Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders
Abstract
1. Introduction
2. Search Strategy
Selection Criteria
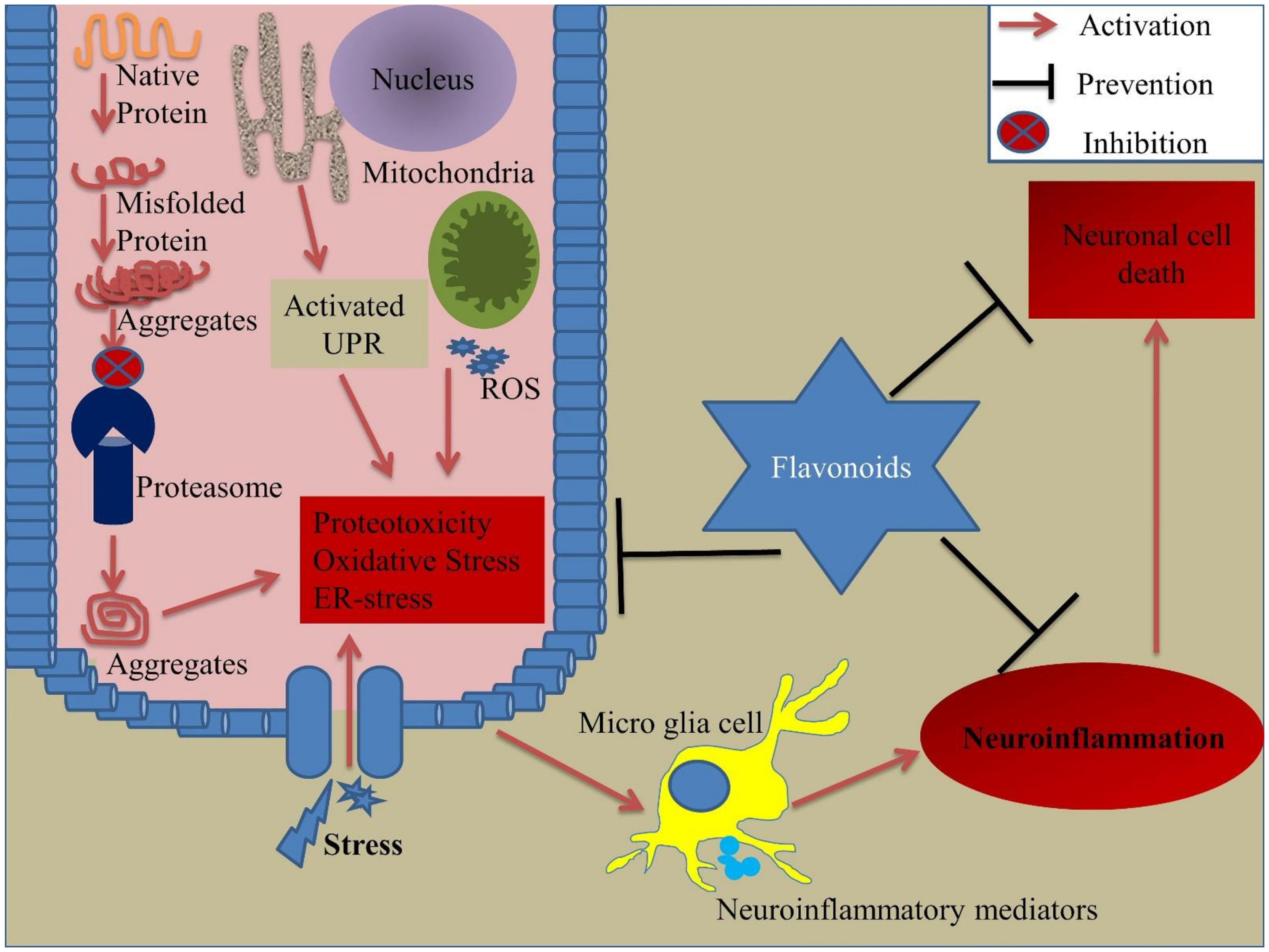
3. Environmental Stress and Cellular Stress Response
3.1. Environmental Stress
3.2. Cellular Stress Response
4. Flavonoids
5. Flavonoids and Cellular Stress Response
5.1. Role of Flavonoids in Neuroinflammation
5.2. Role of Flavonoids in Oxidative Stress
5.3. Role of Flavonoids in Proteotoxicity
5.4. Role of Flavonoids in Endoplasmic Reticulum (ER) Stress
6. Pre-Clinical/Clinical Studies of Flavonoids
7. Flavonoid Metabolism
8. Neuronal Access of Flavonoids
9. Flavonoid Extraction: A Key to Improved Flavonoid Property
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ER | Endoplasmic reticulum |
| PQC | Protein quality control |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| UPR | Unfolded protein response |
| HSP | Heat shock protein |
References
- Abeliovich, A.; Gitler, A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 2016, 539, 207–216. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Focus on neurodegenerative disease. Nat. Neurosci. 2018, 21, 1293. [CrossRef]
- Grippo, A.J.; Scotti, M.A. Stress and neuroinflammation. Mod. Trends Pharm. 2013, 28, 20–32. [Google Scholar] [CrossRef]
- Nakajima, Y.; Suzuki, S. Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner. Int. J. Mol. Sci. 2013, 14, 7771–7783. [Google Scholar] [CrossRef]
- Hohn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Sims, S.G.; Sanchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.H.; Seo, Y.H.; Jang, J.H.; Jeong, C.H.; Lee, S.; Jeong, G.S.; Park, B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019, 27, 145–151. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Chaurasia, J.K.; Mishra, A.; Tripathi, Y.B. Immunomodulation property of hexane fraction of leaves of Cinnamomum tamala Linn. in rats. Cell Biochem. Funct. 2010, 28, 454–460. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, A.; Cui, X.; Zhang, Y.; Xie, J. 6’”-p-Coumaroylspinosin protects PC12 neuronal cells from acrylamide-induced oxidative stress and apoptosis. J. Food Biochem. 2020, 44, e13321. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Lin, T.; Li, J.; Zhang, Y.; Zhou, A.; Xie, J. Ultrasonicated sour Jujube seed flavonoids extract exerts ameliorative antioxidant capacity and reduces Abeta-induced toxicity in Caenorhabditis elegans. J. Ethnopharmacol. 2019, 239, 111886. [Google Scholar] [CrossRef]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant. Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Jones, Q.R.; Warford, J.; Rupasinghe, H.P.; Robertson, G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012, 33, 602–610. [Google Scholar] [CrossRef]
- Maiti, S.; Nazmeen, A.; Medda, N.; Patra, R.; Ghosh, T.K. Flavonoids green tea against oxidant stress and inflammation with related human diseases. Clin. Nutr. Exp. 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Suen, J.; Thomas, J.; Kranz, A.; Vun, S.; Miller, M. Effect of Flavonoids on Oxidative Stress and Inflammation in Adults at Risk of Cardiovascular Disease: A Systematic Review. Healthcare 2016, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmed, N.; Rungatscher, A.; Linardi, D.; Kulsoom, B.; Innamorati, G.; Meo, S.A.; Gebrie, M.A.; Mani, R.; Merigo, F.; et al. Cocoa Flavonoids Reduce Inflammation and Oxidative Stress in a Myocardial Ischemia-Reperfusion Experimental Model. Antioxidants 2020, 9, 167. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverria, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sanchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-ElGhani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Haber-Weiss cycle--70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Rego, A.C.; Oliveira, C.R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003, 28, 1563–1574. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Ibstedt, S.; Sideri, T.C.; Grant, C.M.; Tamas, M.J. Global analysis of protein aggregation in yeast during physiological conditions and arsenite stress. Biol. Open 2014, 3, 913–923. [Google Scholar] [CrossRef]
- Jacobson, T.; Navarrete, C.; Sharma, S.K.; Sideri, T.C.; Ibstedt, S.; Priya, S.; Grant, C.M.; Christen, P.; Goloubinoff, P.; Tamas, M.J. Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast. J. Cell Sci. 2012, 125, 5073–5083. [Google Scholar] [CrossRef]
- Gardarin, A.; Chedin, S.; Lagniel, G.; Aude, J.C.; Godat, E.; Catty, P.; Labarre, J. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbiol. 2010, 76, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.G.; Ishiwata-Kimata, Y.; Kohno, K.; Kimata, Y. Cadmium impairs protein folding in the endoplasmic reticulum and induces the unfolded protein response. FEMS Yeast Res. 2016, 16, fow049. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.L.; Ghosh, E.; Avery, S.V. Chromate-induced sulfur starvation and mRNA mistranslation in yeast are linked in a common mechanism of Cr toxicity. Toxicol Vitr. 2010, 24, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Kumar, V.; Misra, U.K.; Bora, H.K. Memory and Learning Dysfunction Following Copper Toxicity: Biochemical and Immunohistochemical Basis. Mol. Neurobiol. 2018, 55, 3800–3811. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Kumar, V.; Misra, U.K.; Bora, H.K. Movement Disorder in Copper Toxicity Rat Model: Role of Inflammation and Apoptosis in the Corpus Striatum. Neurotox. Res. 2020, 37, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.S.; Melo, T.Q.; Martins, S.A.; Ferrari, M.F. Protein aggregation containing beta-amyloid, alpha-synuclein and hyperphosphorylated tau in cultured cells of hippocampus, substantia nigra and locus coeruleus after rotenone exposure. BMC Neurosci. 2010, 11, 144. [Google Scholar] [CrossRef]
- Deshmukh, R.S.; Chaudhary, R.K.; Roy, I. Effect of pesticides on the aggregation of mutant huntingtin protein. Mol. Neurobiol. 2012, 45, 405–414. [Google Scholar] [CrossRef]
- Yang, W.; Tiffany-Castiglioni, E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. J. Toxicol. Environ. Health A 2007, 70, 1849–1857. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Garrido, C.; Paul, C.; Seigneuric, R.; Kampinga, H.H. The small heat shock proteins family: The long forgotten chaperones. Int. J. Biochem. Cell Biol. 2012, 44, 1588–1592. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yenari, M. Heat Shock Proteins and the Stress Response. In Primer on Cerebrovascular Diseases; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 273–275. [Google Scholar]
- Pockley, A.G.; Henderson, B. Extracellular cell stress (heat shock) proteins-immune responses and disease: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160522. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C. Size matters: Of the small HSP27 and its large oligomers. Cell Death Differ. 2002, 9, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, E.S.; Wang, T.; Luo, K.; Xiao, Z.; Niewiadomska, A.M.; Martinez, T.; Xu, W.; Neckers, L.; Yu, X.F. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2009, 106, 20330–20335. [Google Scholar] [CrossRef]
- Theodoraki, M.A.; Caplan, A.J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta 2012, 1823, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, M.; Matsuyama, T.; Yamaguchi, A.; Mitsuda, N.; Tsukamoto, Y.; Taniguchi, M.; Che, Y.H.; Ozawa, K.; Hori, O.; Nishimura, H.; et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat. Med. 2001, 7, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Samali, A.; Jager, R. Methods for studying ER stress and UPR markers in human cells. Methods Mol. Biol. 2015, 1292, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive Oxygen and Nitrogen Species Regulate Key Metabolic, Anabolic, and Catabolic Pathways in Skeletal Muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Diniz, T.C.; Almeida, J.R.G.D.S.; De Lima-Saraiva, S.R.G.; Ribeiro, F.P.R.D.A.; Pacheco, A.G.M.; De Freitas, R.M.; Quintans-Júnior, L.J.; Quintans, J.D.S.S.; Mendes, D.F.R.; De Almeida, R.F.P.R. The role of flavonoids on oxidative stress in epilepsy. Oxidative Med. Cell. Longev. 2015, 2015, 171756. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017, 4, 19. [Google Scholar] [CrossRef]
- Gratton, G.; Weaver, S.R.; Burley, C.V.; Low, K.A.; Maclin, E.L.; Johns, P.W.; Pham, Q.S.; Lucas, S.J.E.; Fabiani, M.; Rendeiro, C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020, 10, 19409. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.J.; Song, T.T.; Murphy, P.A.; Hendrich, S. Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity. J. Nutr. 1999, 129, 957–962. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the isoflavones genistein and daidzein in non-legume dicot and monocot tissues. Plant. Physiol. 2000, 124, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M.; Biggs, D.R.; Perrin, D.R.; Whittle, C.P. Phaseollin and phaseollidin relationships in infection-droplets on endocarp of Phaseolus vulgaris. Physiol. Plant. Pathol. 1974, 4, 261–276. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, O.A.; Ivashev, M.N.; Ozimina, I.I.; Maslikova, G.V. Diosmetin glycosides from caucasian vetch: Isolation and study of biological activity. Pharm. Chem. J. 1998, 32, 595–597. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; Wilson, B.; Lee, C.; Flood, P.; Langenbach, R.; Hong, J.S. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol. Appl. Pharmacol. 2009, 238, 64–70. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Kim, S.Y.; Suk, K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001, 309, 67–71. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1beta production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Aruna, R.; Geetha, A.; Suguna, P. Rutin modulates ASC expression in NLRP3 inflammasome: A study in alcohol and cerulein-induced rat model of pancreatitis. Mol. Cell. Biochem. 2014, 396, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Lin, Y.S.; Sheu, S.Y.; Sun, J.S. Anti-inflammatory effects of daidzein on primary astroglial cell culture. Nutr. Neurosci. 2009, 12, 123–134. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, L.J.; Tashiro, S.; Gao, H.Y.; Onodera, S.; Ikejima, T. (+)-Catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced DNA damage and cell cycle arrest. J. Pharmacol. Sci. 2005, 98, 16–24. [Google Scholar] [CrossRef]
- Lau, F.C.; Bielinski, D.F.; Joseph, J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007, 85, 1010–1017. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar] [CrossRef]
- Chen, H.Q.; Jin, Z.Y.; Li, G.H. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007, 417, 112–117. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Kim, S.J.; Kim, S.J.; et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef]
- Adams, S.M.; Aksenova, M.V.; Aksenov, M.Y.; Mactutus, C.F.; Booze, R.M. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 Tat(1-86) exposure. PLoS ONE 2012, 7, e37540. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Tikhonova, N.G.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules 2020, 25, 5339. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Jin, Z.Y.; Wang, X.J.; Xu, X.M.; Deng, L.; Zhao, J.W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci. Lett. 2008, 448, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Jeong, K.H.; Jung, U.J.; Kim, S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016, 28, 140–146. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Xue, X.; Liu, H.; Qi, L.; Li, X.; Guo, C.; Gong, D.; Qu, H. Baicalein ameliorated the upregulation of striatal glutamatergic transmission in the mice model of Parkinson’s disease. Brain Res. Bull. 2014, 103, 54–59. [Google Scholar] [CrossRef]
- Hung, K.C.; Huang, H.J.; Wang, Y.T.; Lin, A.M. Baicalein attenuates alpha-synuclein aggregation, inflammasome activation and autophagy in the MPP(+)-treated nigrostriatal dopaminergic system in vivo. J. Ethnopharmacol. 2016, 194, 522–529. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Facheris, M.; Beretta, S.; Ferrarese, C. Peripheral markers of oxidative stress and excitotoxicity in neurodegenerative disorders: Tools for diagnosis and therapy? J. Alzheimers Dis. 2004, 6, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Quilantang, N.G.; Kim, H.Y.; Lee, S.; Cho, E.J. Attenuation of hydrogen peroxide-induced oxidative stress in SH-SY5Y cells by three flavonoids from Acer okamotoanum. Chem. Pap. 2019, 73, 1135–1144. [Google Scholar] [CrossRef]
- Pavlica, S.; Gebhardt, R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010, 86, 79–86. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Tu, J.; He, M.; Jiang, Y.; Yang, B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020, 315, 126236. [Google Scholar] [CrossRef]
- Barreca, D.; Curro, M.; Bellocco, E.; Ficarra, S.; Lagana, G.; Tellone, E.; Giunta, M.L.; Visalli, G.; Caccamo, D.; Galtieri, A.; et al. Neuroprotective effects of phloretin and its glycosylated derivative on rotenone-induced toxicity in human SH-SY5Y neuronal-like cells. BioFactors 2017, 43, 549–557. [Google Scholar] [CrossRef]
- Mu, X.; He, G.; Cheng, Y.; Li, X.; Xu, B.; Du, G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009, 92, 642–648. [Google Scholar] [CrossRef]
- Cheng, Y.; He, G.; Mu, X.; Zhang, T.; Li, X.; Hu, J.; Xu, B.; Du, G. Neuroprotective effect of baicalein against MPTP neurotoxicity: Behavioral, biochemical and immunohistochemical profile. Neurosci. Lett. 2008, 441, 16–20. [Google Scholar] [CrossRef]
- Jiang, M.; Porat-Shliom, Y.; Pei, Z.; Cheng, Y.; Xiang, L.; Sommers, K.; Li, Q.; Gillardon, F.; Hengerer, B.; Berlinicke, C.; et al. Baicalein reduces E46K alpha-synuclein aggregation in vitro and protects cells against E46K alpha-synuclein toxicity in cell models of familiar Parkinsonism. J. Neurochem. 2010, 114, 419–429. [Google Scholar] [CrossRef]
- Lu, J.H.; Ardah, M.T.; Durairajan, S.S.; Liu, L.F.; Xie, L.X.; Fong, W.F.; Hasan, M.Y.; Huang, J.D.; El-Agnaf, O.M.; Li, M. Baicalein inhibits formation of alpha-synuclein oligomers within living cells and prevents Abeta peptide fibrillation and oligomerisation. ChemBioChem 2011, 12, 615–624. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, H.T.; Pu, X.P.; Du, G.H. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules 2013, 18, 14726–14738. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, H.R.; Ji, S.T.; Lee, Y.; Lee, J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-kappaB, ERK, and JNK. J. Neurosci. Res. 2014, 92, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int. J. Mol. Med. 2013, 32, 235–240. [Google Scholar] [CrossRef]
- Li, S.; Pu, X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; De Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2012, 2012, 928643. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef]
- Hu, L.W.; Yen, J.H.; Shen, Y.T.; Wu, K.Y.; Wu, M.J. Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS ONE 2014, 9, e97880. [Google Scholar] [CrossRef]
- Hu, Q.; Uversky, V.N.; Huang, M.; Kang, H.; Xu, F.; Liu, X.; Lian, L.; Liang, Q.; Jiang, H.; Liu, A.; et al. Baicalein inhibits alpha-synuclein oligomer formation and prevents progression of alpha-synuclein accumulation in a rotenone mouse model of Parkinson’s disease. Biochim. Biophys. Acta 2016, 1862, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Poetini, M.R.; Araujo, S.M.; De Paula, M.T.; Bortolotto, V.C.; Meichtry, L.B.; De Almeida, F.P.; Jesse, C.R.; Kunz, S.N.; Prigol, M. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson’s disease. Chem. Biol. Interact. 2018, 279, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.S.; Goes, A.T.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Priya, S. Expanding role of molecular chaperones in regulating alpha-synuclein misfolding; implications in Parkinson’s disease. Cell. Mol. Life Sci. 2017, 74, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.M.; Balch, W.E. Modeling general proteostasis: Proteome balance in health and disease. Curr. Opin. Cell Biol. 2011, 23, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.M.; Samant, R.S.; Frydman, J. Mechanisms and Functions of Spatial Protein Quality Control. Annu. Rev. Biochem. 2017, 86, 97–122. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, S.; Chou, A.P.; Bronstein, J.M. Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson’s disease. Neurobiol. Dis. 2006, 23, 198–205. [Google Scholar] [CrossRef]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M.; et al. Flavonoid-Derived Human Phenyl-gamma-Valerolactone Metabolites Selectively Detoxify Amyloid-beta Oligomers and Prevent Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2020, 64, e1900890. [Google Scholar] [CrossRef]
- Cook, C.; Stetler, C.; Petrucelli, L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009423. [Google Scholar] [CrossRef]
- Joshi, V.; Mishra, R.; Upadhyay, A.; Amanullah, A.; Poluri, K.M.; Singh, S.; Kumar, A.; Mishra, A. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J. Cell. Physiol. 2019, 234, 20900–20914. [Google Scholar] [CrossRef]
- Budagova, K.R.; Zhmaeva, S.V.; Grigor’ev, A.N.; Goncharova, A.Y.; Kabakov, A.E. Flavonoid dihydroquercetin, unlike quercetin, fails to inhibit expression of heat shock proteins under conditions of cellular stress. Biochemistry 2003, 68, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Park, K.C.; Awasthi, A.; Prasad, B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer’s model. CNS Neurol. Disord. Drug Targets 2015, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wu, J.; Wang, X.; Huang, J.; Shen, Z.; Chen, X. Epimedium flavonoids mitigate proteotoxicity and extend healthspan via DAF-16 in C. elegans. Tradit. Med. Mod. Med. 2019, 2, 19–25. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, X.; Zhang, Y.; Xie, J.; Zhou, A. 6‴-Feruloylspinosin alleviated beta-amyloid induced toxicity by promoting mitophagy in Caenorhabditis elegans (GMC101) and PC12 cells. Sci. Total. Environ. 2020, 715, 136953. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Ravanan, P. Kaempferol mitigates Endoplasmic Reticulum Stress Induced Cell Death by targeting caspase 3/7. Sci Rep. 2018, 8, 2189. [Google Scholar] [CrossRef]
- Takano, K.; Tabata, Y.; Kitao, Y.; Murakami, R.; Suzuki, H.; Yamada, M.; Iinuma, M.; Yoneda, Y.; Ogawa, S.; Hori, O. Methoxyflavones protect cells against endoplasmic reticulum stress and neurotoxin. Am. J. Physiol. Cell Physiol. 2007, 292, C353–C361. [Google Scholar] [CrossRef]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Li, L.; Shi, W.; Yuan, X.; Wu, L. The Natural Occurring Compounds Targeting Endoplasmic Reticulum Stress. Evid. Based Complement. Alternat. Med. 2016, 2016, 7831282. [Google Scholar] [CrossRef]
- Uddin, M.S.; Tewari, D.; Sharma, G.; Kabir, M.T.; Barreto, G.E.; Bin-Jumah, M.N.; Perveen, A.; Abdel-Daim, M.M.; Ashraf, G.M. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 2902–2919. [Google Scholar] [CrossRef]
- Chiang, C.K.; Hsu, S.P.; Wu, C.T.; Huang, J.W.; Cheng, H.T.; Chang, Y.W.; Hung, K.Y.; Wu, K.D.; Liu, S.H. Endoplasmic reticulum stress implicated in the development of renal fibrosis. Mol. Med. 2011, 17, 1295–1305. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Paulmurugan, R.; Ramkumar, K.M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014, 47, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.S.; Choi, D.; Han, Y.R.; Kim, N.; Jeong, H.S. Morin has protective potential against ER stress induced apoptosis in renal proximal tubular HK-2 cells. Biomed. Pharmacother. 2019, 112, 108659. [Google Scholar] [CrossRef] [PubMed]
- Dourado, N.S.; Souza, C.D.S.; De Almeida, M.M.A.; Da Silva, A.B.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; Da Silva, J.S.; Souza, D.O.; Costa, M.D.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-kappaB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Cordeiro, L.M.; Machado, M.L.; Da Silva, A.F.; Baptista, F.B.O.; Da Silveira, T.L.; Soares, F.A.A.; Arantes, L.P. Rutin protects Huntington’s disease through the insulin/IGF1 (IIS) signaling pathway and autophagy activity: Study in Caenorhabditis elegans model. Food Chem. Toxicol. 2020, 141, 111323. [Google Scholar] [CrossRef]
- Teles, R.B.D.A.; Diniz, T.C.; Pinto, T.C.C.; Júnior, R.G.D.O.; Silva, M.G.E.; De Lavor, É.M.; Fernandes, A.W.C.; De Oliveira, A.P.; Ribeiro, F.P.R.D.A.; Da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxidative Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2012, 37, 434–444. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Puerta, E.; Suarez-Santiago, J.E.; Santos-Magalhaes, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Thangarajan, S.; Ramachandran, S.; Krishnamurthy, P. Chrysin exerts neuroprotective effects against 3-Nitropropionic acid induced behavioral despair-Mitochondrial dysfunction and striatal apoptosis via upregulating Bcl-2 gene and downregulating Bax-Bad genes in male wistar rats. Biomed. Pharmacother. 2016, 84, 514–525. [Google Scholar] [CrossRef]
- Barriga, G.G.-D.; Giralt, A.; Anglada-Huguet, M.; Gaja-Capdevila, N.; Orlandi, J.G.; Soriano, J.; Canals, J.-M.; Alberch, J. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCgamma1 pathway. Hum. Mol. Genet. 2017, 26, 3144–3160. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef]
- Kreilaus, F.; Spiro, A.S.; Hannan, A.J.; Garner, B.; Jenner, A.M. Therapeutic Effects of Anthocyanins and Environmental Enrichment in R6/1 Huntington’s Disease Mice. J. Huntingt. Dis. 2016, 5, 285–296. [Google Scholar] [CrossRef]
- Menze, E.T.; Tadros, M.G.; Abdel-Tawab, A.M.; Khalifa, A.E. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology 2012, 33, 1265–1275. [Google Scholar] [CrossRef]
- Stephen, M.; Woo, J.; Hasan, N.U.; Whiting, P.H.; Thomson, A.W. Immunosuppressive activity, lymphocyte subset analysis, and acute toxicity of FK-506 in the rat. A comparative and combination study with cyclosporine. Transplantation 1989, 47, 60–65. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, S.; Li, X.; Luo, G.; Li, L.; Le, W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006, 31, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Walgren, R.A.; Lin, J.T.; Kinne, R.K.; Walle, T. Cellular uptake of dietary flavonoid quercetin 4’-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000, 294, 837–843. [Google Scholar] [PubMed]
- Van der Woude, H.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Identification of 14 quercetin phase II mono- and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem. Res. Toxicol. 2004, 17, 1520–1530. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Murota, K.; Kumamoto, S.; Misumi, K.; Bando, N.; Ikushiro, S.; Takahashi, N.; Sekido, K.; Kato, Y.; Terao, J. Plasma metabolites of dietary flavonoids after combination meal consumption with onion and tofu in humans. Mol. Nutr. Food Res. 2014, 58, 310–317. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005, 579, 5343–5346. [Google Scholar] [CrossRef]
- Schaffer, S.; Halliwell, B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with alpha-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid. Based Complement. Altern. Med. 2017, 2017, 3019568. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, L.; Xu, L.; Ma, Y.; Lian, W.; Liu, Y.; Wang, Y. Optimized Extraction of Total Triterpenoids from Jujube (Ziziphus jujuba Mill.) and Comprehensive Analysis of Triterpenic Acids in Different Cultivars. Plants 2020, 9, 412. [Google Scholar] [CrossRef]
- Berkani, F.; Serralheiro, M.L.; Dahmoune, F.; Ressaissi, A.; Kadri, N.; Remini, H. Ultrasound Assisted Extraction of Phenolic Compounds from a Jujube By-Product with Valuable Bioactivities. Processes 2020, 8, 1441. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules 2018, 24, 122. [Google Scholar] [CrossRef] [PubMed]
| Subgroup | Chemical Structure | Plant Source | Example | Ref. |
|---|---|---|---|---|
| Isoflavones | 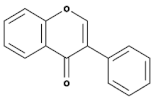 | Soybeans, leguminous plants, microbes, | Genistein, Daidzein, Glycerin, Formanantine | [71,72,73,74] |
| Flavones | 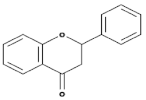 | Leaves, flowers, and fruits | Luteolin, Apigenin | [75,76] |
| Flavanones | 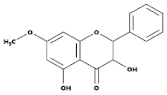 | All citrus fruits | Hesperidin, Naringenin | [77] |
| Flavonols | 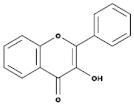 | Onions, berries, lettuce, tomatoes, grapes, and apples | Kaempferol, Quercetin | [78] |
| Neoflavonoids | 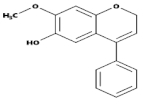 | Sri Lankan endemic plant Mesuathwaitesii | Calophyllolide | [79,80] |
| Flavanols(Flavan-3-ols) | 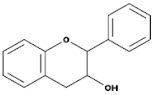 | Peaches, pears, blueberries, bananas, and apples | Catechins, Epicatechins, Epigallocatechin | [81,82] |
| Anthocyanins | 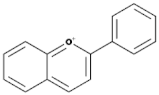 | Bilberries, cranberries, merlot grapes, blackberries, black currants, red grapes, strawberries, blueberries, and raspberries | Cyanidin, Delphinidin, Malvidine | [83] |
| Flavones | 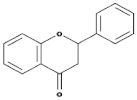 | Leaves, flowers, and fruits | Luteolin, Apigenin | [84,85] |
| Flavanones | 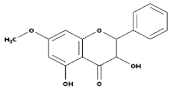 | All citrus fruits | Hesperidin, Naringenin | [77] |
| Flavonoids | Cellular Stress Response | Host Model | Ref |
|---|---|---|---|
| Kaempferol | Inhibits the expression of GRP78 (a chaperone) and CHOP (ER stress associated pro-apoptotic transcription factor) | Human IMR32 | [146] |
| Quercetin | Reduction in the expression of glucose-regulated protein 78 (GRP78) and C/EBP-homologous protein (CHOP) | Human umbilical vein endothelial cells | [152] |
| Morin | Inhibition of the expression of GRP78, Decreased ROS and apoptosis | renal proximal tubular HK-2 cells | [153] |
| Methoxyflavones | Activation of the UPR pathway via activating eIF2α and Nrf2 and induces the expression of downstream genes, such as GRP78, HO-1, and CHOP, without causing ER stress | Mouse insulinoma MIN6 cells | [147] |
| Agathisflavone | Increases the remyelination and alters microglial activation state. Neuroprotective effect via increase the expression of neurotrophic factors ciliary neurotrophic factor (Cntf), epidermal growth factor receptor (Egfr), and neuronal GABA b1 receptor subunit (Gabrb1) | Mice belonging to the C57BL/6 background | [154] |
| Apigenin | Neuroprotection, astrocytes integrity and have an anti-neuro-inflammatory response. These responses are generated via the modulation of inflammatory cytokines mRNA expression and reduce the expression of OX42, IL-6, and gp130. Induces the expression of brain-derived neurotrophic factor (BDNF). | Wistar rats’ hemispheres brain’s Glial cells and neurons | [154] |
| Hesperetin | Reduction of the expression of inflammatory Cytokines by ameliorating Toll-like receptor-4 (TLR4)-mediated ionized calcium-binding adapter molecule 1/glial fibrillary acidic protein (Iba-1/GFAP) expression. Attenuation in the LPS-induced generation of reactive oxygen species/lipid peroxidation (ROS/LPO) and improved the antioxidant protein level, such as nuclear factor erythroid 2-related factor 2 (Nrf2) and Haem-oxygenase (HO-1), in the mouse brain | C57BL/6 N mice | [155] |
| Epimedium | Have anti-proteotoxic potency as it reduces the Aβ1–42- and polyQ-induced paralysis in CL4176 and AM140 | C. elegans human proteotoxic disease models (CL4176, AM140) | [144] |
| Rutin | Rutin treatment reduces polyglutamine (polyQ) protein aggregation in muscle, reduced polyQ-mediated neuronal death in ASH sensory neurons, and extended lifespan. | C. elegans model of Huntington’s disease | [156] |
| phenyl-γ-valerolactones (metabolites of flavan-3-ols) | (4′-OH)-PVL interferes with AβO (but not fibril) assembly and actively remodels performed AβOs into nontoxic amorphous aggregate. | Yeast strains expressing different variants of the human Aβ42 and β23 peptides | [139] |
| Disease | Clinical Onsets | Behavioral Onsets | Disease Model | Flavonoids | Dose | Effect of Flavonoids Treatment on the Animal Model | Ref. |
|---|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Presence of extracellular neuritic plaques containing (Aβ) peptide and intracellular neurofibrillary tangles containing tau | AD results in a progressive loss of cognitive ability and eventually daily function activities | 5 × FAD model | 7,8-dihydroxyflavone (7,8-DHF) | IP injection (5 mg/kg) | Improved memory | [160] |
| Oral administration (5 mg/kg/day) | Improvement in memory and reduction in synapse loss | [161] | |||||
| 2 × FAD model | Apigenin | Oral administration (40 mg/kg/day) | Improvement in learning and memory, reduction in deposition of insoluble Aβ | [162] | |||
| 1 × FAD model, 3 × FAD model, SAMP8 mice | Nobiletin | IP injection (10 mg/kg) | Improvement in memory and reduction in levels of both soluble and insoluble Aβ | [163] | |||
| IP injections (10 and 30 mg/kg) | Improvement in memory; reduction in soluble Aβ levels | [164] | |||||
| 1 × FAD model | Baicalein | IP injections 10 and 50 mg/kg | Improves the memory, reduces some markers of oxidative stress | [162] | |||
| (SAMP8) | Quercetin | IP injections (10 mg/kg) | Improves working memory and reduces the production of Aβ | [165] | |||
| Oral administration (25 mg/kg/day) | Reduces the markers of oxidative stress, LPO and activates the ERK pathway | ||||||
| Huntington’s disease (HD) | Presence of a trinucleotide repeat (CAG) that encodes an abnormally long polyglutamine tract in the huntingtin protein | Movement and psychiatric disturbances, as well as cognitive impairment | 3-NP model of HD in rats | Chrysin | Oral administration (50 mg/kg/day) | Improvement in behavior and reduction in markers of oxidative stress and cell death, and enhancement in the survival of striatal neurons | [166] |
| R6/1 N-terminal transgenic mouse model | 7,8-DHF | Oral administration (5 mg/kg/day) | Delay the development of motor and cognitive deficits, prevention of the loss of striatal volume, enhances the marker of neurotrophic factor signaling, and reduction in some markers of inflammation | [167] | |||
| 3-NP model | Quercetin | oral administration (25 mg/kg/day) | Reduce motor deficits, improve mitochondrial function, and attenuate some markers of oxidative stress | [168] | |||
| R6/1 N-terminal transgenic mouse model | Anthocyanins | 100 mg/kg/day | Delay the loss of motor function | [169] | |||
| 3-NP model in rats | Hesperidin | Oral administration (100 mg/kg/day) | Reduce motor deficits, as well as markers of inflammation and oxidative stress | [170] | |||
| Amyotrophic Lateral Sclerosis (ALS) | Heritable gene mutations | Loss of the motor neurons that control the voluntary movement of muscles, resulting in paralysis and death | SOD1-G93A model | 7,8-DHF | IP injection (5 mg/kg) | Reduction in the age-dependent decrease in motor performance and preserving the total motor neuron count and dendritic spine density on motor neurons | [171] |
| Fisetin | Oral administration (9 mg/kg) | Delay the development of motor deficits, reduction in their rate of progression, and increases lifespan | [172] | ||||
| (−)-epigallocatechin gallate (EGCG) | oral administration (5.8–10 mg/kg) | Delay symptom onset and extend the lifespan | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. https://doi.org/10.3390/biomedicines9020099
Devi S, Kumar V, Singh SK, Dubey AK, Kim J-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines. 2021; 9(2):99. https://doi.org/10.3390/biomedicines9020099
Chicago/Turabian StyleDevi, Shweta, Vijay Kumar, Sandeep Kumar Singh, Ashish Kant Dubey, and Jong-Joo Kim. 2021. "Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders" Biomedicines 9, no. 2: 99. https://doi.org/10.3390/biomedicines9020099
APA StyleDevi, S., Kumar, V., Singh, S. K., Dubey, A. K., & Kim, J.-J. (2021). Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines, 9(2), 99. https://doi.org/10.3390/biomedicines9020099







