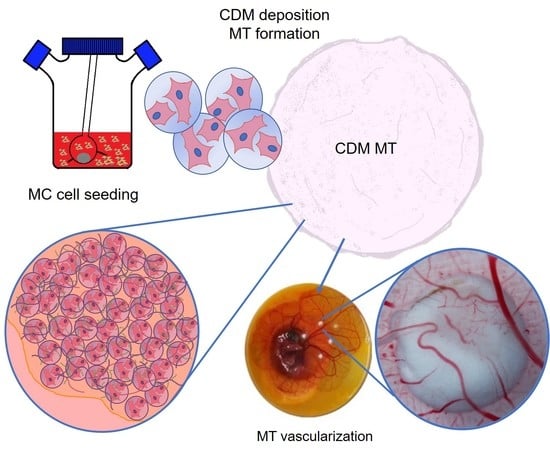
Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Poly-Lactic Acid Microcarriers Fabrication, Biofunctionalization and Characterization
2.3. Cell Culture
2.4. Microcarrier Cell Seeding and Microtissue ProductionCell Seeding &Microtissue Production under Static Conditions
2.5. Microcarrier Cell Seeding and Microtissue ProductionCell Seeding & Microtissue Production under Dynamic Conditions
2.6. Cell-Seeded Microcarriers’ Vital Staining
2.7. Microtissue Size and Morphology Size & Morphology
2.8. Cell Proliferation
2.9. Protein Deposition Quantification
2.10. Immunofluorescence Staining
2.11. Microtissue Decellularization
2.12. Microtissue Angiogenic Potential—CAM Model
2.13. Statistical Analysis
3. Results and Discussion
3.1. PLA Microcarrier Characterization
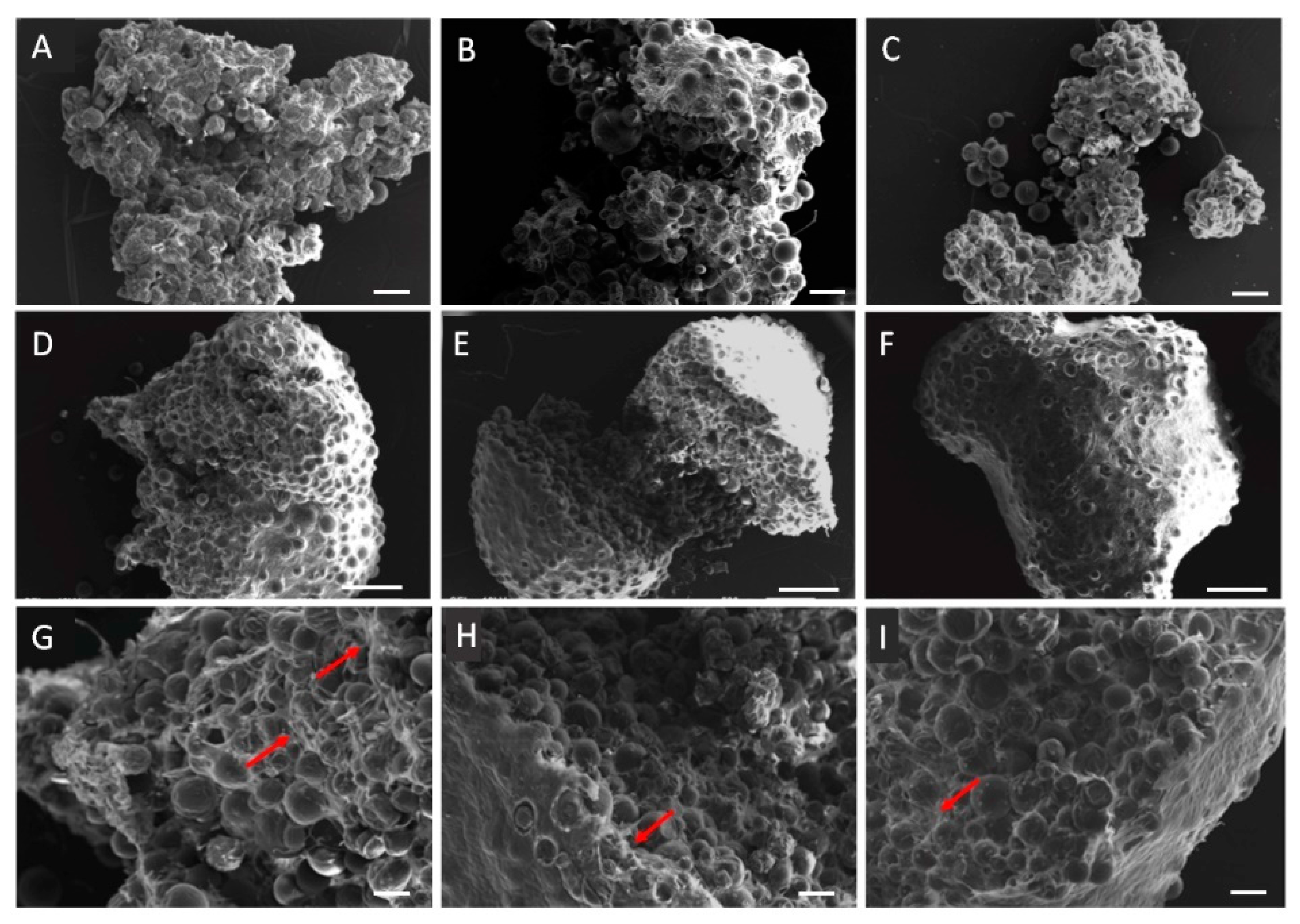
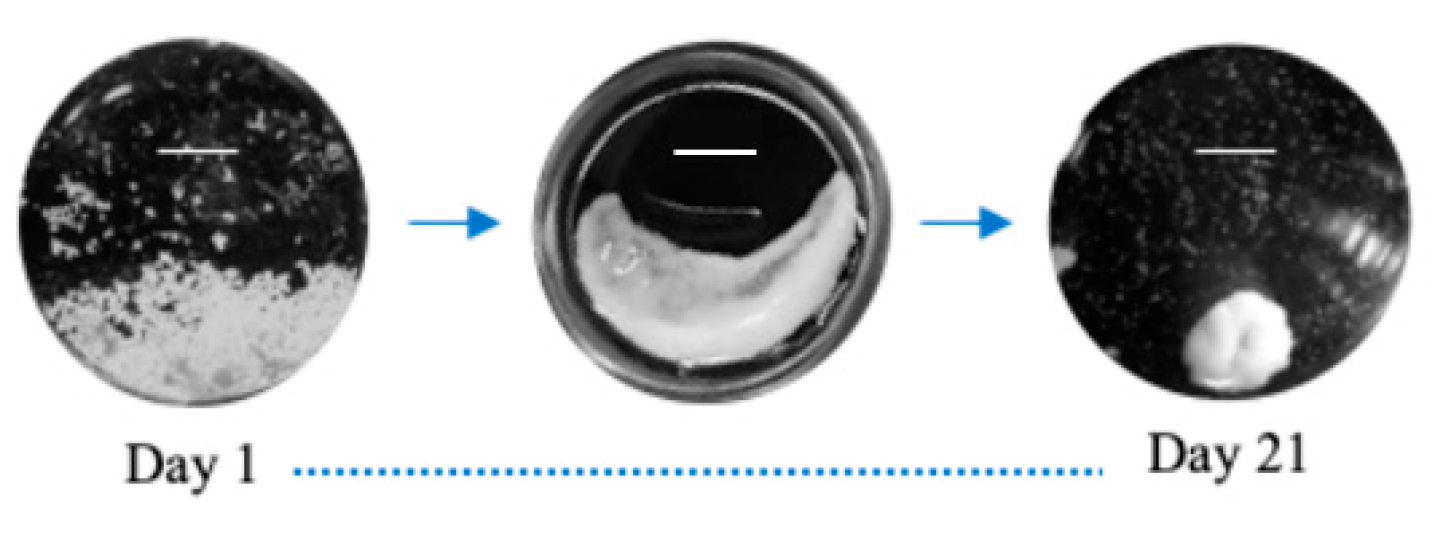
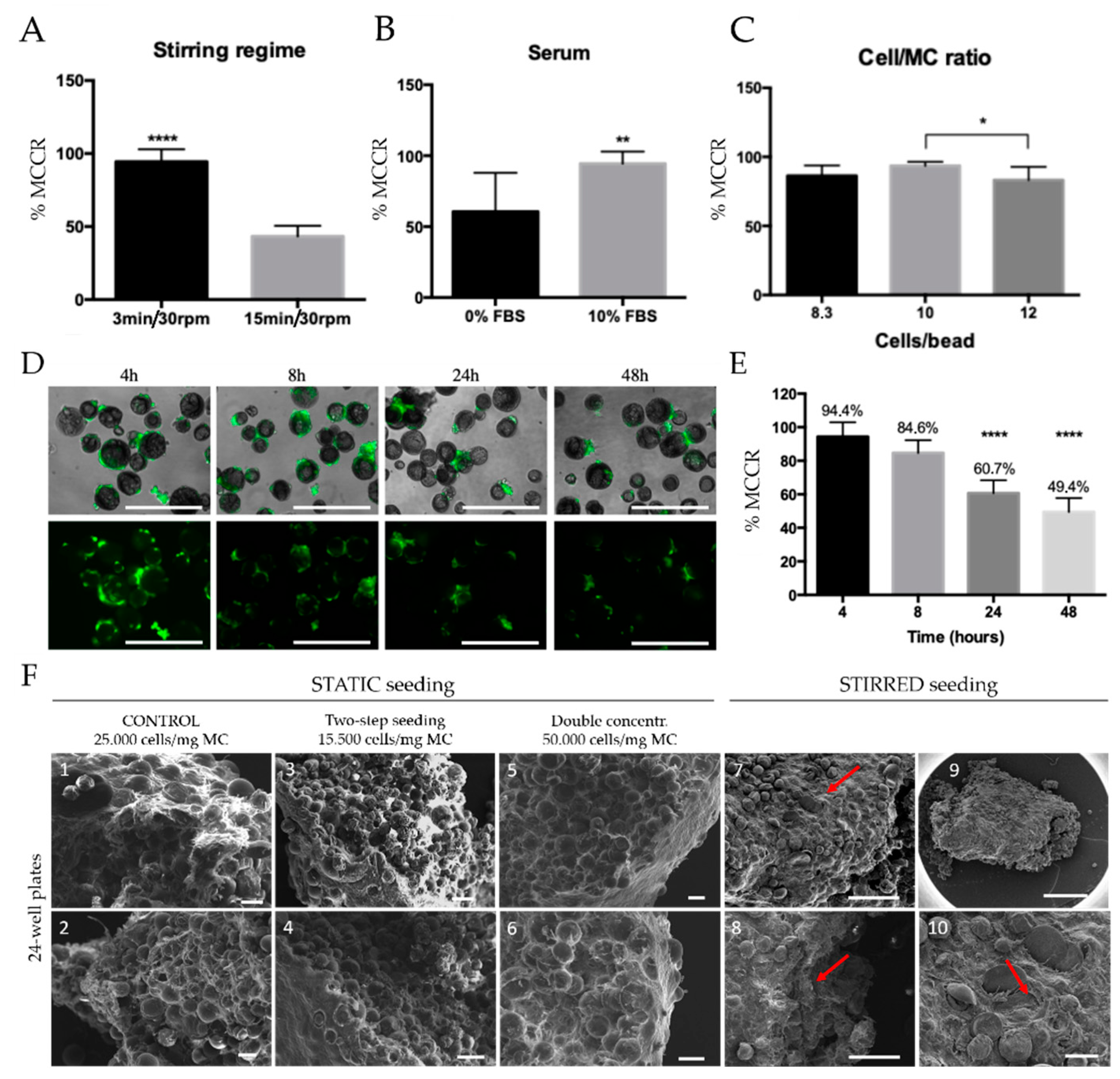
3.2. Microtissue Development and Optimization
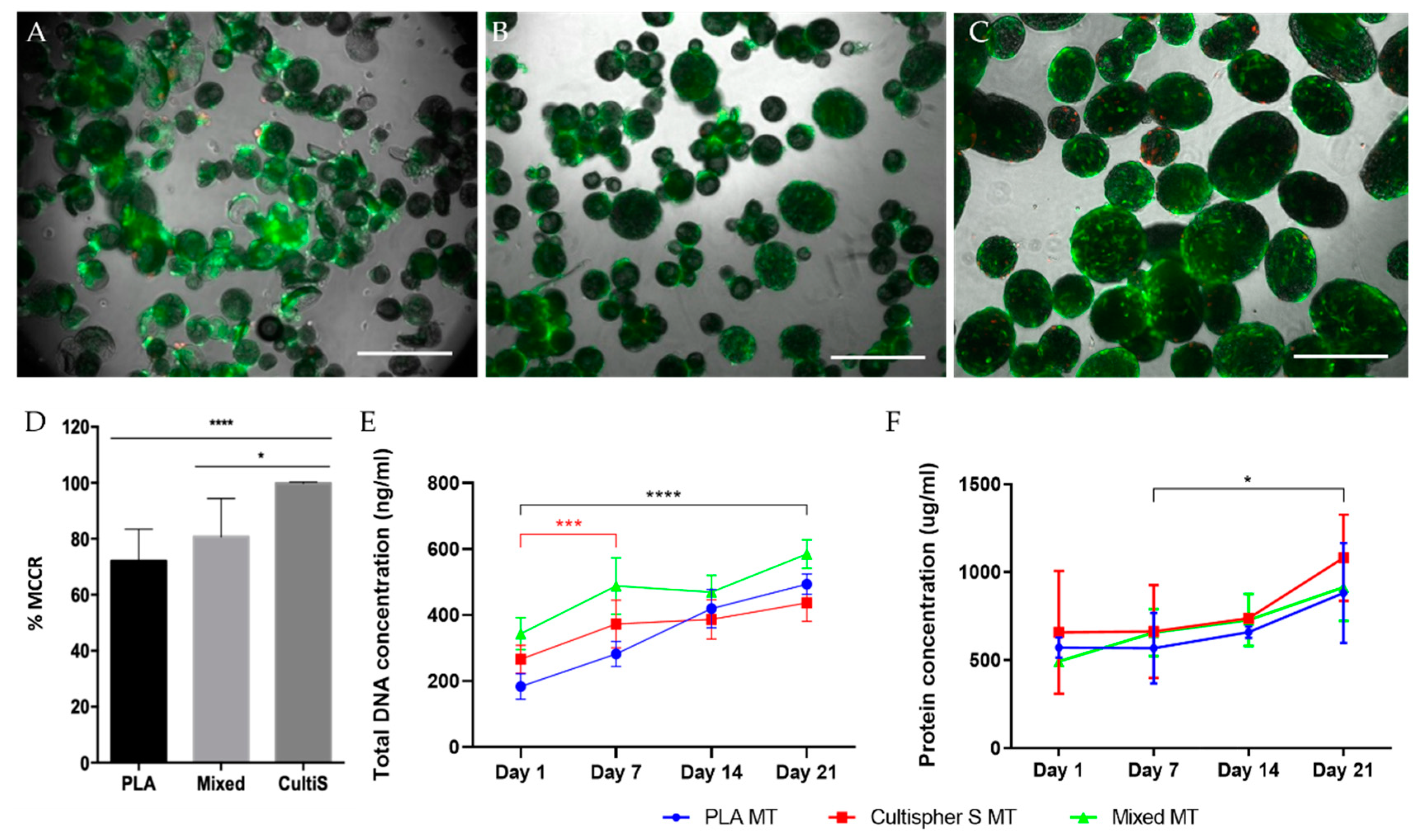
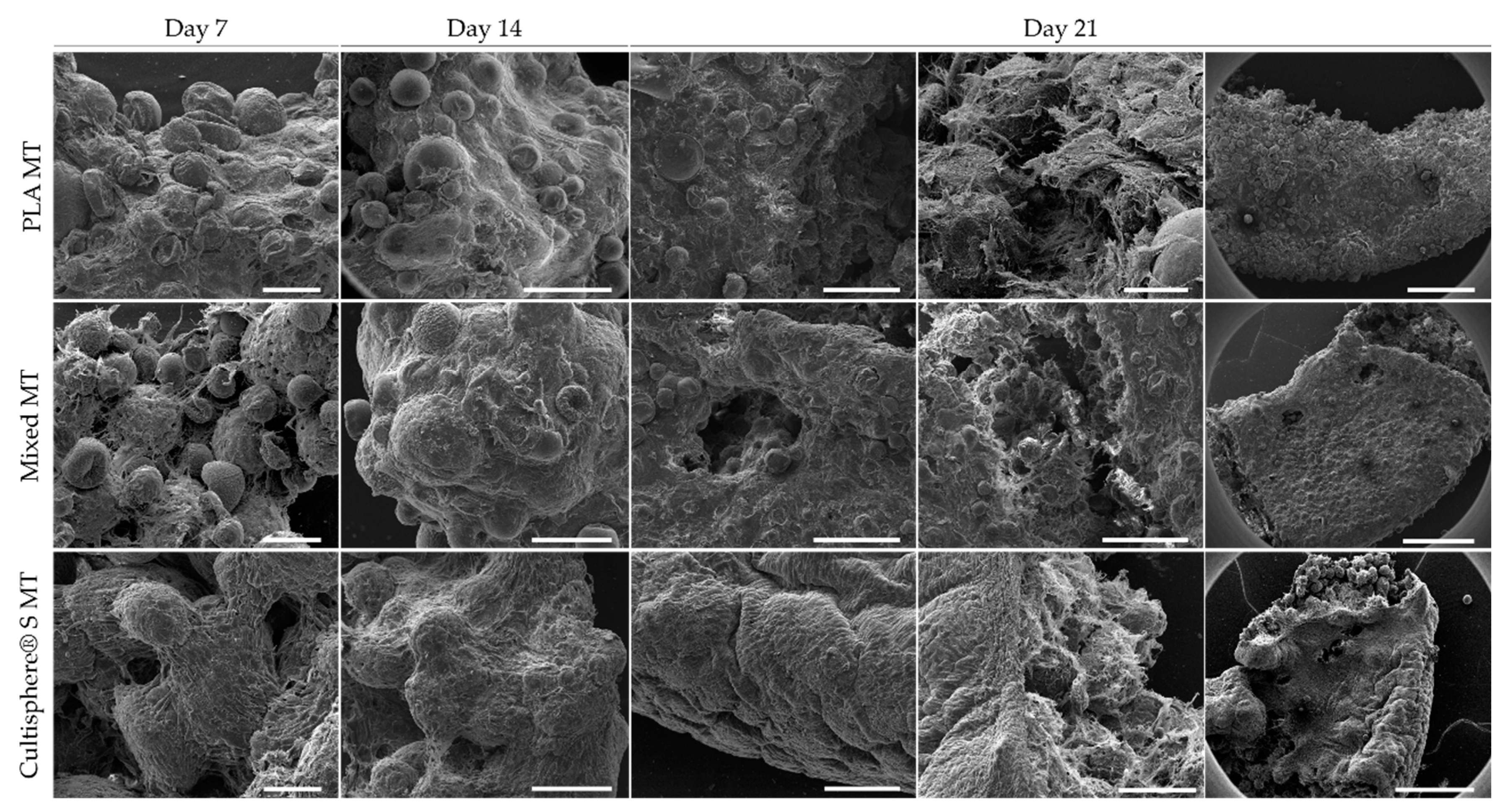
3.3. Microtissue Production and Characterization Using PLA, Cultispher® S, and Combining Both Microcarriersthe Combination of Both Microcarriers
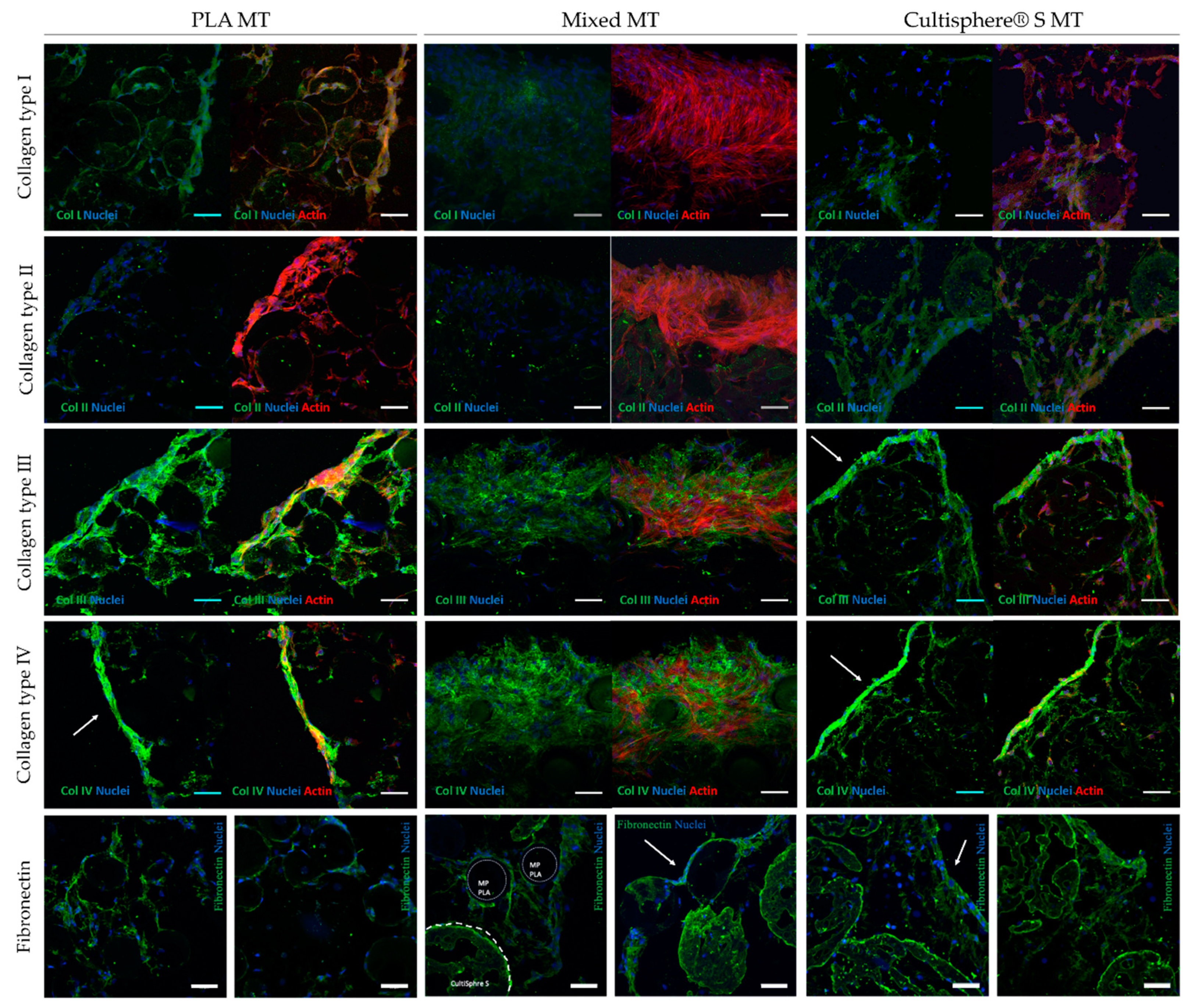
3.4. Microtissue Cell-Derived Matrix Proteins Immunofluorescence Staining
3.5. Microtissues Angiogenesis Ex Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering: Fourth Edition; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Rossi, L.; Attanasio, C.; Vilardi, E.; De Gregorio, M.; Netti, P.A. Vasculogenic Potential Evaluation of Bottom-up, PCL Scaffolds Guiding Early Angiogenesis in Tissue Regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Khan, O.F.; Voice, D.N.; Leung, B.M.; Sefton, M.V. A Novel High-Speed Production Process to Create Modular Components for the Bottom-up Assembly of Large-Scale Tissue-Engineered Constructs. Adv. Healthc. Mater. 2015, 4, 113–120. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotech. 2011, 13–22. [Google Scholar] [CrossRef]
- Waldeck, H.M.; Kao, W.J. Extracellular Matrix: Inspired Biomaterials. Compr. Biomater. 2011, 2, 113–126. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matters 2010, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Duarte Campos, D.F.; Fischer, H. 3D Bioprinting of Cell-Laden Hydrogels for Advanced Tissue Engineering. Curr. Opin. Biomed. Eng. 2017, 58–66. [Google Scholar] [CrossRef]
- Kachouie, N.N.; Du, Y.; Bae, H.; Khabiry, M.; Ahari, A.F.; Zamanian, B.; Fukuda, J.; Khademhosseini, A. Directed Assembly of Cell-Laden Hydrogels for Engineering Functional Tissues. Organogenesis 2010, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Li, Q.; Yang, D.; Fan, Z.; Ma, D.; Ren, L. Effect of Different Cell Sheet ECM Microenvironment on the Formation of Vascular Network. Tissue Cell 2016, 48, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Franco-Barraza, J.; Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr. Protoc. Cell Biol. 2017, 71, 10.9.1–10.9.34. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 108–115. [Google Scholar] [CrossRef]
- Okudaira, T.; Amimoto, N.; Mizumoto, H.; Kajiwara, T. Formation of Three-Dimensional Hepatic Tissue by the Bottom-up Method Using Spheroids. J. Biosci. Bioeng. 2016, 122, 213–218. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takeuchi, S. Three-Dimensional Cell Culture Based on Microfluidic Techniques to Mimic Living Tissues. Biomater. Sci. 2013, 257–264. [Google Scholar] [CrossRef]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of Tissue Constructs by 3D Bioprinting of Cell-Laden Microcarriers. Biofabrication 2014, 6. [Google Scholar] [CrossRef]
- Zhong, M.; Wei, D.; Yang, Y.; Sun, J.; Chen, X.; Guo, L.; Wei, Q.; Wan, Y.; Fan, H.; Zhang, X. Vascularization in Engineered Tissue Construct by Assembly of Cellular Patterned Micromodules and Degradable Microspheres. ACS Appl. Mater. Interfaces 2017, 9, 3524–3534. [Google Scholar] [CrossRef]
- Twal, W.O.; Klatt, S.C.; Harikrishnan, K.; Gerges, E.; Cooley, M.A.; Trusk, T.C.; Zhou, B.; Gabr, M.G.; Shazly, T.; Lessner, S.M.; et al. Cellularized Microcarriers as Adhesive Building Blocks for Fabrication of Tubular Tissue Constructs. Ann. Biomed. Eng. 2014, 42, 1470–1481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Declercq, H.A.; De Caluwé, T.; Krysko, O.; Bachert, C.; Cornelissen, M.J. Bone Grafts Engineered from Human Adipose-Derived Stem Cells in Dynamic 3D-Environments. Biomaterials 2013, 34, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk Fibroin/Gelatin Microcarriers as Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Lippens, E.; Vertenten, G.; Gironès, J.; Declercq, H.; Saunders, J.; Luyten, J.; Duchateau, L.; Schacht, E.; Vlaminck, L.; Gasthuys, F.; et al. Evaluation of Bone Regeneration with an Injectable, in Situ Polymerizable Pluronic® F127 Hydrogel Derivative Combined with Autologous Mesenchymal Stem Cells in a Goat Tibia Defect Model. Tissue Eng. Part A 2010, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Y.; Lin, S.-Z.; Li, Y.-S.; Harn, H.-J.; Chiou, T.-W. Functional Cells Cultured on Microcarriers for Use in Regenerative Medicine Research. Cell Transplant. 2011, 20, 49–62. [Google Scholar] [CrossRef]
- Nweke, C.E.; Stegemann, J.P. Modular Microcarrier Technologies for Cell-Based Bone Regeneration. J. Mater. Chem. B 2020, 3972–3984. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, M.; Ye, Z.; Zhou, Y.; Tan, W. Fabrication of Viable and Functional Pre-vascularized Modular Bone Tissues by Coculturing MSCs and HUVECs on Microcarriers in Spinner Flasks. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Chen, G. Autologous Extracellular Matrix Scaffolds for Tissue Engineering. Biomaterials 2011, 32, 2489–2499. [Google Scholar] [CrossRef]
- Rubi-Sans, G.; Castaño, O.; Cano, I.; Mateos-Timoneda, M.A.; Perez-Amodio, S.; Engel, E. Engineering Cell-Derived Matrices: From 3D Models to Advanced Personalized Therapies. Adv. Funct. Mater. 2020, 2000496. [Google Scholar] [CrossRef]
- Fitzpatrick, L.E.; McDevitt, T.C. Cell-Derived Matrices for Tissue Engineering and Regenerative Medicine Applications. Biomater. Sci. 2015, 3, 12–24. [Google Scholar] [CrossRef]
- Ozguldez, H.O.; Cha, J.; Hong, Y.; Koh, I.; Kim, P. Nanoengineered, Cell-Derived Extracellular Matrix Influences ECM-Related Gene Expression of Mesenchymal Stem Cells. Biomater. Res. 2018, 22, 32. [Google Scholar] [CrossRef]
- Levato, R.; Mateos-Timoneda, M.A.; Planell, J.A. Preparation of Biodegradable Polylactide Microparticles via a Biocompatible Procedure. Macromol. Biosci. 2012, 12, 557–566. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 733–745. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in Tissue Engineering. Trends Biotechnol. 2008, 434–441. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Punet, X.; Mauchauffé, R.; Giannotti, M.I.; Rodríguez-Cabello, J.C.; Sanz, F.; Engel, E.; Mateos-Timoneda, M.A.; Planell, J.A. Enhanced Cell-Material Interactions through the Biofunctionalization of Polymeric Surfaces with Engineered Peptides. Biomacromolecules 2013, 14, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Timoneda, M.A.; Castano, O.; Planell, J.A.; Engel, E. Effect of Structure, Topography and Chemistry on Fibroblast Adhesion and Morphology. J. Mater. Sci. Mater. Med. 2014, 25, 1781–1787. [Google Scholar] [CrossRef]
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular Calcium and CaSR Drive Osteoinduction in Mesenchymal Stromal Cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 1–13. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Li, J.; Guo, Q.; Peng, J.; Liu, S.; Yang, J.; Wang, Y. Cell-Derived Extracellular Matrix: Basic Characteristics and Current Applications in Orthopedic Tissue Engineering. Tissue Eng. Part B Rev. 2016, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L. Bottom-up Tissue Engineering. Curr. Opin. Biotechnol. 2011, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 655–663. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond Molecules: Self-Assembly of Mesoscopic and Macroscopic Components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef]
- Futrega, K.; Palmer, J.S.; Kinney, M.; Lott, W.B.; Ungrin, M.D.; Zandstra, P.W.; Doran, M.R. The Microwell-Mesh: A Novel Device and Protocol for the High Throughput Manufacturing of Cartilage Microtissues. Biomaterials 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv. Mater. 2011, 23, H90–H94. [Google Scholar] [CrossRef]
- Rivron, N.C.; Vrij, E.J.; Rouwkema, J.; Le Gac, S.; van den Berg, A.; Truckenmüller, R.K.; van Blitterswijk, C.A. Tissue Deformation Spatially Modulates VEGF Signaling and Angiogenesis. Proc. Natl. Acad. Sci. USA 2012, 6886–6891. [Google Scholar] [CrossRef]
- Thibault, R.A.; Mikos, A.G.; Kasper, F.K. Scaffold/Extracellular Matrix Hybrid Constructs for Bone-Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Hewitt, C.J. Systematic Microcarrier Screening and Agitated Culture Conditions Improves Human Mesenchymal Stem Cell Yield in Bioreactors. Biotechnol. J. 2016, 11, 473–486. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Ye, Z.; Zhang, Y.; Zhou, Y.; Tan, W.S. A Modular Approach to the Engineering of a Centimeter-Sized Bone Tissue Construct with Human Amniotic Mesenchymal Stem Cells-Laden Microcarriers. Biomaterials 2011, 32, 7532–7542. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, M.; Ye, Z.; Zhou, Y.; Tan, W.S. Ectopic Osteogenesis of Macroscopic Tissue Constructs Assembled from Human Mesenchymal Stem Cell-Laden Microcarriers through In Vitro Perfusion Culture. PLoS ONE 2014, 9, e109214. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Luo, H.; Tang, Q.; Ye, Z.; Zhou, Y.; Tan, W.S. Modulating and Modeling Aggregation of Cell-Seeded Microcarriers in Stirred Culture System for Macrotissue Engineering. J. Biotechnol. 2010, 150, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kallos, M.S.; Hunter, C.; Sen, A. Improved Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in Microcarrier-Based Suspension Culture. J. Tissue Eng. Regen. Med. 2014, 8, 210–225. [Google Scholar] [CrossRef]
- Hlady, V.; Buijs, J. Protein Adsorption on Solid Surfaces. Curr. Opin. Biotechnol. 1996, 7, 72–77. [Google Scholar] [CrossRef]
- Allen, L.T.; Tosetto, M.; Miller, I.S.; O’Connor, D.P.; Penney, S.C.; Lynch, I.; Keenan, A.K.; Pennington, S.R.; Dawson, K.A.; Gallagher, W.M. Surface-Induced Changes in Protein Adsorption and Implications for Cellular Phenotypic Responses to Surface Interaction. Biomaterials 2006, 27, 3096–3108. [Google Scholar] [CrossRef]
- Westman, A.M.; Goldstein, R.L.; Bradica, G.; Goldman, S.M.; Randolph, M.A.; Gaut, J.P.; Vacanti, J.P.; Hoganson, D.M. Decellularized Extracellular Matrix Microparticles Seeded with Bone Marrow Mesenchymal Stromal Cells for the Treatment of Full-Thickness Cutaneous Wounds. J. Biomater. Appl. 2019, 33, 1070–1079. [Google Scholar] [CrossRef]
- Smith, D.; Herman, C.; Razdan, S.; Abedin, M.R.; Van Stoecker, W.; Barua, S. Microparticles for Suspension Culture of Mammalian Cells. ACS Appl. Bio Mater. 2019, 2, 2791–2801. [Google Scholar] [CrossRef]
- Piola, M.; Soncini, M.; Cantini, M.; Sadr, N.; Ferrario, G.; Fiore, G.B. Design and Functional Testing of a Multichamber Perfusion Platform for Three-Dimensional Scaffolds. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.; Couture, S.M.; Ye, J.; Lin, Z.; Hua, G.; Huang, H.I.P.; Wu, J.; Hsu, D.; Carpenter, M.K.; Couture, L.A. Scalable GMP Compliant Suspension Culture System for Human ES Cells. Stem Cell Res. 2012, 8, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 762–798. [Google Scholar] [CrossRef]
- Rafiq, Q.A.; Ruck, S.; Hanga, M.P.; Heathman, T.R.J.; Coopman, K.; Nienow, A.W.; Williams, D.J.; Hewitt, C.J. Qualitative and Quantitative Demonstration of Bead-to-Bead Transfer with Bone Marrow-Derived Human Mesenchymal Stem Cells on Microcarriers: Utilising the Phenomenon to Improve Culture Performance. Biochem. Eng. J. 2018, 135, 11–21. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Block, T.J.; Rakian, R.; Gonzalez, A.O.; Dean, D.D.; Yeh, C.K.; Chen, X.D. Native Extracellular Matrix, Synthesized Ex Vivo by Bone Marrow or Adipose Stromal Cells, Faithfully Directs Mesenchymal Stem Cell Differentiation. Matrix Biol. Plus 2020, 100044. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Current Biology Basement Membranes; Elsevier: Amsterdam, The Netherlands, 2017; Volume 27. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV Is Essential for Basement Membrane Stability but Dispensable for Initiation of Its Assembly during Early Development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Merceron, C.; Portron, S.; Masson, M.; Lesoeur, J.; Hakim Fellah, B.; Gauthier, O.; Geffroy, O.; Weiss, P.; Guicheux, J.; Vinatier, C. The Effect of Two-and Three-Dimensional Cell Culture on the Chondrogenic Potential of Human Adipose-Derived Mesenchymal Stem Cells After Subcutaneous Transplantation with an Injectable Hydrogel. Cell Transplant. 2011, 20, 1575–1588. [Google Scholar] [CrossRef]
- Matsuda, C.; Takagi, M.; Hattori, T.; Wakitani, S.; Yoshida, T. Differentiation of Human Bone Marrow Mesenchymal Stem Cells to Chondrocytes for Construction of Three-Dimensional Cartilage Tissue. Cytotechnology 2005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubí-Sans, G.; Recha-Sancho, L.; Pérez-Amodio, S.; Mateos-Timoneda, M.Á.; Semino, C.E.; Engel, E. Development of a Three-Dimensional Bioengineered Platform for Articular Cartilage Regeneration. Biomolecules 2020, 10, 52. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Yudintceva, N.; Nikonov, P.; Smagina, L.; Yudin, V.; Blinova, M.; Voronkina, I. Protein Expression by Bone Mesenchymal Stem Cells Cultivated in PLLA Scaffolds with Different Pore Geometry. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 248–257. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Fallas, M.E.A.; Piccoli, M.; Franzin, C.; Sgrò, A.; Dedja, A.; Urbani, L.; Bertin, E.; Trevisan, C.; Gamba, P.; Burns, A.J.; et al. Decellularized Diaphragmatic Muscle Drives a Constructive Angiogenic Response in Vivo. Int. J. Mol. Sci. 2018, 19, 1319. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Reilly, G.C.; Claeyssens, F. Boosting the Osteogenic and Angiogenic Performance of Multiscale Porous Polycaprolactone Scaffolds by In Vitro Generated Extracellular Matrix Decoration. ACS Appl. Mater. Interfaces 2020, 12, 12510–12524. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, C.M.; Chen, X.; Harris, J.W.; Suresh, V.; Hughes, C.C.; Li Jeon, N.; Putnam, A.J.; George, S.C. The Effect of Matrix Density on the Regulation of 3-D Capillary Morphogenesis. Biophys. J. 2008, 94, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial Extracellular Matrix: Biosynthesis, Remodeling, and Functions during Vascular Morphogenesis and Neovessel Stabilization. Circ. Res. 2005, 1093–1107. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibodies | Secondary Antibodies | |||||
|---|---|---|---|---|---|---|
| Reactivity | Host | Ref. | Reactivity | Host | Emission | Ref. |
| Collagen type I | Mouse | ab6308 | Mouse | Goat | 488 nm | ab150117 |
| Collagen type II | Rabbit | ab34712 | Rabbit | Goat | 488 nm | ab150081 |
| Collagen type III | Rabbit | ab7778 | Mouse | Donkey | 594 nm | SAB4600098 |
| Collagen type IV | Rabbit | ab6586 | ||||
| Fibronectin | Rabbit | ab2413 | ||||
| Actin | Mouse | sc-47778 | ||||
| Laminin | Rabbit | ab11575 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubí-Sans, G.; Cano-Torres, I.; Pérez-Amodio, S.; Blanco-Fernandez, B.; Mateos-Timoneda, M.A.; Engel, E. Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template. Biomedicines 2021, 9, 232. https://doi.org/10.3390/biomedicines9030232
Rubí-Sans G, Cano-Torres I, Pérez-Amodio S, Blanco-Fernandez B, Mateos-Timoneda MA, Engel E. Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template. Biomedicines. 2021; 9(3):232. https://doi.org/10.3390/biomedicines9030232
Chicago/Turabian StyleRubí-Sans, Gerard, Irene Cano-Torres, Soledad Pérez-Amodio, Barbara Blanco-Fernandez, Miguel A. Mateos-Timoneda, and Elisabeth Engel. 2021. "Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template" Biomedicines 9, no. 3: 232. https://doi.org/10.3390/biomedicines9030232
APA StyleRubí-Sans, G., Cano-Torres, I., Pérez-Amodio, S., Blanco-Fernandez, B., Mateos-Timoneda, M. A., & Engel, E. (2021). Development and Angiogenic Potential of Cell-Derived Microtissues Using Microcarrier-Template. Biomedicines, 9(3), 232. https://doi.org/10.3390/biomedicines9030232