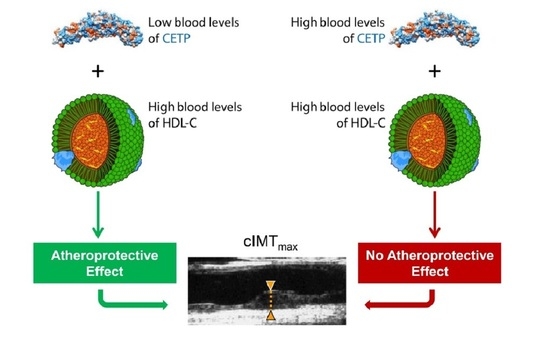
The Association between HDL-C and Subclinical Atherosclerosis Depends on CETP Plasma Concentration: Insights from the IMPROVE Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Biochemical Analyses
2.3. Ultrasonographic Variables
2.4. Genotyping and Quality Control
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Drug-Free Subjects
3.2. Association between CETP Plasma Concentration and cIMT Variables
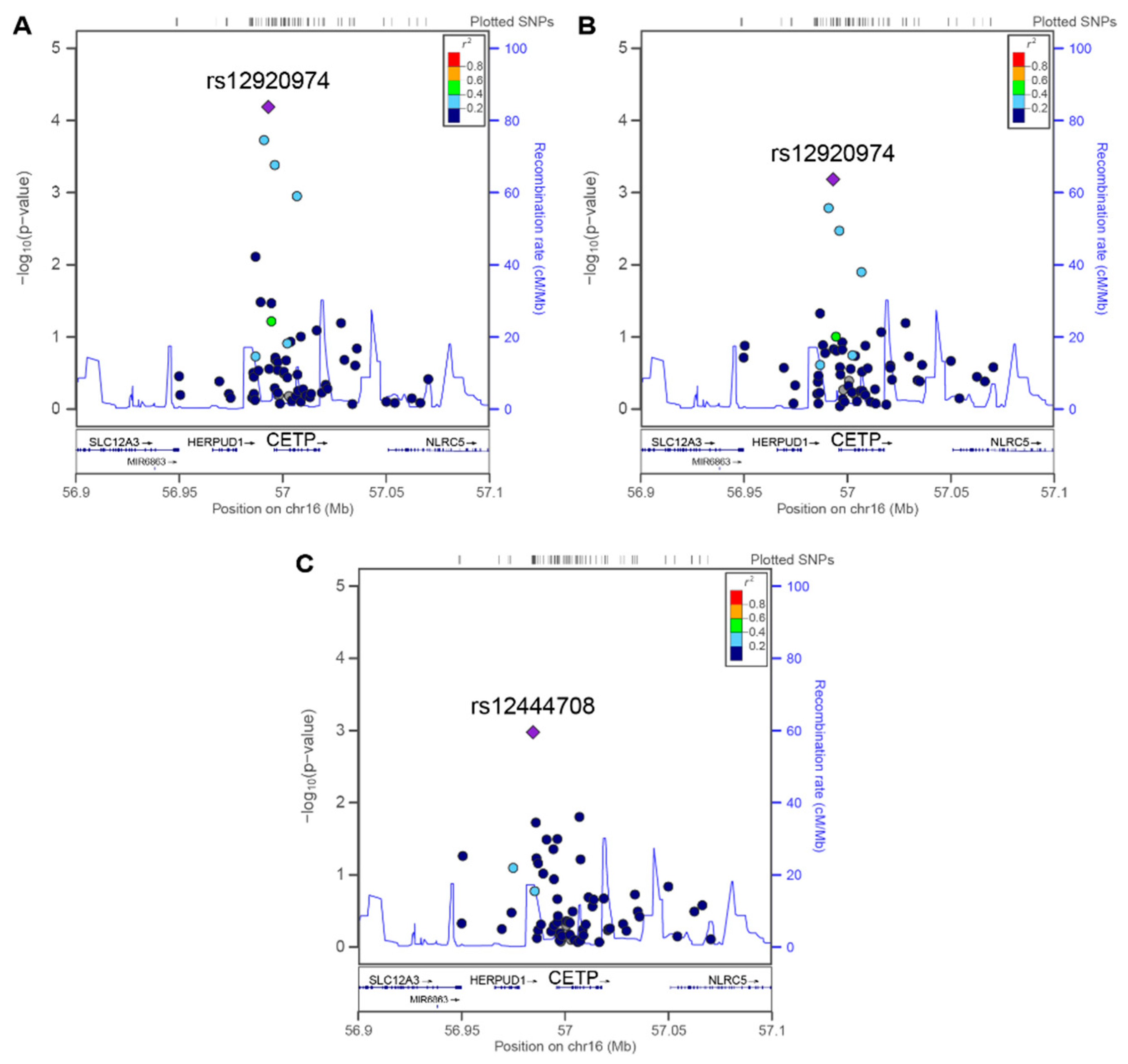
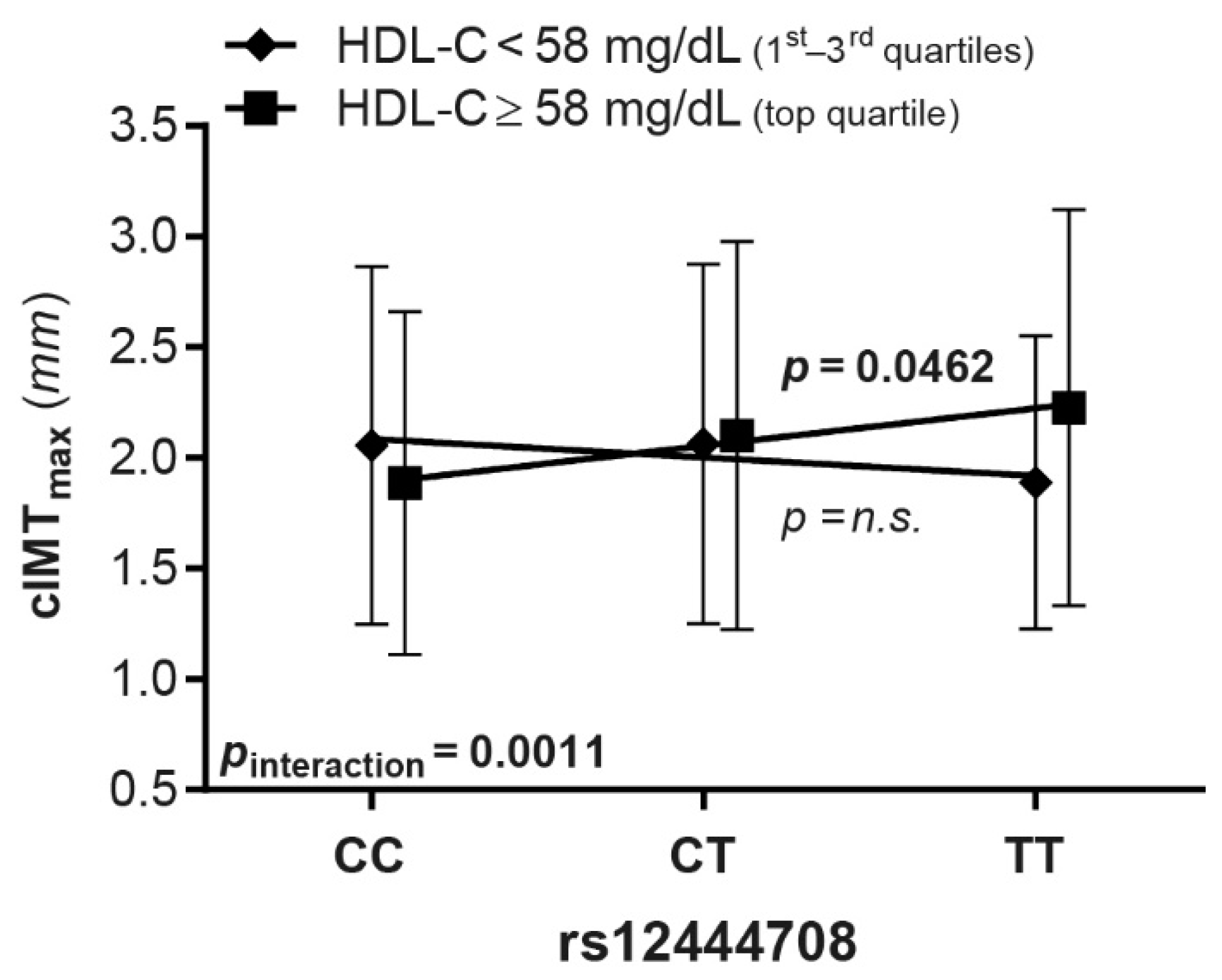
3.3. Association between CETP SNPs and cIMT Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Holm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Armitage, J.; Holmes, M.V.; Preiss, D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Bursill, C.A.; Lambert, G.; Tabet, F.; Barter, P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 2009, 50, S195–S200. [Google Scholar] [CrossRef]
- Cuchel, M.; Rader, D.J. Macrophage reverse cholesterol transport: Key to the regression of atherosclerosis? Circulation 2006, 113, 2548–2555. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Brewer, H.B., Jr.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Klerkx, A.H.; El Harchaoui, K.; van der Steeg, W.A.; Boekholdt, S.M.; Stroes, E.S.; Kastelein, J.J.; Kuivenhoven, J.A. Cholesteryl ester transfer protein (CETP) inhibition beyond raising high-density lipoprotein cholesterol levels: Pathways by which modulation of CETP activity may alter atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.C.; VandenBroek, J.M.; Cooper, P.S. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J. Lipid Res. 2004, 45, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Castilho, L.N.; Oliveira, H.C.; Cazita, P.M.; de Oliveira, A.C.; Sesso, A.; Quintao, E.C. Oxidation of LDL enhances the cholesteryl ester transfer protein (CETP)-mediated cholesteryl ester transfer rate to HDL, bringing on a diminished net transfer of cholesteryl ester from HDL to oxidized LDL. Clin. Chim. Acta 2001, 304, 99–106. [Google Scholar] [CrossRef]
- De Grooth, G.J.; Klerkx, A.H.; Stroes, E.S.; Stalenhoef, A.F.; Kastelein, J.J.; Kuivenhoven, J.A. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 2004, 45, 1967–1974. [Google Scholar] [CrossRef]
- Klerkx, A.H.; de Grooth, G.J.; Zwinderman, A.H.; Jukema, J.W.; Kuivenhoven, J.A.; Kastelein, J.J. Cholesteryl ester transfer protein concentration is associated with progression of atherosclerosis and response to pravastatin in men with coronary artery disease (REGRESS). Eur. J. Clin. Investig. 2004, 34, 21–28. [Google Scholar] [CrossRef]
- De Grooth, G.J.; Smilde, T.J.; Van Wissen, S.; Klerkx, A.H.; Zwinderman, A.H.; Fruchart, J.C.; Kastelein, J.J.; Stalenhoef, A.F.; Kuivenhoven, J.A. The relationship between cholesteryl ester transfer protein levels and risk factor profile in patients with familial hypercholesterolemia. Atherosclerosis 2004, 173, 261–267. [Google Scholar] [CrossRef]
- Okamura, T.; Sekikawa, A.; Kadowaki, T.; El-Saed, A.; Abbott, R.D.; Curb, J.D.; Edmundowicz, D.; Nakamura, Y.; Murata, K.; Kashiwagi, A.; et al. Cholesteryl ester transfer protein, coronary calcium, and intima-media thickness of the carotid artery in middle-age Japanese men. Am. J. Cardiol. 2009, 104, 818–822. [Google Scholar] [CrossRef]
- Tosheska, K.; Labudovic, D.; Jovanova, S.; Jaglikovski, B.; Alabakovska, S. Cholesteryl ester transfer protein, low density lipoprotein particle size and intima media thickness in patients with coronary heart disease. Bosn. J. Basic Med. Sci. 2011, 11, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Kuivenhoven, J.A.; Wareham, N.J.; Peters, R.J.; Jukema, J.W.; Luben, R.; Bingham, S.A.; Day, N.E.; Kastelein, J.J.; Khaw, K.T. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: The prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation 2004, 110, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.; Perton, F.G.; Dallinga-Thie, G.M.; van Roon, A.M.; Wolffenbuttel, B.H.; van Tol, A.; Dullaart, R.P. Plasma cholesteryl ester transfer is a determinant of intima-media thickness in type 2 diabetic and nondiabetic subjects: Role of CETP and triglycerides. Diabetes 2005, 54, 3554–3559. [Google Scholar] [CrossRef] [PubMed]
- Alssema, M.; Dekker, J.M.; Kuivenhoven, J.A.; Nijpels, G.; Teerlink, T.; Scheffer, P.G.; Diamant, M.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J. Elevated cholesteryl ester transfer protein concentration is associated with an increased risk for cardiovascular disease in women, but not in men, with Type 2 diabetes: The Hoorn Study. Diabet. Med. 2007, 24, 117–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borggreve, S.E.; Hillege, H.L.; Dallinga-Thie, G.M.; de Jong, P.E.; Wolffenbuttel, B.H.; Grobbee, D.E.; van Tol, A.; Dullaart, R.P.; Group, P.S. High plasma cholesteryl ester transfer protein levels may favour reduced incidence of cardiovascular events in men with low triglycerides. Eur. Heart J. 2007, 28, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Di Angelantonio, E.; Sarwar, N.; Erqou, S.; Saleheen, D.; Dullaart, R.P.; Keavney, B.; Ye, Z.; Danesh, J. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 2008, 299, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Blauw, L.L.; Li-Gao, R.; Noordam, R.; de Mutsert, R.; Trompet, S.; Berbee, J.F.P.; Wang, Y.; van Klinken, J.B.; Christen, T.; van Heemst, D.; et al. CETP (Cholesteryl Ester Transfer Protein) Concentration: A Genome-Wide Association Study Followed by Mendelian Randomization on Coronary Artery Disease. Circ. Genom. Precis. Med. 2018, 11, e002034. [Google Scholar] [CrossRef] [PubMed]
- Christen, T.; Trompet, S.; Noordam, R.; Blauw, L.L.; Gast, K.B.; Rensen, P.C.N.; Willems van Dijk, K.; Rosendaal, F.R.; de Mutsert, R.; Jukema, J.W.; et al. Mendelian randomization analysis of cholesteryl ester transfer protein and subclinical atherosclerosis: A population-based study. J. Clin. Lipidol. 2018, 12, 137–144.e131. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tardif, J.C.; Nicholls, S.J.; Revkin, J.H.; Shear, C.L.; Duggan, W.T.; Ruzyllo, W.; Bachinsky, W.B.; Lasala, G.P.; Tuzcu, E.M.; et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 2007, 356, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Fayad, Z.A.; Mani, V.; Woodward, M.; Kallend, D.; Abt, M.; Burgess, T.; Fuster, V.; Ballantyne, C.M.; Stein, E.A.; Tardif, J.C.; et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): A randomised clinical trial. Lancet 2011, 378, 1547–1559. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Group, H.T.R.C.; Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Tuzcu, E.M.; Brennan, D.M.; Tardif, J.C.; Nissen, S.E. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: Insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 2008, 118, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, D.; Nyyssonen, K.; Rauramaa, R.; de Faire, U.; Hamsten, A.; Smit, A.J.; Mannarino, E.; Humphries, S.E.; Giral, P.; Grossi, E.; et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: The IMPROVE study. Eur. Heart J. 2010, 31, 614–622. [Google Scholar] [CrossRef]
- Murakami, T.; Michelagnoli, S.; Longhi, R.; Gianfranceschi, G.; Pazzucconi, F.; Calabresi, L.; Sirtori, C.R.; Franceschini, G. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Stein, J.H. Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: A recommended update to the editorial policy at Atherosclerosis. Atherosclerosis 2020, 312, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Gertow, K.; Sennblad, B.; Strawbridge, R.J.; Ohrvik, J.; Zabaneh, D.; Shah, S.; Veglia, F.; Fava, C.; Kavousi, M.; McLachlan, S.; et al. Identification of the BCAR1-CFDP1-TMEM170A locus as a determinant of carotid intima-media thickness and coronary artery disease risk. Circ. Cardiovasc. Genet. 2012, 5, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Westfall, P.H.; Young, S.S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Purcell, S. PLINK (1.07) Documentation. 2010. Available online: http://zzz.bwh.harvard.edu/plink/dist/plink-doc-1.07.pdf (accessed on 19 February 2021).
- Kee, P.; Caiazza, D.; Rye, K.A.; Barrett, P.H.; Morehouse, L.A.; Barter, P.J. Effect of inhibiting cholesteryl ester transfer protein on the kinetics of high-density lipoprotein cholesteryl ester transport in plasma: In vivo studies in rabbits. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, M.; Twisk, J.; Hoekstra, M.; Van Rij, B.T.; Van der Lans, C.A.; Bos, I.S.; Kruijt, J.K.; Kuipers, F.; Van Berkel, T.J. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J. Biol. Chem. 2003, 278, 23699–23705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luu, T.; Emfinger, C.H.; Parks, B.A.; Shi, J.; Trefts, E.; Zeng, F.; Kuklenyik, Z.; Harris, R.C.; Wasserman, D.H.; et al. CETP Inhibition Improves HDL Function but Leads to Fatty Liver and Insulin Resistance in CETP-Expressing Transgenic Mice on a High-Fat Diet. Diabetes 2018, 67, 2494–2506. [Google Scholar] [CrossRef]
- Chantepie, S.; Bochem, A.E.; Chapman, M.J.; Hovingh, G.K.; Kontush, A. High-density lipoprotein (HDL) particle subpopulations in heterozygous cholesteryl ester transfer protein (CETP) deficiency: Maintenance of antioxidative activity. PLoS ONE 2012, 7, e49336. [Google Scholar] [CrossRef]
- Pasqualini, L.; Cortese, C.; Marchesi, S.; Siepi, D.; Pirro, M.; Vaudo, G.; Liberatoscioli, L.; Gnasso, A.; Schillaci, G.; Mannarino, E. Paraoxonase-1 activity modulates endothelial function in patients with peripheral arterial disease. Atherosclerosis 2005, 183, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Vaudo, G.; Lupattelli, G.; Pasqualini, L.; Mannarino, M.R.; Schillaci, G.; Alaeddin, A.; Paciullo, F.; Fallarino, F.; Bagaglia, F.; et al. On-treatment C-reactive protein and HDL cholesterol levels in patients at intermediate cardiovascular risk: Impact on carotid intima-media thickness. Life Sci. 2013, 93, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Manolio, T.A.; Boerwinkle, E.; O’Donnell, C.J.; Wilson, A.F. Genetics of ultrasonographic carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Wood, L.S.; Pickering, E.H.; Dechairo, B.; Hyde, C.L. High-density genotyping and functional SNP localization in the CETP gene. J. Lipid Res. 2007, 48, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, E.M.; Huffman, J.E.; Bis, J.C.; Isaacs, A.; Mulder, M.; Sabo, A.; Smith, A.V.; Demissie, S.; Manichaikul, A.; Brody, J.A.; et al. Fine mapping the CETP region reveals a common intronic insertion associated to HDL-C. NPJ Aging Mech. Dis. 2015, 1, 15011. [Google Scholar] [CrossRef]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, M.F.; Wojczynski, M.K.; Straka, R.; Kammerer, C.M.; Lee, J.H.; Kraja, A.T.; Christensen, K.; Newman, A.B.; Province, M.A.; Borecki, I.B. Genetic analysis of long-lived families reveals novel variants influencing high density-lipoprotein cholesterol. Front. Genet. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Noordam, R.; Bos, M.M.; Wang, H.; Winkler, T.W.; Bentley, A.R.; Kilpelainen, T.O.; de Vries, P.S.; Sung, Y.J.; Schwander, K.; Cade, B.E.; et al. Multi-ancestry sleep-by-SNP interaction analysis in 126,926 individuals reveals lipid loci stratified by sleep duration. Nat. Commun. 2019, 10, 5121. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Makino, N.; Sawada, S.; Otsuka, S.; Watanabe, M.; Okamoto, H.; Kamada, M.; Mizushima, A. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J. Biol. Chem. 1998, 273, 5033–5036. [Google Scholar] [CrossRef] [PubMed]

| Whole Sample (n = 552) | CETP < 1.38 µg/mL (n = 274) | CETP ≥ 1.38 µg/mL (n = 278) | p-Value | |
|---|---|---|---|---|
| Age, years | 63.8 ± 5.0 | 64.0 ± 5.0 | 63.6 ± 4.9 | 0.43 |
| Male sex, n (%) | 304 (55.1) | 151 (55.1) | 153 (55.0) | 0.53 |
| Hypertensive, n (%) | 271 (49.1) | 139 (50.7) | 132 (47.5) | 0.25 |
| Diabetes, n (%) | 67 (12.1) | 30 (10.9) | 37 (13.3) | 0.24 |
| Smoking status | ||||
| Never, n (%) | 240 (43.5) | 128 (46.7) | 112 (40.3) | |
| Former, n (%) | 195 (35.3) | 97 (35.4) | 98 (35.3) | 0.13 |
| Current, n (%) | 117 (21.2) | 49 (17.9) | 68 (24.5) | |
| BMI, kg/m2 | 26.3 ± 3.8 | 26.2 ± 3.5 | 26.4 ± 4.1 | 0.59 |
| Total cholesterol, mg/dL | 233 ± 39 | 231 ± 39 | 234 ± 39 | 0.49 |
| LDL cholesterol, mg/dL | 158 ± 34 | 156 ± 35 | 159 ± 33 | 0.37 |
| HDL cholesterol, mg/dL | 50 ± 15 | 48 ± 15 | 52 ± 15 | 0.007 |
| Triglycerides, mg/dLa | 110 (77–158) | 119 (82–170) | 102 (74–142) | <0.0001 |
| CETP, µg/mL | 1.40 ± 0.33 | 1.14 ± 0.18 | 1.65 ± 0.23 | - |
| cIMTmax | 1.76 (1.35–2.31) | 1.76 (1.39–2.31) | 1.84 (1.35–2.31) | 0.87 |
| cIMTmean–max | 1.32 (1.12–1.57) | 1.31 (1.13–1.54) | 1.33 (1.12–1.64) | 0.69 |
| PF CC-IMTmean | 0.72 (0.65–0.78) | 0.72 (0.65–0.78) | 0.72 (0.65–0.76) | 0.26 |
| Full Cohort (n = 3436) | HDL-C < 58 mg/dL (n = 2673) | HDL-C ≥ 58 mg/dL (n = 763) | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Position | A1 | β | puncorr | EMP2 | β | puncorr | EMP2 | β | puncorr | EMP2 | β | p-Value |
| rs12444708 | 56950570, intergenic | T | 0.005 | 0.3410 | 1 | −0.005 | 0.4592 | 1 | 0.037 | 0.0011 | 0.0462 | 0.041 | 0.0011 |
| rs9938160 | 56950678, intergenic | C | −0.002 | 0.6733 | 1 | 0.004 | 0.3746 | 1 | −0.021 | 0.0205 | 0.5504 | −0.025 | 0.0112 |
| rs72786786 | 56951602, intergenic | A | −0.011 | 0.0085 | 0.3084 | −0.009 | 0.0515 | 0.8517 | −0.015 | 0.0758 | 0.9403 | −0.005 | 0.6148 |
| rs173539 | 56954132, intergenic | T | −0.009 | 0.0360 | 0.7479 | −0.006 | 0.1826 | 0.9993 | −0.014 | 0.1043 | 0.9788 | −0.007 | 0.4636 |
| rs34760410 | 56955723, intergenic | T | 0.023 | 0.0002 | 0.0100 | 0.022 | 0.0018 | 0.0740 | 0.028 | 0.0358 | 0.7430 | 0.004 | 0.7794 |
| rs12920974 | 56959113, promoter | T | 0.016 | 0.0001 | 0.0034 | 0.016 | 0.0007 | 0.0310 | 0.019 | 0.0479 | 0.8311 | 0.001 | 0.9171 |
| rs3764261 | 56959412, promoter | A | −0.009 | 0.0372 | 0.7587 | −0.007 | 0.1691 | 0.9980 | −0.013 | 0.1254 | 0.9892 | −0.006 | 0.5493 |
| rs12708968 | 56960907, promoter | C | 0.021 | 0.0005 | 0.0213 | 0.019 | 0.0037 | 0.1458 | 0.026 | 0.0351 | 0.7363 | 0.006 | 0.6664 |
| rs12708974 | 56971638, intron 7 | T | 0.019 | 0.0012 | 0.0553 | 0.016 | 0.0137 | 0.4151 | 0.030 | 0.0171 | 0.4945 | 0.011 | 0.4152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, G.I.; Bianconi, V.; Bonomi, A.; Simonelli, S.; Amato, M.; Frigerio, B.; Ravani, A.; Vitali, C.; Sansaro, D.; Coggi, D.; et al. The Association between HDL-C and Subclinical Atherosclerosis Depends on CETP Plasma Concentration: Insights from the IMPROVE Study. Biomedicines 2021, 9, 286. https://doi.org/10.3390/biomedicines9030286
Colombo GI, Bianconi V, Bonomi A, Simonelli S, Amato M, Frigerio B, Ravani A, Vitali C, Sansaro D, Coggi D, et al. The Association between HDL-C and Subclinical Atherosclerosis Depends on CETP Plasma Concentration: Insights from the IMPROVE Study. Biomedicines. 2021; 9(3):286. https://doi.org/10.3390/biomedicines9030286
Chicago/Turabian StyleColombo, Gualtiero I., Vanessa Bianconi, Alice Bonomi, Sara Simonelli, Mauro Amato, Beatrice Frigerio, Alessio Ravani, Cecilia Vitali, Daniela Sansaro, Daniela Coggi, and et al. 2021. "The Association between HDL-C and Subclinical Atherosclerosis Depends on CETP Plasma Concentration: Insights from the IMPROVE Study" Biomedicines 9, no. 3: 286. https://doi.org/10.3390/biomedicines9030286
APA StyleColombo, G. I., Bianconi, V., Bonomi, A., Simonelli, S., Amato, M., Frigerio, B., Ravani, A., Vitali, C., Sansaro, D., Coggi, D., Mannarino, M. R., Savonen, K. P., Kurl, S., Gigante, B., Smit, A. J., Giral, P., Tremoli, E., Calabresi, L., Veglia, F., ... on behalf of the IMPROVE Study Group. (2021). The Association between HDL-C and Subclinical Atherosclerosis Depends on CETP Plasma Concentration: Insights from the IMPROVE Study. Biomedicines, 9(3), 286. https://doi.org/10.3390/biomedicines9030286