The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis
Abstract
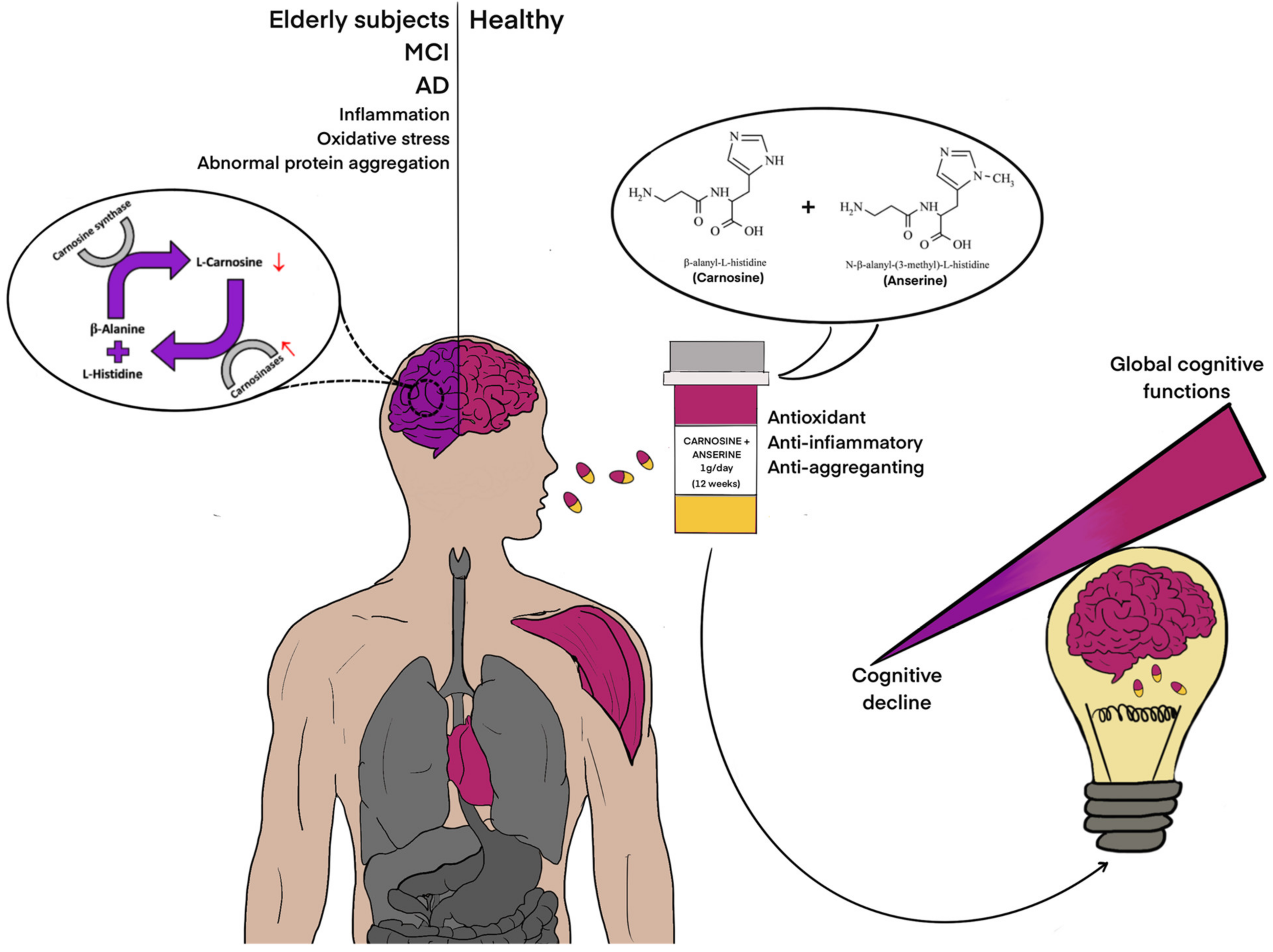
1. Introduction
2. Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Study Quality and Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
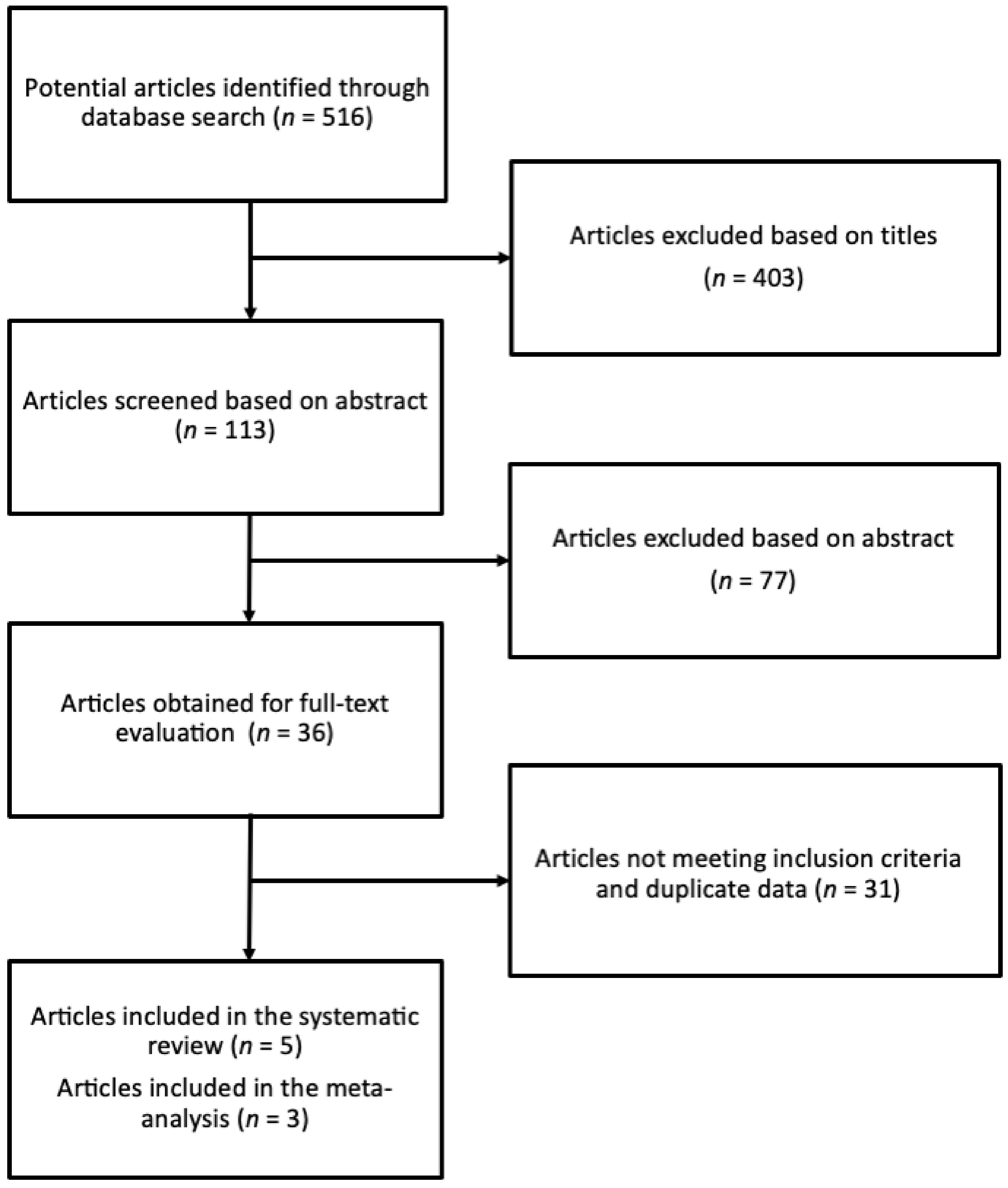
3.1. Study Identification and Selection Process
3.2. Study Quality Assessment
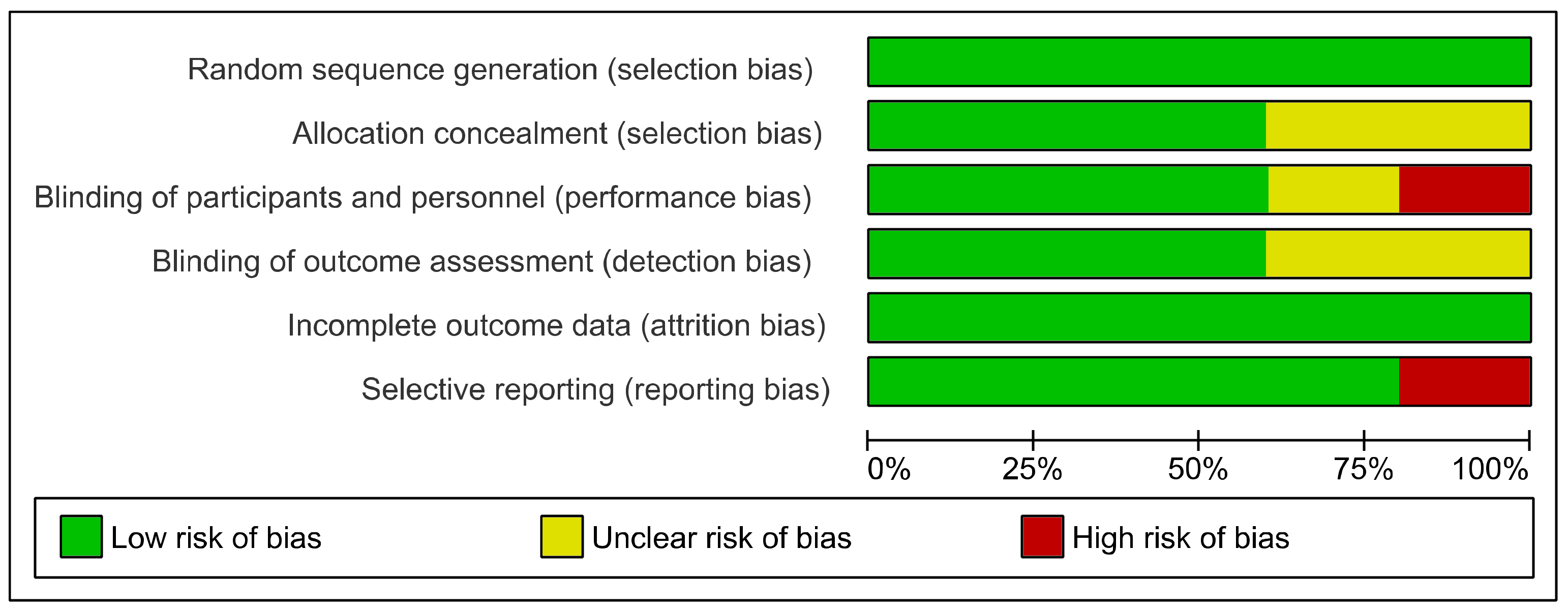
3.3. Risk of Bias
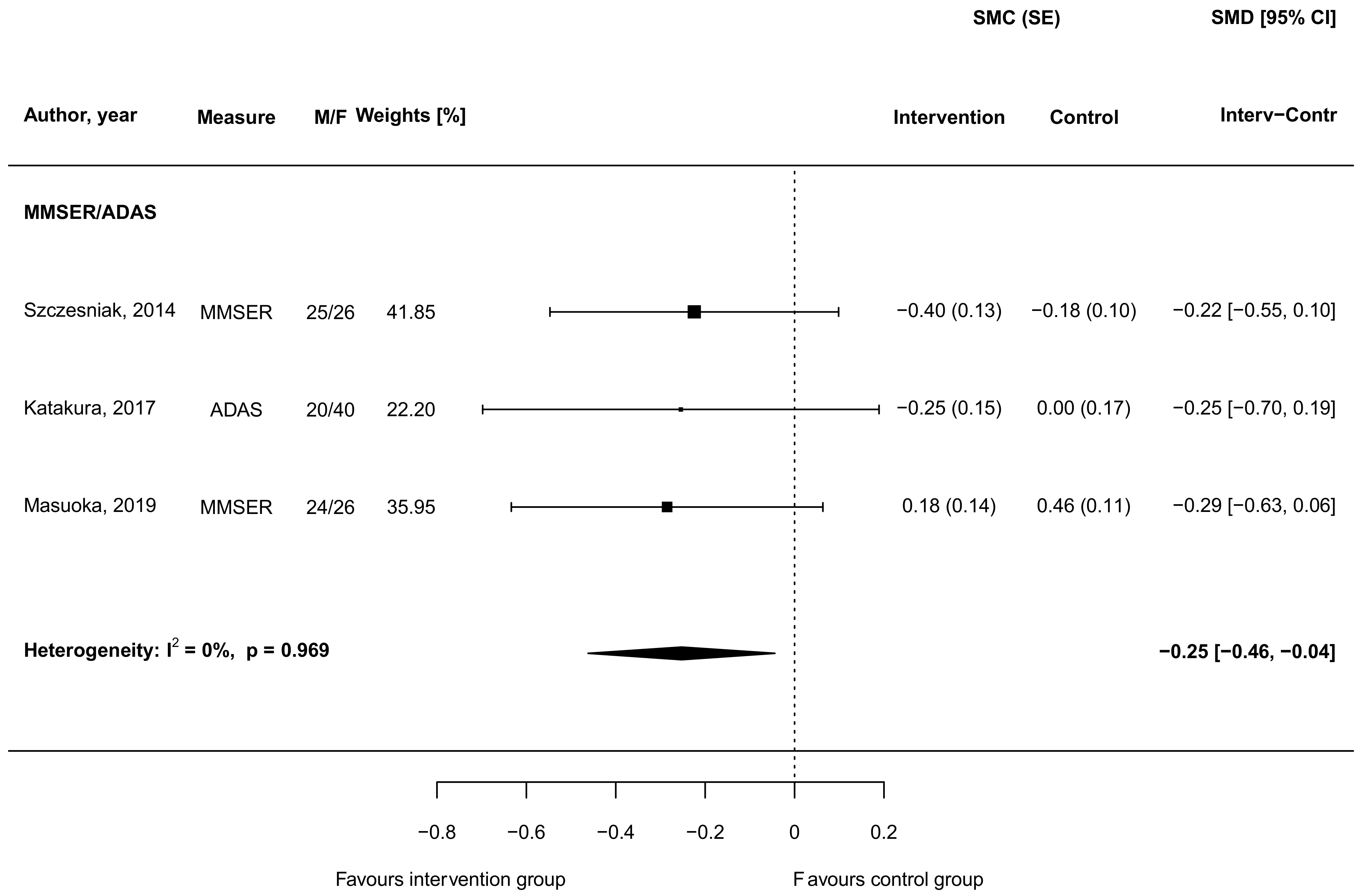
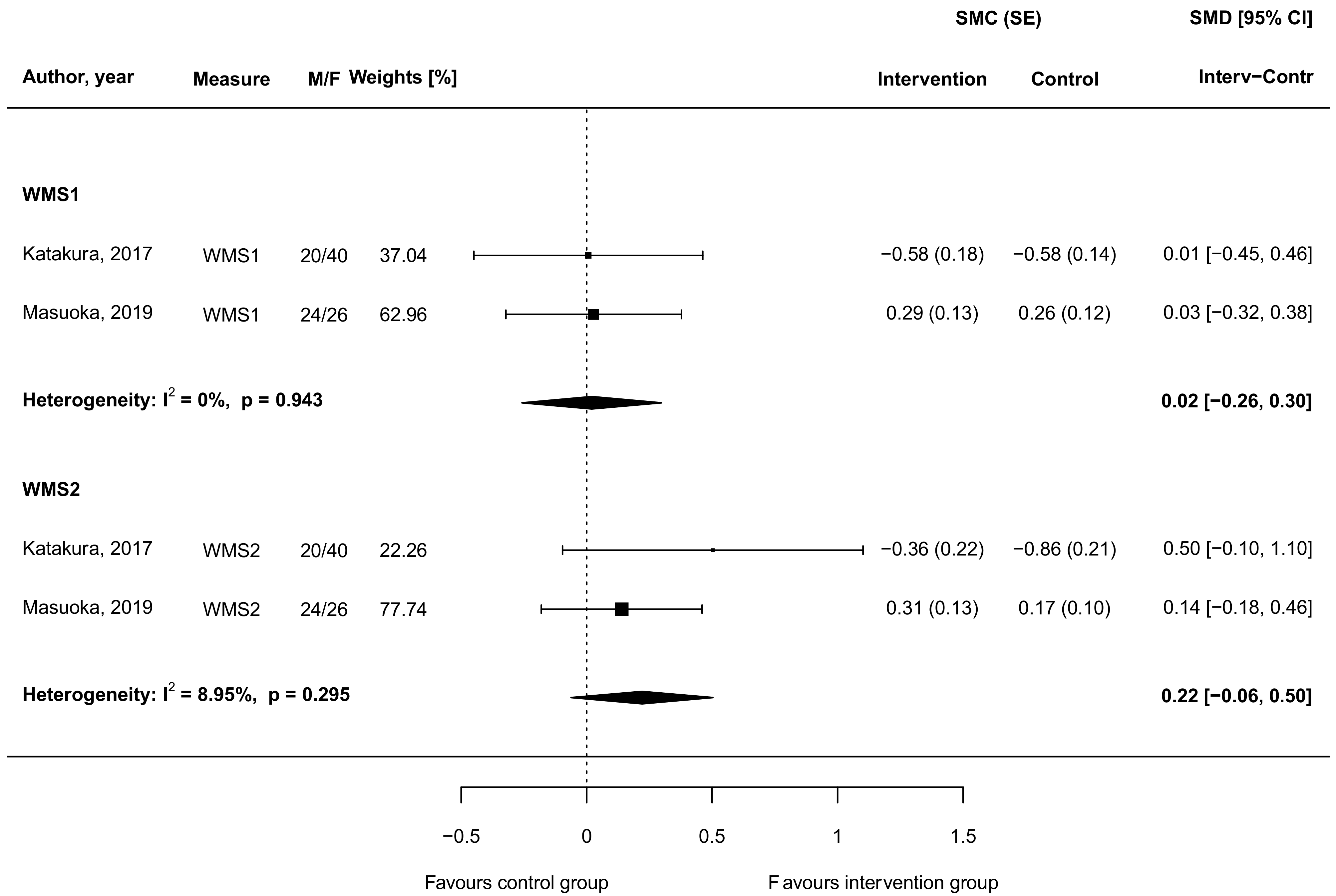
3.4. Carnosine Supplementation and Cognitive and Memory Function
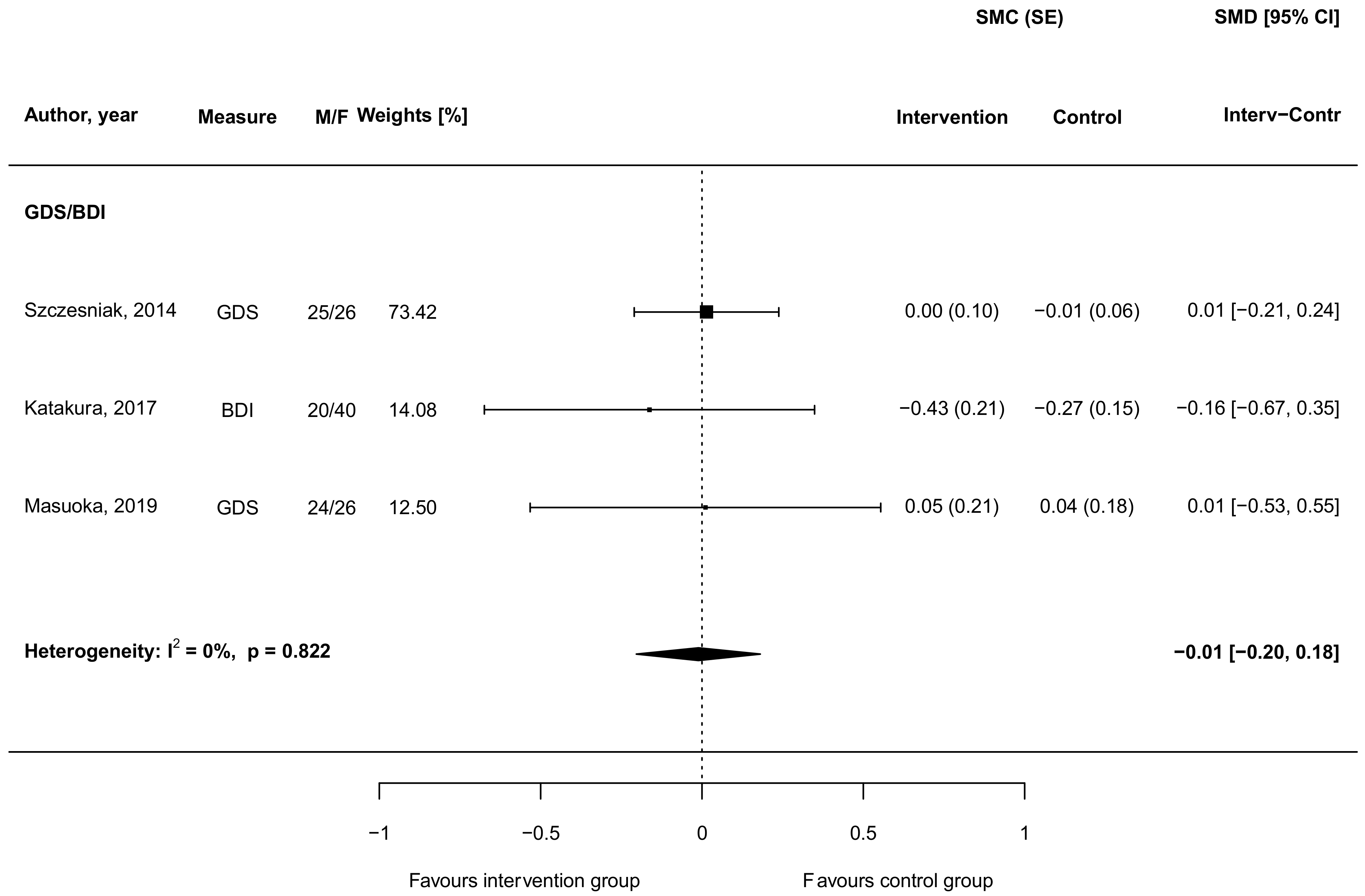
3.5. Carnosine Supplementation and Depressive Symptoms
3.6. Carnosine Supplementation and Mood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gulewitsch, W.; Amiradžibi, S. Ueber das carnosin, eine neue organische base des fleischextractes. Ber. Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef]
- Kalyankar, G.D.; Meister, A. Enzymatic synthesis of carnosine and related β-alanyl and γ-aminobutyryl peptides. J. Biol. Chem. 1959, 234, 3210–3218. [Google Scholar] [CrossRef]
- Winnick, R.; Winnick, T. Carnosine-anserine synthetase of muscle i. Preparation and properties of a soluble enyzme from chick muscle. Biochim. Biophys. Acta 1959, 31, 47–55. [Google Scholar] [CrossRef]
- Menon, K.; Mousa, A.; de Courten, B. Effects of supplementation with carnosine and other histidine-containing dipeptides on chronic disease risk factors and outcomes: Protocol for a systematic review of randomised controlled trials. BMJ Open 2018, 8, e020623. [Google Scholar] [CrossRef]
- Parker, C.J., Jr.; Ring, E. A comparative study of the effect of carnosine on myofibrillar-atpase activity of vertebrate and invertebrate muscles. Comp. Biochem. Physiol. 1970, 37, 413–419. [Google Scholar] [CrossRef]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as atp-grasp domain-containing protein 1 (atpgd1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Preston, J.E.; Himsworth, D.T.; Worthington, V.C.; Keown, M.; Michaelis, J.; Lawrence, J.; Mateen, A.; Allende, L.; Eagles, P.A.; et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. [Google Scholar] [CrossRef]
- Mannion, A.F.; Jakeman, P.M.; Dunnett, M.; Harris, R.C.; Willan, P.L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 47–50. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Calabrese, V.; Guarino, F.; Cavallaro, M.; Cornelius, C.; De Pinto, V.; Rizzarelli, E. Carnosinase levels in aging brain: Redox state induction and cellular stress response. Antioxid. Redox Signal. 2009, 11, 2759–2775. [Google Scholar] [CrossRef] [PubMed]
- Lenney, J.F.; George, R.P.; Weiss, A.M.; Kucera, C.M.; Chan, P.W.; Rinzler, G.S. Human serum carnosinase: Characterization, distinction from cellular carnosinase, and activation by cadmium. Clin. Chim. Acta 1982, 123, 221–231. [Google Scholar] [CrossRef]
- Lenney, J.F.; Peppers, S.C.; Kucera-Orallo, C.M.; George, R.P. Characterization of human tissue carnosinase. Biochem. J. 1985, 228, 653–660. [Google Scholar] [CrossRef]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.L.; Hansen, S.; Tischler, B.; Bunting, R.; Berry, K. Carnosinemia. A new metabolic disorder associated with neurologic disease and mental defect. N. Engl. J. Med. 1967, 277, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Bessman, S.P.; Baldwin, R. Imidazole aminoaciduria in cerebromacular degeneration. Science 1962, 135, 789–791. [Google Scholar] [CrossRef]
- Levenson, J.; Lindahl-Kiessling, K.; Rayner, S. Carnosine excretion in juvenile amaurotic idiocy. Lancet 1964, 284, 756–757. [Google Scholar] [CrossRef]
- Banerjee, S.; Poddar, M.K. Carnosine research in relation to aging brain and neurodegeneration: A blessing for geriatrics and their neuronal disorders. Arch. Gerontol. Geriatr. 2020, 91, 104239. [Google Scholar] [CrossRef]
- Tiedje, K.; Stevens, K.; Barnes, S.; Weaver, D. B-alanine as a small molecule neurotransmitter. Neurochem. Int. 2010, 57, 177–188. [Google Scholar] [CrossRef]
- Mal’tseva, V.V.; Sergienko, V.V.; Stvolinskii, S.L. The effect of carnosine on hematopoietic stem cell activity in irradiated animals. Biokhimiia 1992, 57, 1378–1382. [Google Scholar] [PubMed]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine raw 264.7 macrophages. Mol. Cell Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef]
- Hasanein, P.; Felegari, Z. Chelating effects of carnosine in ameliorating nickel-induced nephrotoxicity in rats. Can. J. Physiol. Pharm. 2017, 95, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Antholine, W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Phys. Chem. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of pro-oxidant and pro-inflammatory activities of m1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases pma-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Pepper, E.D.; Farrell, M.J.; Nord, G.; Finkel, S.E. Antiglycation effects of carnosine and other compounds on the long-term survival of escherichia coli. Appl. Environ. Microbiol. 2010, 76, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Gallant, S.C.; Sukhich, G.T. Carnosine, the protective, anti-aging peptide. Biosci. Rep. 1999, 19, 581–587. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, Y.; Bao, Y.; Xu, H.; Cheng, J.; Wang, B.; Shen, Y.; Chen, Z.; Lyu, J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to ogd/recovery. Brain Res. Bull. 2016, 124, 76–84. [Google Scholar] [CrossRef]
- Marsland, A.L.; Gianaros, P.J.; Kuan, D.C.; Sheu, L.K.; Krajina, K.; Manuck, S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015, 48, 195–204. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the common biological link between depression and cardiovascular diseases: Can carnosine exert a protective role? Curr. Med. Chem. 2019, 27, 1782–1880. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Kramer, J.H. Longitudinal inflammation, cognitive decline, and alzheimer’s disease: A mini-review. Clin. Pharmacol. Ther. 2014, 96, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased nrf2 signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef]
- Droge, W.; Schipper, H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein aggregation in the brain: The molecular basis for alzheimer’s and parkinson’s diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Espa, E.; Clemensson, E.K.H.; Luk, K.C.; Heuer, A.; Björklund, T.; Cenci, M.A. Seeding of protein aggregation causes cognitive impairment in rat model of cortical synucleinopathy. Mov. Disord. 2019, 34, 1699–1710. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. Beta-alanyl-l-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Ahshin-Majd, S.; Zamani, S.; Kiamari, T.; Kiasalari, Z.; Baluchnejadmojarad, T.; Roghani, M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 2016, 86, 102–111. [Google Scholar] [CrossRef]
- Ma, J.; Xiong, J.Y.; Hou, W.W.; Yan, H.J.; Sun, Y.; Huang, S.W.; Jin, L.; Wang, Y.; Hu, W.W.; Chen, Z. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci. Ther. 2012, 18, 745–753. [Google Scholar] [CrossRef]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xtg-ad mice. PLoS ONE 2011, 6, e17971. [Google Scholar]
- Boldyrev, A.A. Carnosine and Oxidative Stress in Cells and Tissues; Nova Publishers: Hauppauge, NY, USA, 2007. [Google Scholar]
- Gardner, M.L.; Illingworth, K.M.; Kelleher, J.; Wood, D. Intestinal absorption of the intact peptide carnosine in man, and comparison with intestinal permeability to lactulose. J. Physiol. 1991, 439, 411–422. [Google Scholar] [CrossRef]
- Goto, K.; Maemura, H.; Takamatsu, K.; Ishii, N. Hormonal responses to resistance exercise after ingestion of carnosine and anserine. J. Strength Cond. Res. 2011, 25, 398–405. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: A randomized controlled trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of anserine/carnosine supplementation on mild cognitive impairment with apoe4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef]
- Small, B.J.; Rawson, K.S.; Martin, C.; Eisel, S.L.; Sanberg, C.D.; McEvoy, C.L.; Sanberg, P.R.; Shytle, R.D.; Tan, J.; Bickford, P.C. Nutraceutical intervention improves older adults’ cognitive functioning. Rejuvenation Res. 2014, 17, 27–32. [Google Scholar] [CrossRef]
- Cornelli, U. Treatment of alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener. Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily carnosine and anserine supplementation alters verbal episodic memory and resting state network connectivity in healthy elderly adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of anserine/carnosine supplementation on verbal episodic memory in elderly people. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; de Courten, B. The potential of carnosine in brain-related disorders: A comprehensive review of current evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.J. Synthesizing standardized mean-change measures. Br. J. Math. Stat. Psychol. 1988, 41, 257–278. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine ccl24 in peripheral blood mononuclear cells from elderly people. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Shirotsuki, K.; Nonaka, Y.; Abe, K.; Adachi, S.I.; Adachi, S.; Kuboki, T.; Nakao, M. The effect for japanese workers of a self-help computerized cognitive behaviour therapy program with a supplement soft drink. Biopsychosoc. Med. 2017, 11, 23. [Google Scholar] [CrossRef]
- Prokopieva, V.D.; Yarygina, E.G.; Bokhan, N.A.; Ivanova, S.A. Use of carnosine for oxidative stress reduction in different pathologies. Oxid. Med. Cell Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef]
- Kubota, M.; Kobayashi, N.; Sugizaki, T.; Shimoda, M.; Kawahara, M.; Tanaka, K.-i. Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of parkinson’s disease. PLoS ONE 2020, 15, e0240448. [Google Scholar] [CrossRef]
- Fresta, C.G.; Hogard, M.L.; Caruso, G.; Melo Costa, E.E.; Lazzarino, G.; Lunte, S.M. Monitoring carnosine uptake by raw 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents aβ-induced oxidative stress and inflammation in microglial cells: A key role of tgf-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B. Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Ann. N. Y. Acad. Sci. 2010, 1207, 155–162. [Google Scholar] [CrossRef]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafò, A.; Torrisi, S.A.; Leggio, G.M.; et al. Neurobiological links between depression and ad: The role of tgf-β1 signaling as a new pharmacological target. Pharm. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Kipnis, J. Learning and memory… And the immune system. Learn Mem. 2013, 20, 601–606. [Google Scholar] [CrossRef]
- Fung, I.T.H.; Sankar, P.; Zhang, Y.; Robison, L.S.; Zhao, X.; D’Souza, S.S.; Salinero, A.E.; Wang, Y.; Qian, J.; Kuentzel, M.L.; et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J. Exp. Med. 2020, 217, 217. [Google Scholar] [CrossRef]
- Constantinidou, F.; Zaganas, I.; Papastefanakis, E.; Kasselimis, D.; Nidos, A.; Simos, P.G. Age-related decline in verbal learning is moderated by demographic factors, working memory capacity, and presence of amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2014, 20, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.I.; Smart, C.M.; Gawryluk, J.R. Subjective cognitive decline and apoe ɛ4: A systematic review. J. Alzheimers Dis. 2018, 65, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Kubomura, D.; Matahira, Y.; Masui, A.; Matsuda, H. Intestinal absorption and blood clearance of l-histidine-related compounds after ingestion of anserine in humans and comparison to anserine-containing diets. J. Agric. Food Chem. 2009, 57, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, F.; Convertino, M.; Magno, A.; Caflisch, A.; Corazza, A.; Haridas, H.; Esposito, G.; Cataldo, S.; Pignataro, B.; Milardi, D.; et al. Carnosine inhibits aβ(42) aggregation by perturbing the h-bond network in and around the central hydrophobic cluster. Chembiochem 2013, 14, 583–592. [Google Scholar] [CrossRef]
- Aloisi, A.; Barca, A.; Romano, A.; Guerrieri, S.; Storelli, C.; Rinaldi, R.; Verri, T. Anti-aggregating effect of the naturally occurring dipeptide carnosine on aβ1-42 fibril formation. PLoS ONE 2013, 8, e68159. [Google Scholar] [CrossRef] [PubMed]
- Kepchia, D.; Huang, L.; Dargusch, R.; Rissman, R.A.; Shokhirev, M.N.; Fischer, W.; Schubert, D. Diverse proteins aggregate in mild cognitive impairment and alzheimer’s disease brain. Alzheimers Res. Ther. 2020, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, K.; Fujii, K.; Matsumoto, T.; Sato, M.; Morimatsu, F.; Tashiro, K.; Kuhara, S.; Katakura, Y. Mechanisms and consequences of carnosine-induced activation of intestinal epithelial cells. J. Funct. Foods 2015, 13, 32–37. [Google Scholar] [CrossRef]
- Bosco, P.; Ferri, R.; Salluzzo, M.G.; Castellano, S.; Signorelli, M.; Nicoletti, F.; Nuovo, S.D.; Drago, F.; Caraci, F. Role of the transforming-growth-factor-β1 gene in late-onset alzheimer’s disease: Implications for the treatment. Curr. Genom. 2013, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Siuda, J.; Patalong-Ogiewa, M.; Żmuda, W.; Targosz-Gajniak, M.; Niewiadomska, E.; Matuszek, I.; Jędrzejowska-Szypułka, H.; Lewin-Kowalik, J.; Rudzińska-Bar, M. Cognitive impairment and bdnf serum levels. Neurol. Neurochir. Polska 2017, 51, 24–32. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. L-carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-alpha levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N. Bidirectional interactions between diabetes and alzheimer’s disease. Neurochem. Int. 2017, 108, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell Biochem. 2017, 425, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro Costa, E.; Diniz, B.S. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: Systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2016, 31, 905–911. [Google Scholar] [CrossRef]
- Baune, B.T.; Sluth, L.B.; Olsen, C.K. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: A short-term, randomized, double-blind, exploratory study. J. Affect. Disord. 2018, 229, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Araminia, B.; Shalbafan, M.; Mortezaei, A.; Shirazi, E.; Ghaffari, S.; Sahebolzamani, E.; Mortazavi, S.H.; Shariati, B.; Ardebili, M.E.; Aqamolaei, A.; et al. L-carnosine combination therapy for major depressive disorder: A randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 2020, 267, 131–136. [Google Scholar] [CrossRef] [PubMed]
| PICOS | Description |
|---|---|
| P (Population) | Men and/or women, adults. |
| I (Intervention) | Carnosine supplementation (carnosine alone or combined with other treatment). |
| C (Comparison) | Carnosine supplementation group (carnosine alone or combined with other treatment) versus placebo/control group. |
| O (Outcomes) | Changes in cognitive function, depressive symptoms, and overall mental health. Long term changes rather than acute effect. |
| S (Study design) | Systematic review with meta-analysis. |
| Author and Publication Year | Study Design, Country | Sample Size, Trial Duration | Sex, Age (mean ± SD) | Population Characteristics | Intervention (and Doses) | Measured Outcomes of Interest | NIH Quality Assessment |
|---|---|---|---|---|---|---|---|
| Szcześniak et al. 2014 | Double-blind randomized placebo-controlled trial, Poland | 51, 13 weeks | MF, 81.0 ± 7.0 y intervention group, 80.5 ± 7.5 y control group | Nursing home residents, MMSE score >15 | 1 g of anserine/carnosine (2:1 ratio); once a day | Cognitive function (MMSE, STMS), depressive symptoms (GDS), dementia (CDR) | Good |
| Cornelli 2010 | Double-blind randomized controlled trial, USA | 48, 6 months | MF, 75.0 ± 4.2 y intervention group, 74.0 ± 4.9 y control group | Patients with diagnosis of probable AD, MMSE score >21 | Formula F (100 mg carnosine and antioxidant compounds: vitamins B, vitamin C and E, coenzyme Q10, beta-carotene, selenium, l-cysteine, Ginko biloba); once per day | Cognitive function (MMSE) | Good |
| Katakura et al. 2017 | Double-blind randomized placebo-controlled trial, Japan | 60, 3 months | MF, 60.4 ± 2.1 y intervention group, 65.3 ± 1.6 y control group | Healthy elderly volunteers | 1 g of anserine/carnosine (3:1 ratio); twice a day | Cognitive function (MMSE, MCS), Alzheimer’s disease (ADAS), memory (WMS-LM1, WMS-LM2), depressive symptoms (BDI) | Good |
| Masuoka et al. 2019 | Double-blind randomized placebo-controlled trial, Japan | 50, 12 weeks | MF, 72.9 ± 8.8 y intervention group, 73.6 ± 6.1 y control group | Outpatients with MCI, MMSE >23 | 750 mg anserine and 250 mg carnosine; once a day | Cognitive function (MMSE), Alzheimer’s disease (ADAS), dementia (CDR), memory (WMS), depressive symptoms (GDS), | Good |
| Shirotsuki et al. 2017 | Randomized placebo- controlled trial, Japan | 72, 6 weeks | MF, 37.88 ± 9.15 y intervention group, 38.35 ± 8.83 y control group | Healthy full-time office workers | Supplement drink with 200 mg carnosine and computerized cognitive behavior therapy; once a day | Mood (POMS) | Fair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. https://doi.org/10.3390/biomedicines9030253
Caruso G, Godos J, Castellano S, Micek A, Murabito P, Galvano F, Ferri R, Grosso G, Caraci F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines. 2021; 9(3):253. https://doi.org/10.3390/biomedicines9030253
Chicago/Turabian StyleCaruso, Giuseppe, Justyna Godos, Sabrina Castellano, Agnieszka Micek, Paolo Murabito, Fabio Galvano, Raffaele Ferri, Giuseppe Grosso, and Filippo Caraci. 2021. "The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis" Biomedicines 9, no. 3: 253. https://doi.org/10.3390/biomedicines9030253
APA StyleCaruso, G., Godos, J., Castellano, S., Micek, A., Murabito, P., Galvano, F., Ferri, R., Grosso, G., & Caraci, F. (2021). The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines, 9(3), 253. https://doi.org/10.3390/biomedicines9030253










