiyama, M.; Ikawa, M.; Okabe, M.; et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J. Mol. Cell. Cardiol. 2008, 44, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Flögel, U.; Jacoby, C.; Hartwig, H.; Thewissen, M.; Merx, M.W.; Molojavyi, A.; Heller-Stilb, B.; Schrader, J.; Häussinger, D. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004, 18, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Webb, S.M.; Arthur, P.G.; Hackett, M.J. Investigation of the effect of taurine supplementation on muscle taurine content in the mdx mouse model of Duchenne muscular dystrophy using chemically specific synchrotron imaging. Analyst 2020, 145, 7242–7251. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Cozzoli, A.; Mantuano, P.; Camerino, G.M.; Massari, A.M.; Sblendorio, V.T.; De Bellis, M.; Tamma, R.; Giustino, A.; Nico, B.; et al. Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: A comparison with the gold standard, α-methyl prednisolone. Pharmacol. Res. 2016, 106, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. [MD-16-0004R1] Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Pinniger, G.J.; Nair, K.V.; Grounds, M.D.; Arthur, P.G. Beneficial effects of high dose taurine treatment in juvenile dystrophic mdx mice are offset by growth restriction. PLoS ONE 2017, 12, e0187317. [Google Scholar] [CrossRef]
- Terrill, J.R.; Pinniger, G.J.; Graves, J.A.; Grounds, M.D.; Arthur, P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J. Physiol. 2016, 594, 3095–3110. [Google Scholar] [CrossRef]
- Murphy, R.M.; Barker, R.G.; Horvath, D.; Van Der Poel, C. Benefits of Pre-natal Taurine Supplementation in Preventing the Onset of Acute Damage in the Mdx Mouse. PLoS Curr. 2017, 9, 9. [Google Scholar] [CrossRef]
- Voss, J.W.; Pedersen, S.F.; Christensen, S.T.; Lambert, I.H. Regulation of the expression and subcellular localization of the taurine transporter TauT in mouse NIH3T3 fibroblasts. JBIC J. Biol. Inorg. Chem. 2004, 271, 4646–4658. [Google Scholar] [CrossRef]
- Bitoun, M.; Tappaz, M. Taurine Down-Regulates Basal and Osmolarity-Induced Gene Expression of Its Transporter, but Not the Gene Expression of Its Biosynthetic Enzymes, in Astrocyte Primary Cultures. J. Neurochem. 2002, 75, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Matsell, D.G.; Bennett, T.; Han, X.; Budreau, A.M.; Chesney, R.W. Regulation of the taurine transporter gene in the S3 segment of the proximal tubule. Kidney Int. 1997, 52, 748–754. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Fraysse, B.; Mirabella, M.; Servidei, S.; Rüegg, U.T.; Camerino, D.C. Enhanced Dystrophic Progression in mdx Mice by Exercise and Beneficial Effects of Taurine and Insulin-Like Growth Factor-1. J. Pharmacol. Exp. Ther. 2003, 304, 453–463. [Google Scholar] [CrossRef]
- Cozzoli, A.; Rolland, J.-F.; Capogrosso, R.F.; Sblendorio, V.T.; Longo, V.; Simonetti, S.; Nico, B.; De Luca, A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of Duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011, 37, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, L.; Zhang, P.; Tan, Z.; Cheng, X.; Luo, J.; Zhao, X.; Yang, Q.; Gu, H.; Jiang, A.; et al. The regulation of skeletal muscle fiber-type composition by betaine is associated with NFATc1/MyoD. J. Mol. Med. 2018, 96, 685–700. [Google Scholar] [CrossRef]
- Senesi, P.; Luzi, L.; Montesano, A.; Mazzocchi, N.; Terruzzi, I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J. Transl. Med. 2013, 11, 174. [Google Scholar] [CrossRef]
- Chen, R.; Zhuang, S.; Chen, Y.; Cheng, Y.; Wen, C.; Zhou, Y. Betaine improves the growth performance and muscle growth of partridge shank broiler chickens via altering myogenic gene expression and insulin-like growth factor-1 signaling pathway. Poult. Sci. 2018, 97, 4297–4305. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Xu, Z.; Wang, X.; Feng, J.; Shan, T.; Wang, Y. Betaine promotes lipid accumulation in adipogenic-differentiated skeletal muscle cells through ERK/PPARγ signalling pathway. Mol. Cell. Biochem. 2018, 447, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-A.; Zhong, R.-H.; Chen, S.; Wang, F.; Luo, Y.; Lu, X.-T.; Yishake, D.; Chen, Y.-M.; Fang, A.-P.; Zhu, H.-L. Dietary betaine intake is associated with skeletal muscle mass change over 3 years in middle-aged adults: The Guangzhou Nutrition and Health Study. Br. J. Nutr. 2021, 125, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Randell, E.; Pedram, P.; Yi, Y.; Gulliver, W.; Sun, G. Higher Dietary Choline and Betaine Intakes Are Associated with Better Body Composition in the Adult Population of Newfoundland, Canada. PLoS ONE 2016, 11, e0155403. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; A Ratamess, N.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. J. Int. Soc. Sports Nutr. 2009, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, A.O.; Ozkoc, M.; Ol, K.K.; Karimkhani, H.; Senturk, H.; Burukoglu, D.; Kanbak, G. Protective effect of betaine against skeleton muscle apoptosis in rats induced by chronic alcohol and statin consumption. Bratisl. Med. J. 2020, 121, 589–599. [Google Scholar] [CrossRef]
- Berry, G.T.; Mallee, J.J.; Kwon, H.; Rim, J.S.; Mulla, W.R.; Muenke, M.; Spinner, N.B. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): Molecular cloning and localization to chromosome 21. Genomics 1995, 25, 507–513. [Google Scholar] [CrossRef]
- Huber, K.; Walk, C.; Kühn, I.; Stein, H.; Kidd, M.; Rodehutscord, M. Chapter 4 Cellular myo-inositol metabolism. In Phytate Destruction—Consequences for Precision Animal Nutrition; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 53–60. [Google Scholar]
- Dang, N.T.; Mukai, R.; Yoshida, K.-I.; Ashida, H. D-Pinitol andmyo-Inositol Stimulate Translocation of Glucose Transporter 4 in Skeletal Muscle of C57BL/6 Mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef]
- Ortmeyer, H.K. Dietary Myoinositol Results in Lower Urine Glucose and in Lower Postprandial Plasma Glucose in Obese Insulin Resistant Rhesus Monkeys. Obes. Res. 1996, 4, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Nishiumi, S.; Yoshida, K.-I.; Ashida, H. Rat L6 myotubes as an in vitro model system to study GLUT4-dependent glucose uptake stimulated by inositol derivatives. Cytotechnology 2007, 55, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Srivastava, S.K. Aldose reductase: A novel therapeutic target for inflammatory pathologies. Int. J. Biochem. Cell Biol. 2010, 42, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Petrash, J.M. All in the family: Aldose reductase and closely related aldo-keto reductases. Cell. Mol. Life Sci. 2004, 61, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.D.; Clements, R.S.; Travis, S.B.; Oski, F.; Winegrad, A.I. Glucose utilization by the polyol pathway in human erythrocytes. Biochem. Biophys. Res. Commun. 1970, 40, 199–205. [Google Scholar] [CrossRef]
- González, R.G.; Barnett, P.; Aguayo, J.; Cheng, H.-M.; Chylack, L.T. Direct Measurement of Polyol Pathway Activity in the Ocular Lens. Diabetes 1984, 33, 196–199. [Google Scholar] [CrossRef]
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–34. [Google Scholar]
- Rittner, H.L.; Hafner, V.; Klimiuk, P.A.; Szweda, L.I.; Goronzy, J.J.; Weyand, C.M. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J. Clin. Investig. 1999, 103, 1007–1013. [Google Scholar] [CrossRef]
- Choudhary, S.; Xiao, T.; Srivastava, S.; Zhang, W.; Chan, L.; Vergara, L.; Van Kuijk, F.; Ansari, N. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol. Appl. Pharmacol. 2005, 204, 122–134. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.; Ireland, L.S.; Harrison, D.J.; Hayes, J.D. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999, 343, 487–504. [Google Scholar] [CrossRef]
- Holland, A.; Dowling, P.; Zweyer, M.; Swandulla, D.; Henry, M.; Clynes, M.; Ohlendieck, K. Proteomic profiling of cardiomyopathic tissue from the aged mdx model of Duchenne muscular dystrophy reveals a drastic decrease in laminin, nidogen and annexin. Proteomics 2013, 13, 2312–2323. [Google Scholar] [CrossRef]
- Cotter, M.; Cameron, N.; Robertson, S.; Ewing, I. Polyol pathway-related skeletal muscle contractile and morphological abnormalities in diabetic rats. Exp. Physiol. 1993, 78, 139–155. [Google Scholar] [CrossRef]
- Sanchez, O.A.; Snow, L.M.; Lowe, D.A.; Serfass, R.C.; Thompson, L.V. Effects of endurance exercise-training on single-fiber contractile properties of insulin-treated streptozotocin-induced diabetic rats. J. Appl. Physiol. 2005, 99, 472–478. [Google Scholar] [CrossRef]
- Sanchez, O.A.; Walseth, T.F.; Snow, L.M.; Serfass, R.C.; Thompson, L.V. Skeletal Muscle Sorbitol Levels in Diabetic Rats with and without Insulin Therapy and Endurance Exercise Training. Exp. Diabetes Res. 2009, 2009, 1–6. [Google Scholar] [CrossRef][Green Version]
- Ramana, K.V.; Friedrich, B.; Srivastava, S.; Bhatnagar, A. Activation of Nulcear Factor- B by Hyperglycemia in Vascular Smooth Muscle Cells Is Regulated by Aldose Reductase. Diabetes 2004, 53, 2910–2920. [Google Scholar] [CrossRef]
- Ramana, K.V.; Srivastava, S.K. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine 2006, 36, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Srivastava, S.K.; Ramana, K.V. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine 2009, 48, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S.; et al. PTC124 targets genetic disorders caused by nonsense mutations. Nat. Cell Biol. 2007, 447, 87–91. [Google Scholar] [CrossRef]
- Roy, B.; Friesen, W.J.; Tomizawa, Y.; Leszyk, J.D.; Zhuo, J.; Johnson, B.; Dakka, J.; Trotta, C.R.; Xue, X.; Mutyam, V.; et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc. Natl. Acad. Sci. USA 2016, 113, 12508–12513. [Google Scholar] [CrossRef]
- Iftikhar, M.; Frey, J.; Shohan, J.; Malek, S.; Mousa, S.A. Current and emerging therapies for Duchenne muscular dystrophy and spinal muscular atrophy. Pharmacol. Ther. 2021, 220, 107719. [Google Scholar] [CrossRef]
- Parkes, D.G.; Weisinger, R.S.; May, C.N. Cardiovascular actions of CRH and urocortin: An update. Peptides 2001, 22, 821–827. [Google Scholar] [CrossRef]
- Latchman, D.S. Urocortin. Int. J. Biochem. Cell Biol. 2002, 34, 907–910. [Google Scholar] [CrossRef]
- Fidahic, M.; Kadic, A.J.; Radić, M. Celecoxib for rheumatoid arthritis. Cochrane Datab. Syst. Rev. 2016, 6, 012095. [Google Scholar] [CrossRef]
- Millay, D.P.; Goonasekera, S.A.; Sargent, M.A.; Maillet, M.; Aronow, B.J.; Molkentin, J.D. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 19023–19028. [Google Scholar] [CrossRef]
- De Paepe, B.; De Bleecker, J.L. Cytokines and Chemokines as Regulators of Skeletal Muscle Inflammation: Presenting the Case of Duchenne Muscular Dystrophy. Mediat. Inflamm. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, B.; Creus, K.K.; Weis, J.; De Bleecker, J.L. Heat shock protein families 70 and 90 in Duchenne muscular dystrophy and inflammatory myopathy: Balancing muscle protection and destruction. Neuromuscul. Disord. 2012, 22, 26–33. [Google Scholar] [CrossRef]
- Kumar, R.; Serrette, J.; Khan, S.; Miller, A.; Thompson, E. Effects of different osmolytes on the induced folding of the N-terminal activation domain (AF1) of the glucocorticoid receptor. Arch. Biochem. Biophys. 2007, 465, 452–460. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, J.C.; Bolen, D.W.; Thompson, E.B. The Conformation of the Glucocorticoid Receptor AF1/tau1 Domain Induced by Osmolyte Binds Co-regulatory Proteins. J. Biol. Chem. 2001, 276, 18146–18152. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.G.; Van Der Poel, C.; Horvath, D.; Murphy, R.M. Taurine and Methylprednisolone Administration at Close Proximity to the Onset of Muscle Degeneration Is Ineffective at Attenuating Force Loss in the Hind-Limb of 28 Days Mdx Mice. Sports 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.D.R.; Talanian, J.L.; Shoveller, A.K.; Heigenhauser, G.J.F.; Spriet, L.L. Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J. Appl. Physiol. 2008, 105, 643–651. [Google Scholar] [CrossRef] [PubMed]

| Main Components of the DAPC Complex | DMD Patients | mdx Mouse |
|---|---|---|
| α-dystroglycan | weak | unstable |
| β-dystroglycan | unstable | |
| α-sarcoglycan | weak | weak |
| β-sarcoglycan | weak | |
| δ-sarcoglycan | weak | |
| γ-sarcoglycan | weak | |
| sarcospan | weak | weak |
| dystrophin | absent | absent |
| α1-syntrophin | weak | weak |
| β1-syntrophin | absent | |
| α-dystrobrevin | weak | absent |
| Organic Osmolyte | Physiological Role | Expression in DMD Patients/mdx Mouse | Clinical Trials |
|---|---|---|---|
| Taurine | Osmoregulation, antioxidant, modulation of Ca2+ signaling, conjugation of bile acids | DMD: ↑ taurine excretion ↑ taurine trans-porter (TauT) mdx: ↓ muscle taurine ↓ TauT | Peripartum cardiomyopathy, thalassemia major, diabetes, etc. |
| Betaine | Osmoregulation, methyldonor | DMD: expression in small regenerative and atrophic fibers | Homocystinuria, non-alcoholic fatty liver disease, etc. |
| Myo-inositol (MI) | Osmoregulation, structural base for 2nd messengers (signal transduction, component of plasma membrane) | DMD: ↑ sodium/myo-inositol transporter (SMIT) | Polycystic ovary syndrome, bipolar disorders, etc. |
| Sorbitol | Osmoregulation | DMD: ↑ Aldose reductase mdx: ↑ Aldose reductase (cardiac muscle) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbelet, S.; Merckx, C.; De Paepe, B. The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target. Biomedicines 2021, 9, 350. https://doi.org/10.3390/biomedicines9040350
Herbelet S, Merckx C, De Paepe B. The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target. Biomedicines. 2021; 9(4):350. https://doi.org/10.3390/biomedicines9040350
Chicago/Turabian StyleHerbelet, Sandrine, Caroline Merckx, and Boel De Paepe. 2021. "The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target" Biomedicines 9, no. 4: 350. https://doi.org/10.3390/biomedicines9040350
APA StyleHerbelet, S., Merckx, C., & De Paepe, B. (2021). The PKA-p38MAPK-NFAT5-Organic Osmolytes Pathway in Duchenne Muscular Dystrophy: From Essential Player in Osmotic Homeostasis, Inflammation and Skeletal Muscle Regeneration to Therapeutic Target. Biomedicines, 9(4), 350. https://doi.org/10.3390/biomedicines9040350








