Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Husbandry
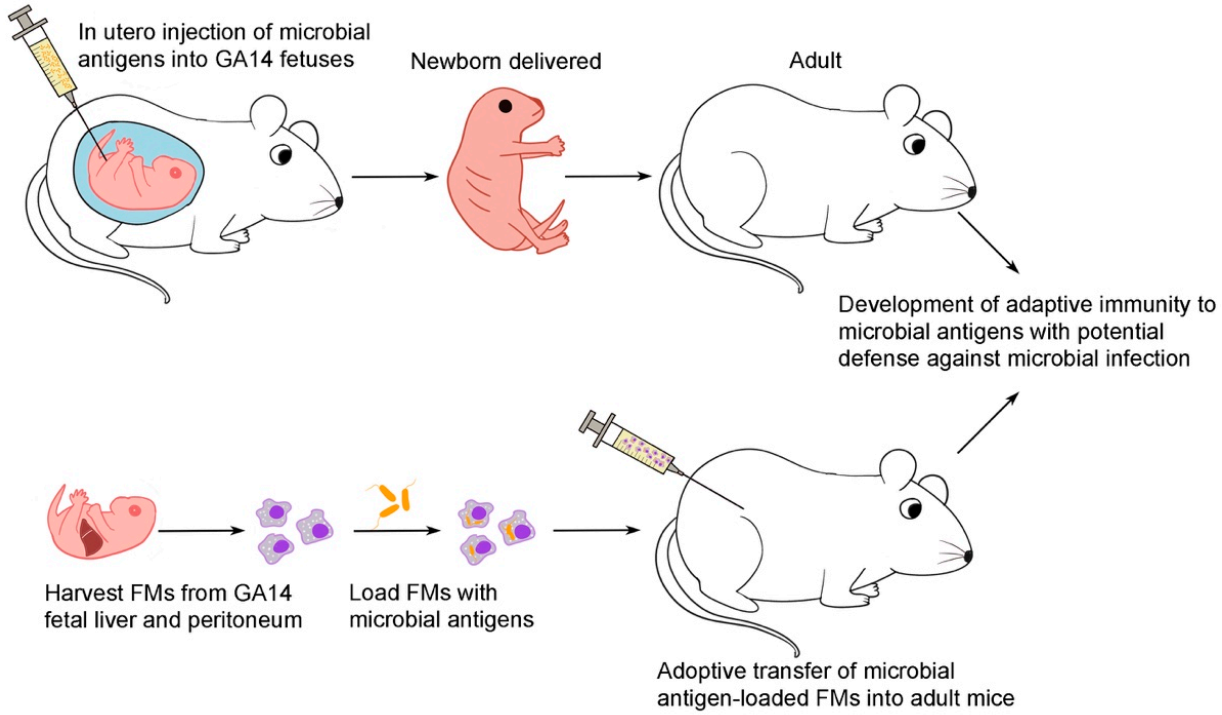
2.2. In Utero Injection [20,21]
2.3. Harvest of Gestational Day 14 FMs from the Liver and Peritoneum [17]
2.4. Adoptive Transfer of Flagellin or Bacteria-Loaded FMs
2.5. Intraperitoneal Challenge of S. typhimurium in Mice
2.6. Electron Microscopic Examination for Endocytosis of FMs
2.7. ELISA for Serum Anti-Flagellin and Anti-LPS IgG1 Levels
2.8. Proliferation of Lymphocytes in Response to Flagellin
2.9. Quantification of IFNγ, IL4, and IL5 Cytokines
2.10. Statistical Analyses
3. Results
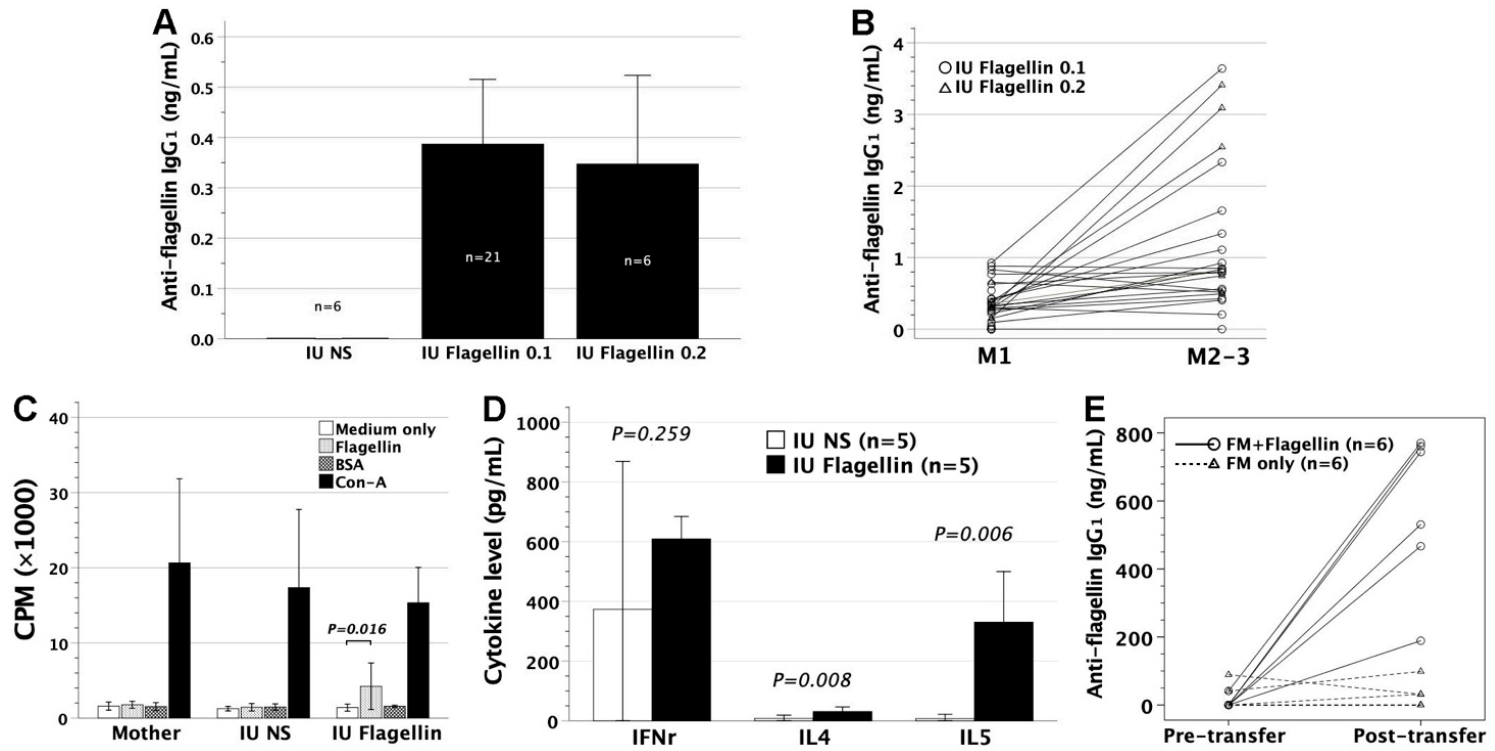
3.1. In Utero Injection of Flagellin in FVB/N Mice
3.2. Adoptive Transfer of Gestational Day 14 FMs Pulsed with Flagellin into FVB/N Recipients
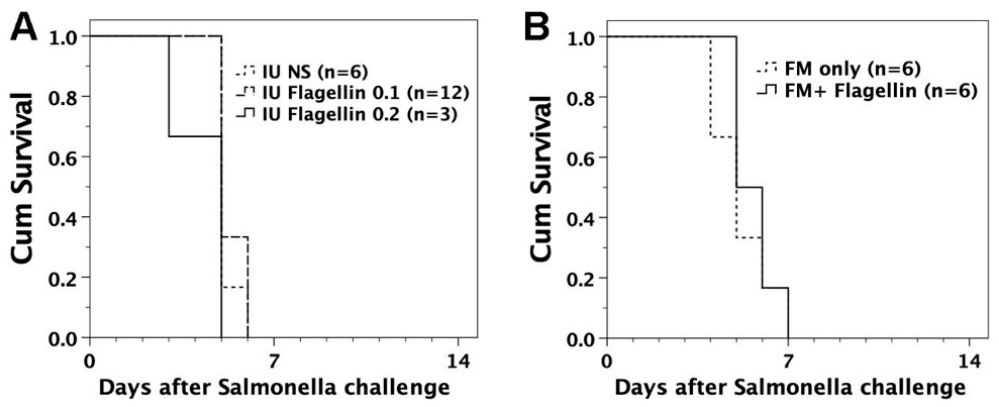
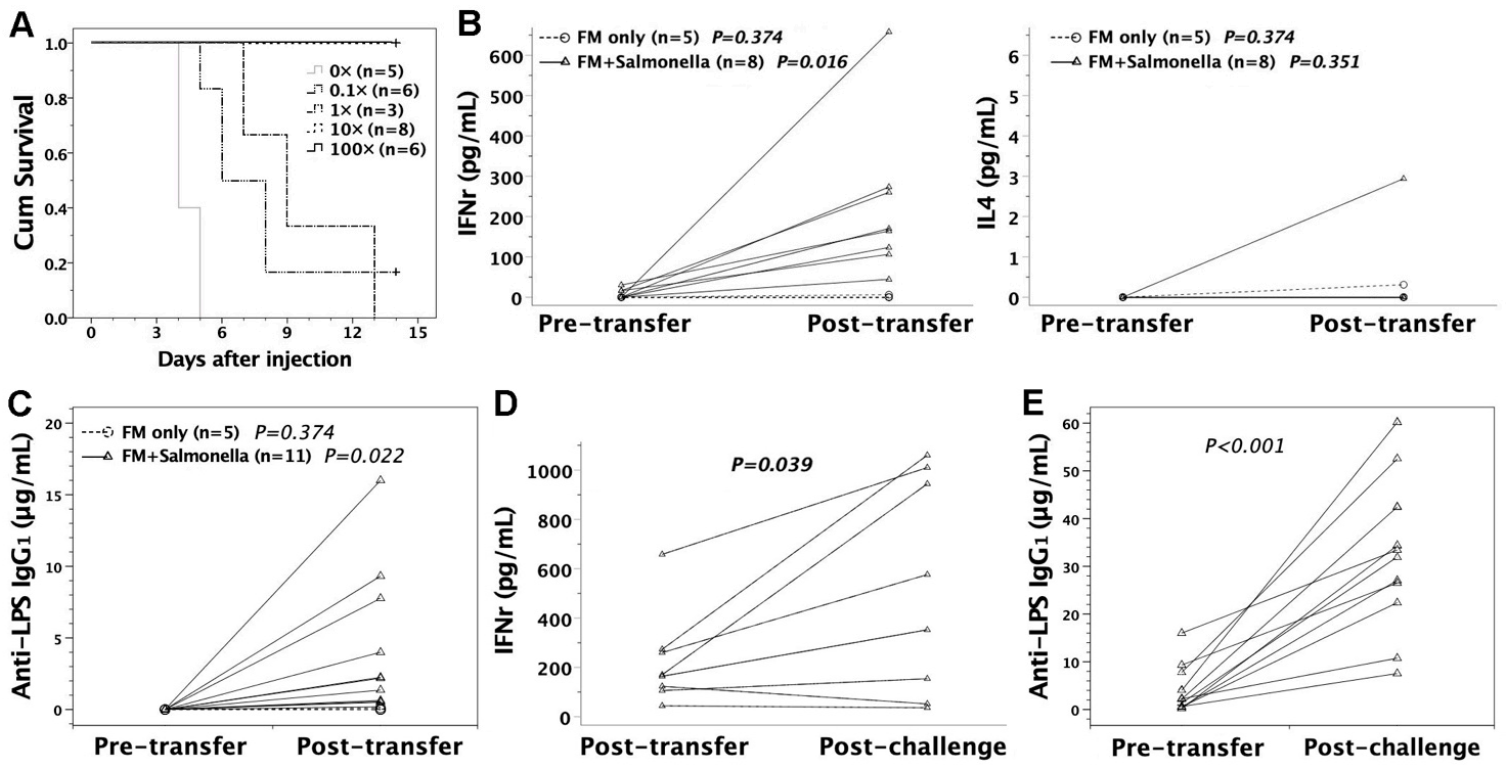
3.3. Susceptibility of FVB/N Mice to S. typhimurium Infection
3.4. Intraperitoneal Challenge of S. typhimurium in Murine Recipients
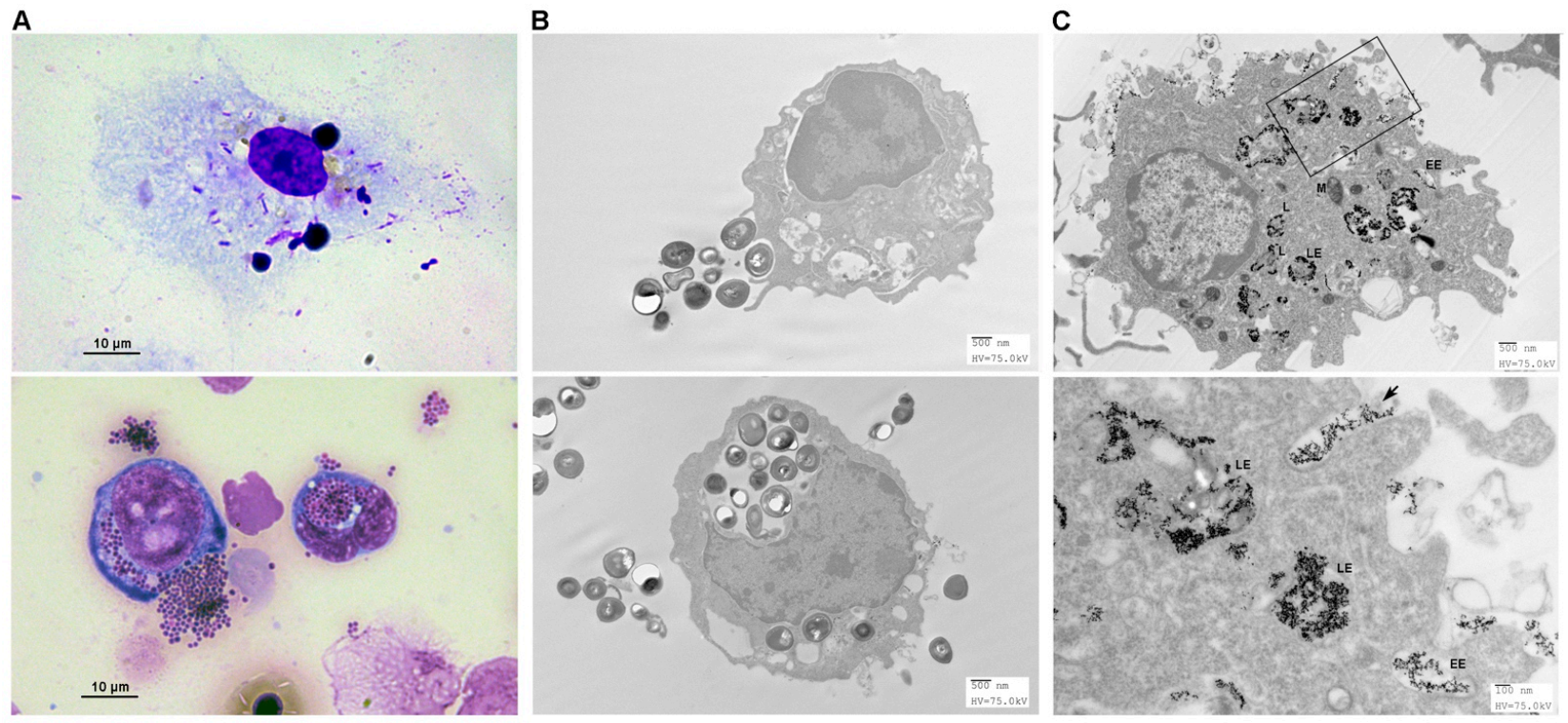
3.5. Endocytosis of Pathogens by FMs
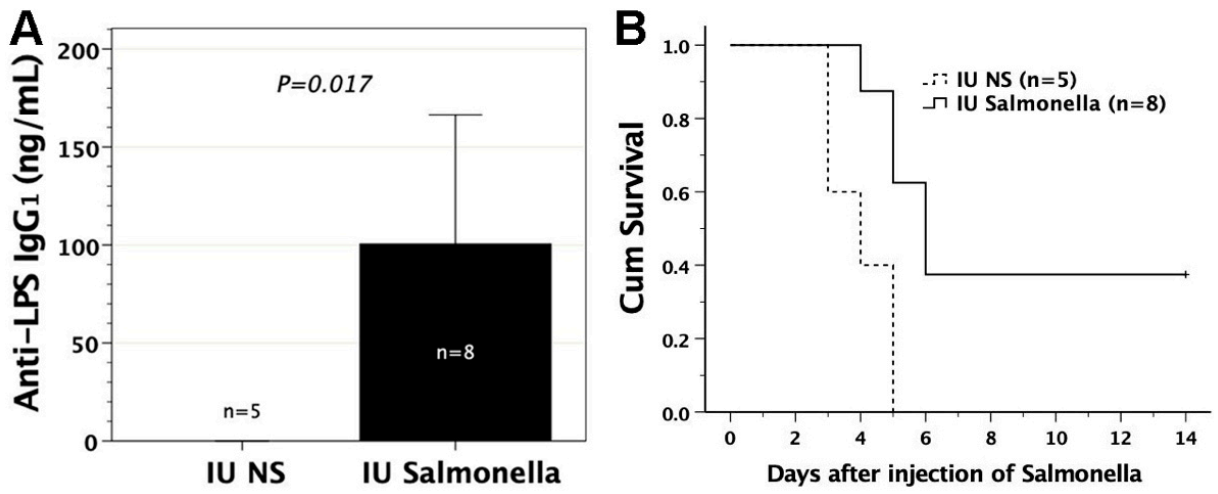
3.6. In Utero Injection of Heat-Killed S. typhimurium into FVB/N Murine Fetuses
3.7. Adoptive Transfer of FMs Loaded with Heat-Killed S. typhimurium
3.8. Protective Effects of Sera or Lymphocytes from Mice Immunized by Salmonella-Loaded FMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Pizzo, J. Focus on diagnosis: Congenital infections (TORCH). Pediatr. Rev. 2011, 32, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Neu, N.; Duchon, J.; Zachariah, P. TORCH infections. Clin. Perinatol. 2015, 42, 77–103. [Google Scholar] [CrossRef]
- Dauby, N.; Goetghebuer, T.; Kollmann, T.R.; Levy, J.; Marchant, A. Uninfected but not unaffected: Chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect. Dis. 2012, 12, 330–340. [Google Scholar] [CrossRef]
- Chougnet, C.A. Human fetal immune cells fight back. Sci. Transl. Med. 2018, 10, eaat3910. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Jones, C.E. Beyond Passive Immunity: Is There Priming of the Fetal Immune System Following Vaccination in Pregnancy and What Are the Potential Clinical Implications? Front. Immunol. 2018, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Billingham, R.E.; Brent, L.; Medawar, P.B. Actively acquired tolerance of foreign cells. Nature 1953, 172, 603–606. [Google Scholar] [CrossRef]
- Marchant, A.; Appay, V.; Van Der Sande, M.; Dulphy, N.; Liesnard, C.; Kidd, M.; Kaye, S.; Ojuola, O.; Gillespie, G.M.; Vargas Cuero, A.L.; et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J. Clin. Investig. 2003, 111, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, I.; Mungai, P.L.; Wamachi, A.N.; Tisch, D.; Kioko, J.M.; Ouma, J.H.; Muchiri, E.; Kazura, J.W.; King, C.L. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J. Infect. Dis. 2006, 193, 1005–1013. [Google Scholar] [CrossRef]
- Malhotra, I.; Dent, A.; Mungai, P.; Wamachi, A.; Ouma, J.H.; Narum, D.L.; Muchiri, E.; Tisch, D.J.; King, C.L. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009, 6, e1000116. [Google Scholar] [CrossRef]
- Kim, H.B.; Shaaban, A.F.; Yang, E.Y.; Liechty, K.W.; Flake, A.W. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J. Surg. Res. 1998, 77, 1–5. [Google Scholar] [CrossRef]
- Chen, J.C.; Kuo, M.L.; Ou, L.S.; Chang, P.Y.; Muench, M.O.; Shen, C.R.; Chang, H.L.; Yu, H.Y.; Fu, R.H. Characterization of tolerance induction through prenatal marrow transplantation: The requirement for a threshold level of chimerism to establish rather than maintain postnatal skin tolerance. Cell Transpl. 2010, 19, 1609–1622. [Google Scholar] [CrossRef]
- Carrier, E.; Lee, T.H.; Busch, M.P.; Cowan, M.J. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood 1995, 86, 4681–4690. [Google Scholar] [CrossRef] [PubMed]
- Sefrioui, H.; Donahue, J.; Srivastava, A.S.; Gilpin, E.; Lee, T.H.; Carrier, E. Alloreactivity following in utero transplantation of cytokine-stimulated hematopoietic stem cells: The role of recipient CD4(-) cells. Exp. Hematol. 2002, 30, 617–624. [Google Scholar] [CrossRef]
- Carrier, E.; Gilpin, E.; Lee, T.H.; Busch, M.P.; Zanetti, M. Microchimerism does not induce tolerance after in utero transplantation and may lead to the development of alloreactivity. J. Lab. Clin. Med. 2000, 136, 224–235. [Google Scholar] [CrossRef]
- Donahue, J.; Gilpin, E.; Lee, T.H.; Busch, M.P.; Croft, M.; Carrier, E. Microchimerism does not induce tolerance and sustains immunity after in utero transplantation. Transplantation 2001, 71, 359–368. [Google Scholar] [CrossRef]
- Chen, J.C.; Chan, C.C.; Wu, C.J.; Ou, L.S.; Yu, H.Y.; Chang, H.L.; Tseng, L.Y.; Kuo, M.L. Fetal Phagocytes Take up Allergens to Initiate T-Helper Cell Type 2 Immunity and Facilitate Allergic Airway Responses. Am. J. Respir. Crit. Care Med. 2016, 194, 934–947. [Google Scholar] [CrossRef]
- Chen, J.C.; Ou, L.S.; Kuo, M.L.; Tseng, L.Y.; Chang, H.L. Fetal exposure to oncoantigen elicited antigen-specific adaptive immunity against tumorigenesis. J. Immunother. Cancer 2020, 8, e000137. [Google Scholar] [CrossRef]
- Roll, C.; Schmid, E.N.; Menken, U.; Hanssler, L. Fatal Salmonella enteritidis sepsis acquired prenatally in a premature infant. Obstet. Gynecol. 1996, 88, 692–693. [Google Scholar] [CrossRef]
- Mollo, B.; Hobson, C.A.; Le Hello, S.; Azria, E.; Le Monnier, A.; Pilmis, B.; Mizrahi, A. Intrauterine infection caused by nontyphoidal Salmonella: A literature review. J. Matern. Fetal Neonatal Med. 2019, 1–5. [Google Scholar] [CrossRef]
- Chen, J.C.; Chang, M.L.; Lee, H.; Muench, M.O. Haploidentical donor T cells fail to facilitate engraftment but lessen the immune response of host T cells in murine fetal transplantation. Br. J. Haematol. 2004, 126, 377–384. [Google Scholar] [CrossRef][Green Version]
- Chen, J.C.; Chang, M.L.; Lee, H.; Muench, M.O. Prevention of graft rejection by donor type II CD8(+) T cells (Tc2 cells) is not sufficient to improve engraftment in fetal transplantation. Fetal Diagn. Ther. 2005, 20, 35–43. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.W.; Peng, C.J.; Chen, M.C.; Hsu, M.H.; Liang, Y.H.; Chiu, C.H.; Fang, J.M.; Lee, Y.C. Constructing conjugate vaccine against Salmonella Typhimurium using lipid-A free lipopolysaccharide. J. Biomed. Sci. 2020, 27, 89. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Aase, J.M.; Noren, G.R.; Reddy, D.V.; Geme, J.W., Jr. Mumps-virus infection in pregnant women and the immunologic response of their offspring. N. Engl. J. Med. 1972, 286, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Andrews, N.; Campbell, H.; Ribeiro, S.; Kara, E.; Donegan, K.; Fry, N.K.; Miller, E.; Ramsay, M. Effectiveness of maternal pertussis vaccination in England: An observational study. Lancet 2014, 384, 1521–1528. [Google Scholar] [CrossRef]
- Labeaud, A.D.; Malhotra, I.; King, M.J.; King, C.L.; King, C.H. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl. Trop. Dis. 2009, 3, e442. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Andrews, N.J.; Southern, J.; Jones, C.E.; Amirthalingam, G.; Waight, P.A.; England, A.; Matheson, M.; Bai, X.; Findlow, H.; et al. Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: Single arm observational study with a historical comparator. Clin. Infect. Dis. 2015, 61, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Rahman, M.J.; Degano, I.R.; Singh, M.; Fernandez, C. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLoS ONE 2010, 5, e9699. [Google Scholar] [CrossRef]
- Malek, A.; Sager, R.; Schneider, H. Transport of proteins across the human placenta. Am. J. Reprod. Immunol. 1998, 40, 347–351. [Google Scholar] [CrossRef]
- McSorley, S.J.; Cookson, B.T.; Jenkins, M.K. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J. Immunol. 2000, 164, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Tabaraie, B.; Sharma, B.K.; Sharma, P.R.; Sehgal, R.; Ganguly, N.K. Evaluation of Salmonella porins as a broad spectrum vaccine candidate. Microbiol. Immunol. 1994, 38, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, Q.; Luo, H.; Liang, K.; Han, Y.; Roland, K.L.; Curtiss, R., 3rd; Kong, Q. Bi-valent polysaccharides of Vi capsular and O9 O-antigen in attenuated Salmonella Typhimurium induce strong immune responses against these two antigens. NPJ Vaccines 2018, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.; Tennant, S.M.; Boyd, M.A.; Wang, J.Y.; Tulapurkar, M.E.; Pasetti, M.F.; Levine, M.M.; Simon, R. Functional Activity of Antibodies Directed towards Flagellin Proteins of Non-Typhoidal Salmonella. PLoS ONE 2016, 11, e0151875. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; MacLennan, C.A.; Simon, R.; Martin, L.B.; Khan, M.I. Nontyphoidal salmonella disease: Current status of vaccine research and development. Vaccine 2016, 34, 2907–2910. [Google Scholar] [CrossRef]
- Snodgrass, H.R.; Kisielow, P.; Kiefer, M.; Steinmetz, M.; von Boehmer, H. Ontogeny of the T-cell antigen receptor within the thymus. Nature 1985, 313, 592–595. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Mittrucker, H.W.; Kaufmann, S.H. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000, 67, 457–463. [Google Scholar] [CrossRef]
- Mizuno, Y.; Takada, H.; Nomura, A.; Jin, C.H.; Hattori, H.; Ihara, K.; Aoki, T.; Eguchi, K.; Hara, T. Th1 and Th1-inducing cytokines in Salmonella infection. Clin. Exp. Immunol. 2003, 131, 111–117. [Google Scholar] [CrossRef]
- Schaible, U.E.; Collins, H.L.; Kaufmann, S.H. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 1999, 71, 267–377. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.F.; Khan, M.; Ball, J.; Toellner, K.M.; Serre, K.; Mohr, E.; MacLennan, I.C. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur. J. Immunol. 2004, 34, 2986–2995. [Google Scholar] [CrossRef]
- Bobat, S.; Flores-Langarica, A.; Hitchcock, J.; Marshall, J.L.; Kingsley, R.A.; Goodall, M.; Gil-Cruz, C.; Serre, K.; Leyton, D.L.; Letran, S.E.; et al. Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur. J. Immunol. 2011, 41, 1606–1618. [Google Scholar] [CrossRef]
- Barrow, P.A. Salmonella infections: Immune and non-immune protection with vaccines. Avian Pathol. 2007, 36, 1–13. [Google Scholar] [CrossRef]
- Mastroeni, P.; Menager, N. Development of acquired immunity to Salmonella. J. Med. Microbiol. 2003, 52, 453–459. [Google Scholar] [CrossRef]
- McSorley, S.J.; Jenkins, M.K. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect. Immun. 2000, 68, 3344–3348. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Gaspal, F.; Serre, K.; Mohr, E.; Henderson, I.R.; Scott-Tucker, A.; Kenny, S.M.; Khan, M.; Toellner, K.M.; Lane, P.J.; et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 2007, 178, 6200–6207. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; McSorley, S.J. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology 2005, 114, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.J.; McSorley, S.J. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 2011, 4, 371–382. [Google Scholar] [CrossRef]
- Jouanguy, E.; Doffinger, R.; Dupuis, S.; Pallier, A.; Altare, F.; Casanova, J.L. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 1999, 11, 346–351. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.; Kumararatne, D.; Casanova, J.L. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 1998, 19, 491–494. [Google Scholar] [CrossRef]
- Mastroeni, P.; Harrison, J.A.; Chabalgoity, J.A.; Hormaeche, C.E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect. Immun. 1996, 64, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, P.; Villarreal-Ramos, B.; Hormaeche, C.E. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro—Salmonella vaccines. Microb. Pathog. 1992, 13, 477–491. [Google Scholar] [CrossRef]
- Bueno, S.M.; Gonzalez, P.A.; Schwebach, J.R.; Kalergis, A.M. T cell immunity evasion by virulent Salmonella enterica. Immunol. Lett. 2007, 111, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ugrinovic, S.; Menager, N.; Goh, N.; Mastroeni, P. Characterization and development of T-Cell immune responses in B-cell-deficient (Igh-6(-/-)) mice with Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2003, 71, 6808–6819. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-C.; Ou, L.-S.; Kuo, M.-L.; Tseng, L.-Y.; Chang, H.-L.; Chen, S.-C.; Chiu, C.-H. Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model. Biomedicines 2021, 9, 245. https://doi.org/10.3390/biomedicines9030245
Chen J-C, Ou L-S, Kuo M-L, Tseng L-Y, Chang H-L, Chen S-C, Chiu C-H. Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model. Biomedicines. 2021; 9(3):245. https://doi.org/10.3390/biomedicines9030245
Chicago/Turabian StyleChen, Jeng-Chang, Liang-Shiou Ou, Ming-Ling Kuo, Li-Yun Tseng, Hsueh-Ling Chang, Shiang-Chi Chen, and Cheng-Hsun Chiu. 2021. "Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model" Biomedicines 9, no. 3: 245. https://doi.org/10.3390/biomedicines9030245
APA StyleChen, J.-C., Ou, L.-S., Kuo, M.-L., Tseng, L.-Y., Chang, H.-L., Chen, S.-C., & Chiu, C.-H. (2021). Fetal Macrophages Exposed to Salmonella Antigens Elicit Protective Immunity Against Overwhelming Salmonella Challenge in A Murine Model. Biomedicines, 9(3), 245. https://doi.org/10.3390/biomedicines9030245






