Pancreastatin Reduces Alternatively Activated Macrophages, Disrupts the Epithelial Homeostasis and Aggravates Colonic Inflammation. A Descriptive Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Active UC Participants and Healthy Individuals
2.2. Animals
2.3. Peptides
2.4. In Vivo Acute Dextran Sulfate Sodium (DSS)-Induced Colitis
2.5. External Disease Activity Index and Microscopic Assessment of Colitis
2.6. Macrophage Cell Culture
2.6.1. Macrophages Isolation from Colitic PST, sPST, and Non-PST Treated Groups
2.6.2. Macrophage Isolation from Naïve Mice
2.7. Human Intestinal Epithelial Cell Line
2.7.1. Lipopolysaccharides (LPS)- and DSS- Stimulated Epithelial Cells in the Presence or Absence of PST-Treated Polarized AAM Supernatants
2.7.2. Epithelial Cell Migration Assessed Using a Wound-Healing Assay
2.7.3. Epithelial Proliferation and Viability Assessed Using Cell Numbers and MTT Assay
2.7.4. Epithelial Cell Survival Using an Oxidative Stress Assay
2.8. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Data Analysis
3. Results
3.1. PST Is Increased in Participants with Active UC and Correlates with mRNA Expression of AAM, TJ Proteins, Epithelial Cells Associated Cytokines and Collagen in Human
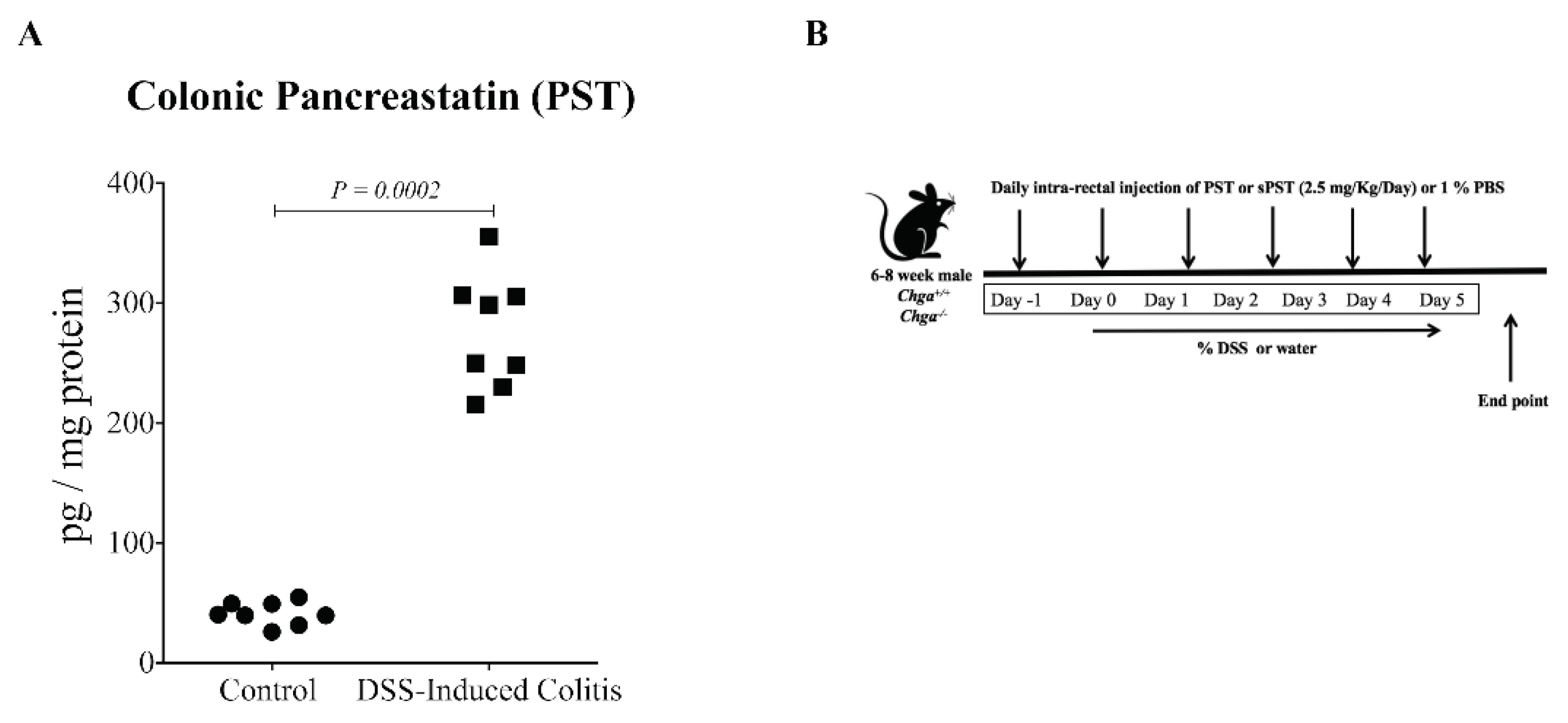
3.2. PST Is Increased, and Treatment Aggravates the Disease Activity in DSS-Induced Colitis
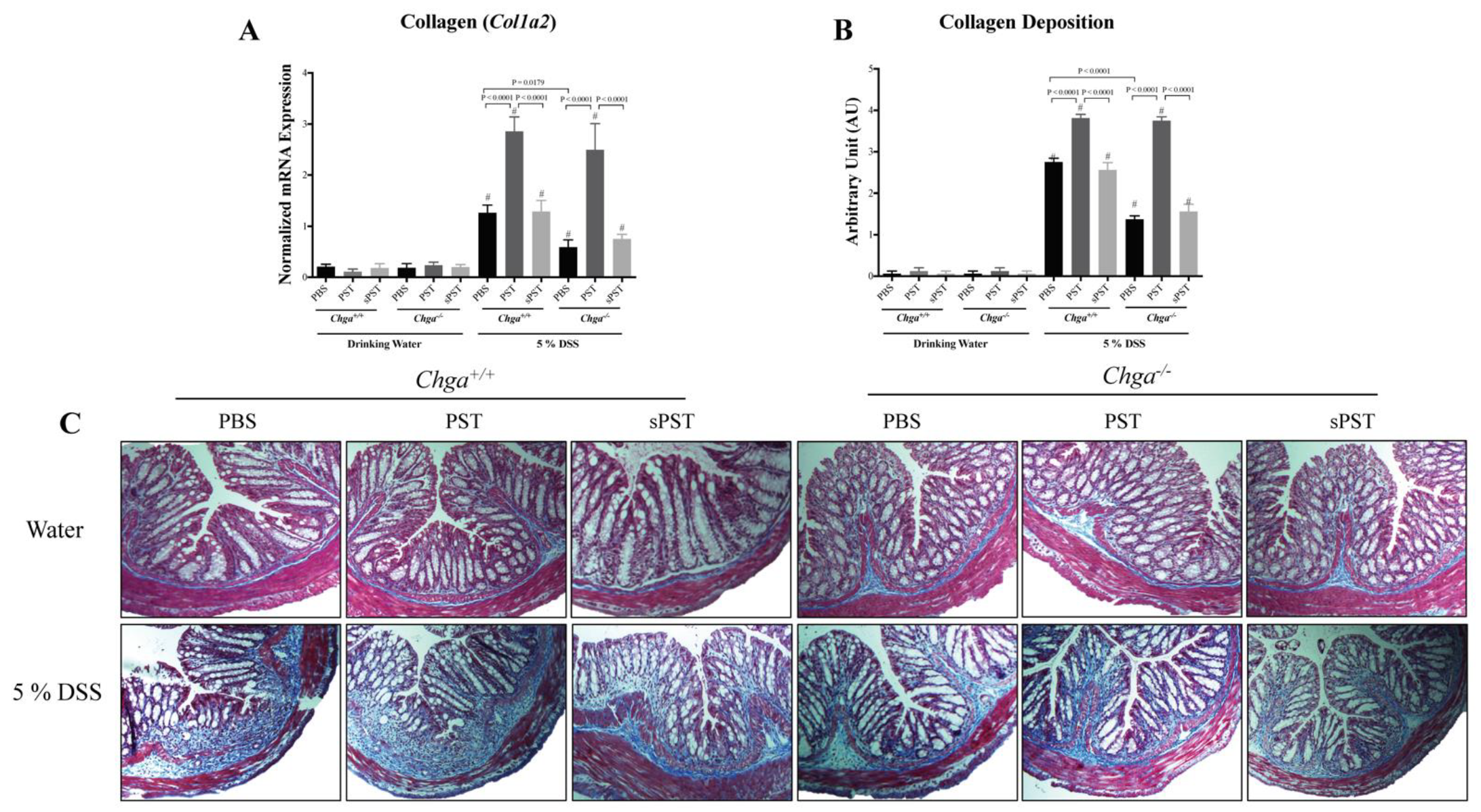
3.3. PST Treatment Promotes Colonic Expression and Deposition of Collagen in DSS-Induced Colitis
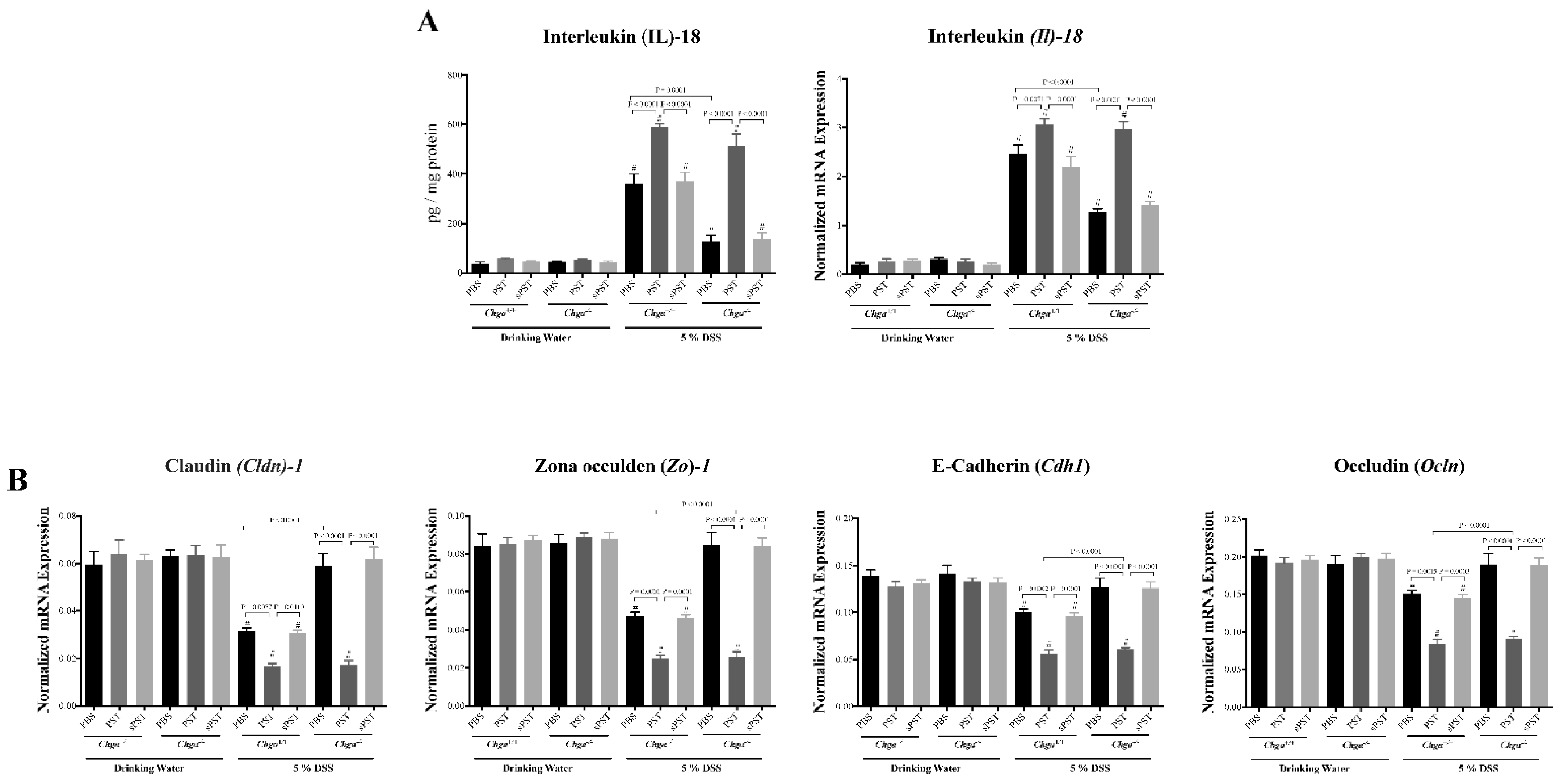
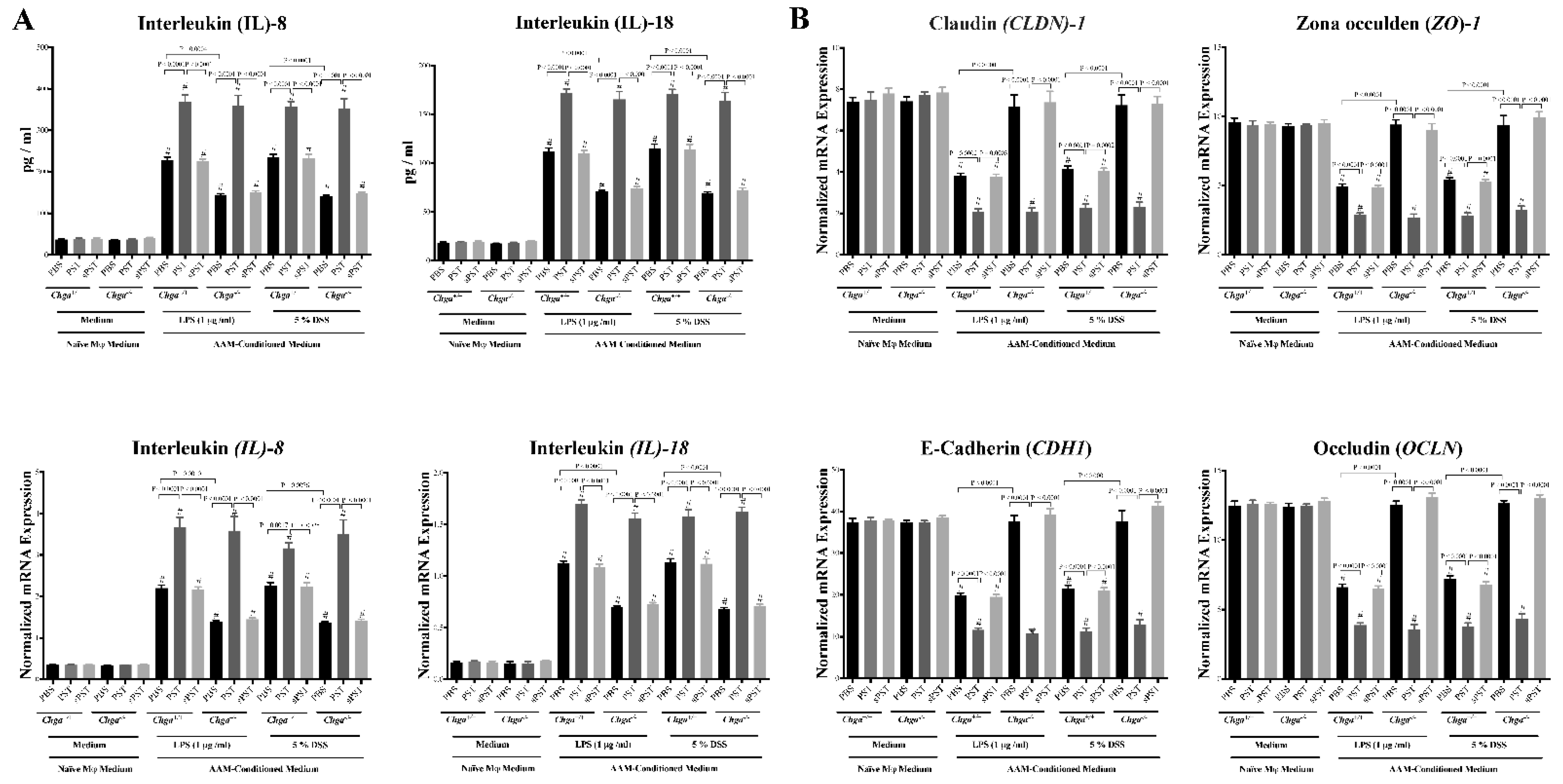
3.4. PST Treatment Promotes IL-18 Colonic Expression and Alters TJ Proteins Colonic Expression in DSS-Induced Colitis
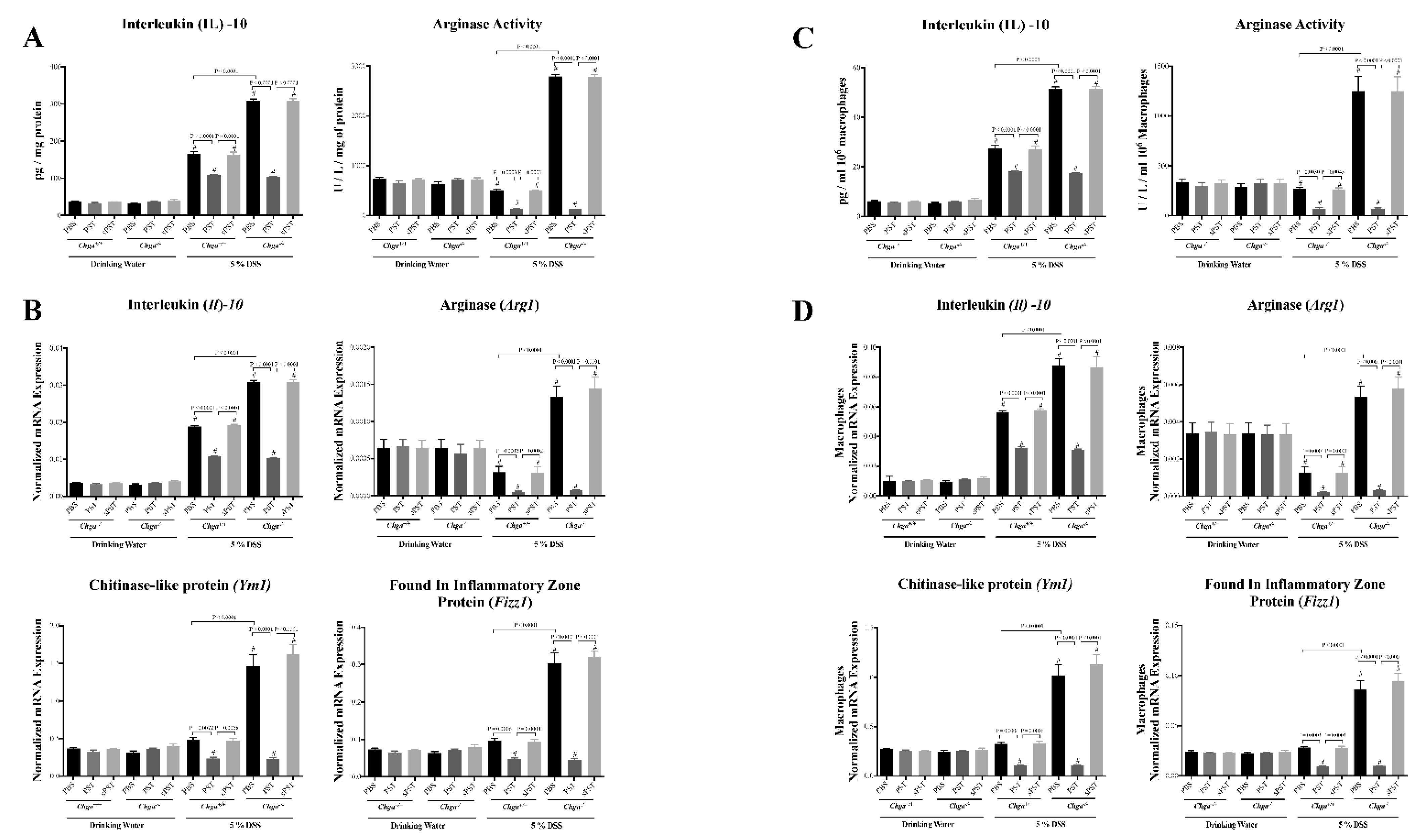
3.5. PST Treatment Decreases Colonic AAM Markers and Decreases AAM-Associated Anti-Inflammatory Mediators in DSS-Induced Colitis
3.6. In Vitro, PST Treatment Decreases the Functional Capacity of AAM
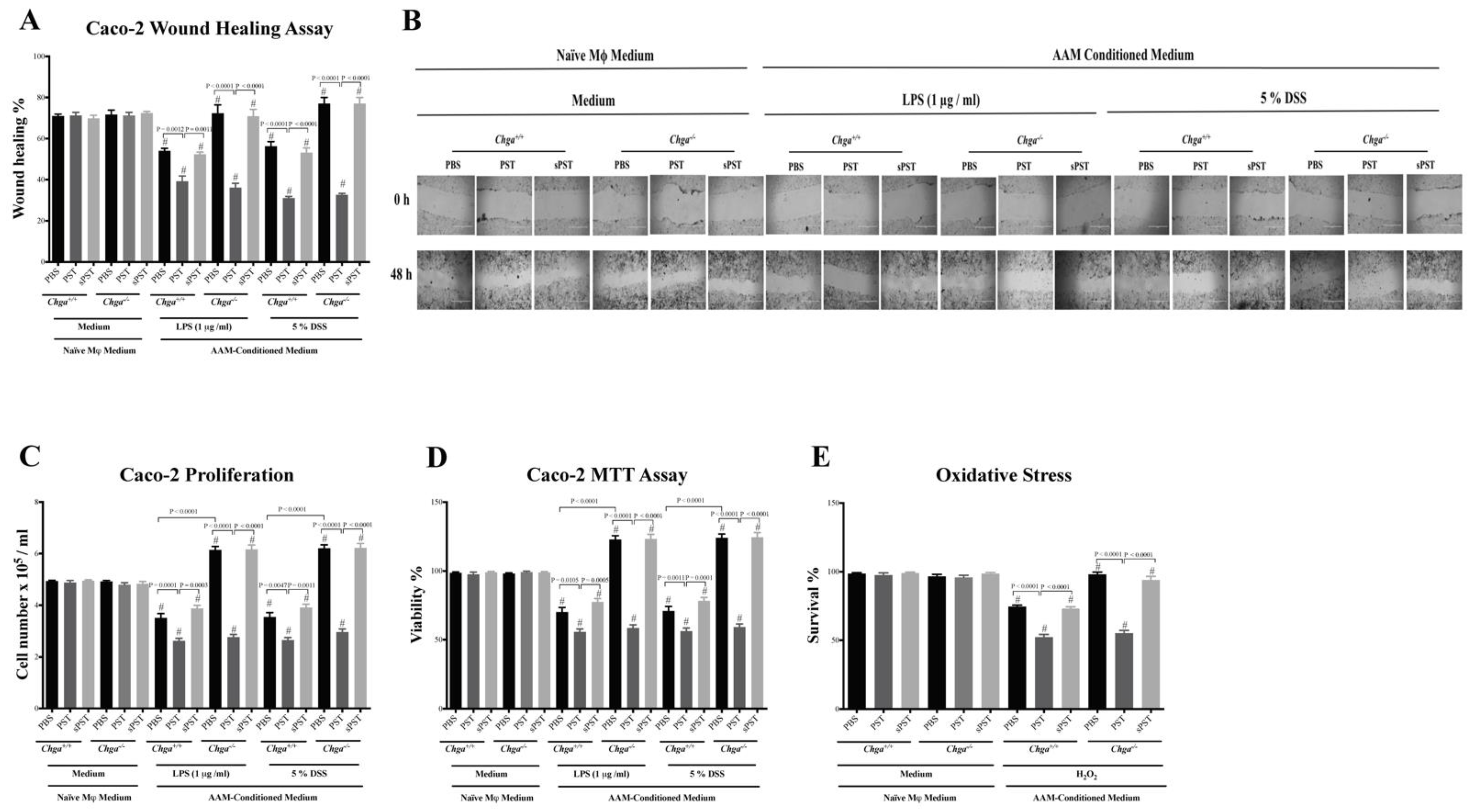
3.7. Conditioned Medium from PST-Pretreated Macrophages Polarized to an AAM Profile Increases IL-8 and IL-18 Release and Disrupts TJ Proteins’ Gene Expression in LPS- and DSS-Stimulated Colonic Epithelial Cells
3.8. Conditioned Medium from PST-Pretreated Macrophages Polarized to an AAM Profile Delays the Intestinal Repair
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cader, M.Z.; Kaser, A. Recent advances in inflammatory bowel disease: Mucosal immune cells in intestinal inflammation. Gut 2013, 62, 1653–1664. [Google Scholar] [CrossRef]
- Smith, P.; Smythies, L.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Ghia, J.-E. A Gene Expression Analysis of M1 and M2 Polarized Macrophages. In Immunometabolism; Springer: Berlin/Heidelberg, Germany, 2020; pp. 131–144. [Google Scholar]
- Eissa, N.; Hussein, H.; Tshikudi, D.M.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.-E. Interdependence between Chromogranin-A, Alternatively Activated Macrophages, Tight Junction Proteins and the Epithelial Functions. A Human and In-Vivo/In-Vitro Descriptive Study. Int. J. Mol. Sci. 2020, 21, 7976. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Roger, J.; Ortiz-Masiá, D.; Calatayud, S.; Hernández, C.; Álvarez, A.; Hinojosa, J.; Esplugues, J.V.; Barrachina, M.D. M2 macrophages activate WNT signaling pathway in epithelial cells: Relevance in ulcerative colitis. PLoS ONE 2013, 8, e78128. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Hisamatsu, T.; Kamada, N.; Kitazume, M.T.; Honda, H.; Oshima, Y.; Saito, R.; Takayama, T.; Kobayashi, T.; Chinen, H. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10–producing regulatory macrophage subset. J. Immunol. 2010, 184, 2671–2676. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Eissa, N.; Mujawar, Q.; Abdul-Salam, T.; Zohni, S.; El-Matary, W. The Immune-Sleep Crosstalk in Inflammatory Bowel Disease. Sleep. Med. 2020, 73, 38–46. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Kermarrec, L.; Grover, J.; Metz-Boutigue, M.-H.E.; Bernstein, C.N.; Ghia, J.-E. chromofungin ameliorates the Progression of colitis by regulating alternatively activated Macrophages. Front. Immunol. 2017, 8, 1131. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Kermarrec, L.; Elgazzar, O.; Metz-Boutigue, M.-H.; Bernstein, C.N.; Ghia, J.-E. Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses proinflammatory macrophage function through the inhibition of NF-κB signaling. Biochem. Pharm. 2017, 145, 102–113. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Kermarrec, L.; Ali, A.Y.; Marshall, A.; Metz-Boutigue, M.-H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.-E. Chromogranin-A regulates macrophage function and the apoptotic pathway in murine DSS colitis. J. Mol. Med. 2018, 96, 183–198. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Danielsson, Å.; Stenling, R.; Grimelius, L. Colonic endocrine cells in inflammatory bowel disease. J. Intern. Med. 1997, 242, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Sciola, V.; Massironi, S.; Conte, D.; Caprioli, F.; Ferrero, S.; Ciafardini, C.; Peracchi, M.; Bardella, M.T.; Piodi, L. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Rabbi, M.F.; Munyaka, P.M.; Khafipour, A.; Bernstein, C.N.; Ghia, J.-E. Mo1929 Critical Role of Chromogranin-A on Macrophage Intrinsic Apoptotic Pathway in Colitis: Human and Animal studies. Gastroenterology 2016, 150, S819. [Google Scholar] [CrossRef]
- D’amico, M.A.; Ghinassi, B.; Izzicupo, P.; Manzoli, L.; Di Baldassarre, A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr. Connect. 2014, 3, R45–R54. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.-E. Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem. Pharmacol. 2018, 152, 315–326. [Google Scholar] [CrossRef]
- Eissa, N.; Kermarrec, L.; Metz-Boutigue, M.-H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.-E. 654-Chromofungin Treatment Promotes Alternatively Activated Macrophages, Suppresses Classically Activated Macrophages and Improves Epithelial Cell Functions during Colitis. Gastroenterology 2017, 152, S143. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Eissa, N.; Munyaka, P.M.; Khafipour, A.; Khafipour, E.; Ghia, J.-E. Tu1893 Human Catestatin Represses Reactivation of Intestinal Inflammation in a Murine Model of Colitis Through the M1 Macrophages and Not the Gut Microbiota. Gastroenterology 2016, 150, S969. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Labis, B.; Metz-Boutigue, M.-H.; Bernstein, C.N.; Ghia, J.-E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharm. 2014, 89, 386–398. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Eissa, N.; Munyaka, P.M.; Kermarrec, L.; Elgazzar, O.; Khafipour, E.; Bernstein, C.N.; Ghia, J.E. Reactivation of intestinal inflammation is suppressed by catestatin in a murine model of colitis via M1 macrophages and not the gut microbiota. Front. Immunol. 2017, 8, 985. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Lu, M.; Avolio, E.; Siddiqui, J.A.; Gayen, J.R.; Wollam, J.; Vu, C.U.; Chi, N.W.; O’Connor, D.T.; Mahata, S.K. Pancreastatin-dependent inflammatory signaling mediates obesity-induced insulin resistance. Diabetes 2015, 64, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, M.F.; Munyaka, P.M.; Eissa, N.; Metz-Boutigue, M.-H.; Khafipour, E.; Ghia, J.E. Human Catestatin Alters Gut Microbiota Composition in Mice. Front. Microbiol. 2016, 7, 2151. [Google Scholar] [CrossRef] [PubMed]
- Rumio, C.; Dusio, G.F.; Colombo, B.; Gasparri, A.; Cardani, D.; Marcucci, F.; Corti, A. The N-terminal fragment of chromogranin A, vasostatin-1 protects mice from acute or chronic colitis upon oral administration. Dig. Dis. Sci. 2012, 57, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Mesgna, R.; Bonin, S.; Hendy, G.N.; Metz-Boutigue, M.-H.; Bernstein, C.N.; Ghia, J.-E. Catestatin regulates epithelial cell dynamics to improve intestinal inflammation. Vaccines 2018, 6, 67. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Rabbi, M.F.; Munyaka, P.M.; Khafipour, A.; Bernstein, C.N.; Ghia, J.-E. Tu1832 Stability of Reference Genes for Messenger RNA Quantification by Real-Time PCR in Mouse Dextran Sodium Sulfate Experimental Colitis. Gastroenterology 2016, 150, S955–S956. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.; Shah, R.; Sedergran, D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Kermarrec, L.; Eissa, N.; Wang, H.; Kapoor, K.; Diarra, A.; Gounni, A.S.; Bernstein, C.N.; Ghia, J.E. Semaphorin-3E attenuates intestinal inflammation through the regulation of the communication between splenic CD11C+ and CD4+ CD25− T-cells. Br. J. Pharm. 2019, 176, 1235–1250. [Google Scholar] [CrossRef]
- Eissa, N.; Hussein, H.; Diarra, A.; Elgazzar, O.; Gounni, A.S.; Bernstein, C.N.; Ghia, J.-E. Semaphorin 3E regulates apoptosis in the intestinal epithelium during the development of colitis. Biochem. Pharm. 2019, 166, 264–273. [Google Scholar] [CrossRef]
- Johnson, L.A.; Luke, A.; Sauder, K.; Moons, D.S.; Horowitz, J.C.; Higgins, P.D. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: Impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm. Bowel Dis. 2012, 18, 460–471. [Google Scholar] [CrossRef]
- Ding, S.; Walton, K.L.; Blue, R.E.; MacNaughton, K.; Magness, S.T.; Lund, P.K. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen α1 (I)-promoter-GFP reporter mice. PLoS ONE 2012, 7, e42568. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Activation of Murine Macrophages. In Current Protocols in Immunology; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Walsh-Reitz, M.M.; Huang, E.F.; Musch, M.W.; Chang, E.B.; Martin, T.E.; Kartha, S.; Toback, F.G. AMP-18 protects barrier function of colonic epithelial cells: Role of tight junction proteins. Am. J. Physiol.-Gastrointest. Liverp. Physiol. 2005, 289, G163–G171. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Wang, H.; Rabbi, M.F.; Bernstein, C.N.; Ghia, J.-E. Stability of Reference Genes for Messenger RNA Quantification by Real-Time PCR in Mouse Dextran Sodium Sulfate Experimental Colitis. PLoS ONE 2016, 11, e0156289. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Kermarrec, L.; Hussein, H.; Bernstein, C.N.; Ghia, J.-E. Appropriateness of reference genes for normalizing messenger RNA in mouse 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis using quantitative real time PCR. Sci. Rep. 2017, 7, 42427. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Westrich, G.; Kanaly, S.; Garka, K.; Born, T.; Derry, J.; Viney, J. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: Blocking interleukin 18 attenuates intestinal damage. Gut 2002, 50, 812–820. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef]
- Eissa, N.; Ghia, J. Immunomodulatory effect of ghrelin in the intestinal mucosa. Neurogastroenterol. Motil. 2015, 27, 1519–1527. [Google Scholar] [CrossRef]
- Eissa, N.; Rabbi, M.; Bernstein, C.; Ghia, J. Chromofungin & pancreastatin co-regulate migration and functional plasticity of murine peritoneal macrophages. Neurogastroenterol. Motil. 2016, 28, 103–104. [Google Scholar]
- Haribhai, D.; Ziegelbauer, J.; Jia, S.; Upchurch, K.; Yan, K.; Schmitt, E.G.; Salzman, N.H.; Simpson, P.; Hessner, M.J.; Chatila, T.A. Alternatively activated macrophages boost induced regulatory T and Th17 cell responses during immunotherapy for colitis. J. Immunol. 2016, 196, 3305–3317. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, L.; Tian, Y.; He, Y.; Zhang, Z.; Guo, P.; Ge, Y.; Hu, J. Spontaneous colitis in IL-10-deficient mice was ameliorated via inhibiting glutaminase1. J. Cell. Mol. Med. 2019, 23, 5632–5641. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M. Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-deficient mice. J. Crohn’s Colitis 2020, 14, 831–840. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in IBD—A dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 228–235. [Google Scholar] [CrossRef]
- Prasse, A.; Pechkovsky, D.V.; Toews, G.B.; Jungraithmayr, W.; Kollert, F.; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Res. Crit. Care Med. 2006, 173, 781–792. [Google Scholar] [CrossRef]
- Pesce, J.T.; Ramalingam, T.R.; Mentink-Kane, M.M.; Wilson, M.S.; El Kasmi, K.C.; Smith, A.M.; Thompson, R.W.; Cheever, A.W.; Murray, P.J.; Wynn, T.A. Arginase-1–expressing macrophages suppress Th2 cytokine–driven inflammation and fibrosis. PLoS Pathog. 2009, 5, e1000371. [Google Scholar] [CrossRef]
- Thomas, J.A.; Pope, C.; Wojtacha, D.; Robson, A.J.; Gordon-Walker, T.T.; Hartland, S.; Ramachandran, P.; Van Deemter, M.; Hume, D.A.; Iredale, J.P. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 2011, 53, 2003–2015. [Google Scholar] [CrossRef]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by Toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am. J. Patho. 2017, 187, 2698–2710. [Google Scholar] [CrossRef]
- Stremmel, W.; Staffer, S.; Schneider, M.J.; Gan-Schreier, H.; Wannhoff, A.; Stuhrmann, N.; Gauss, A.; Wolburg, H.; Mahringer, A.; Swidsinski, A. Genetic mouse models with intestinal-specific tight junction deletion resemble an ulcerative colitis phenotype. J. Crohn’s Colitis 2017, 11, 1247–1257. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Bianco, F.; Scott, R.B.; Keenan, C.M.; Linton, A.A.; O’Neill, C.H.; Bonora, E.; Dicay, M.; Lavoie, B.; Wilcox, R.L. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 2016, 151, 933–944. e933. [Google Scholar] [CrossRef] [PubMed]
- Valicherla, G.R.; Hossain, Z.; Mahata, S.K.; Gayen, J.R. Pancreastatin is an endogenous peptide that regulates glucose homeostasis. Physiol. Genom. 2013, 45, 1060–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munyaka, P.M.; Eissa, N.; Bernstein, C.N.; Khafipour, E.; Ghia, J.-E. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: A role of the gut microbiota. PLoS ONE 2015, 10, e0142536. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Khafipour, A.; Wang, H.; Eissa, N.; Khafipour, E.; Ghia, J.-E. Mo1774 Prenatal Antibiotic Treatment Increases Offspring’s Susceptibility to Experimental Colitis: A Role of the Gut Microbiota. Gastroenterology 2015, 148, S-708. [Google Scholar] [CrossRef]



| Gene Name | Forward | Reverse |
|---|---|---|
| IL10 | GACTTTAAGGGTTACCTGGGTTG | TCACATGCGCCTTGATGTCTG |
| MR | GGAGTGATGGTTCTCCTGTTTC | CCTTTCAGCTCACCACAGTATT |
| CD1B | ACTCAGGAAATCCAATCCTCCTA | ATAGCAGGCTGTGAGCTACAT |
| OCLDN | ACAAGCGGTTTTATCCAGAGTC | GTCATCCACAGGCGAAGTTAAT |
| TBP | CCCGAAACGCCGAATATAATCC | AATCAGTGCCGTGGTTCGTG |
| CLDN1 | AGGTGCTATCTGTTCAGTGATG | TGGCTGACTTTCCTTGTGTAG |
| CADH1 | CTTCTGCTGATCCTGTCTGATG | TGCTGTGAAGGGAGATGTATTG |
| ZO1 | CCAGCCTGCTAAACCTACTAAA | ATCTCTTGCTGCCAAACTATCT |
| COL1A2 | GAGCGGTAACAAGGGTGAGC | CTTCCCCATTAGGGCCTCTC |
| IL8 | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| IL18 | GCGTCACTACACTCAGCTAAT | GCGTCACTACACTCAGCTAAT |
| CHGA Exon-VII | GTTCCATGAAGCTCTCCTTCC | TCAAGGCTGTCCTCCCA |
| Gene | Forward | Reverse |
|---|---|---|
| Il10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Arg1 | TTGGGTGGATGCTCACACTG | GTACACGATGTCTTTGGCAGA |
| Il18 | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| Ym1 | CAGGTCTGGCAATTCTTCTGAA | GTCTTGCTCATGTGTGTAAGTGA |
| Fizz1 | AAGCCTACACTGTGTTTCCTTTT | GCTTCCTTGATCCTTTGATCCAC |
| Col1a2 | GGTGAGCCTGGTCAAACGG | ACTGTGTCCTTTCACGCCTTT |
| Eef2 | TGTCAGTCATCGCCCATGTG | CATCCTTGCGAGTGTCAGTGA |
| Ocldn | TTGAAAGTCCACCTCCTTACAGA | CCGGATAAAAAGAGTACGCTGG |
| Cldn1 | GGGGACAACATCGTGACCG | AGGAGTCGAAGACTTTGCACT |
| Zo1 | GCCGCTAAGAGCACAGCAA | TCCCCACTCTGAAAATGAGGA |
| Cadh1 | CATCCCAGAACCTCGAAACA | TGGGTTAGCTCAGCAGTAAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, N.; Elgazzar, O.; Hussein, H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.-E. Pancreastatin Reduces Alternatively Activated Macrophages, Disrupts the Epithelial Homeostasis and Aggravates Colonic Inflammation. A Descriptive Analysis. Biomedicines 2021, 9, 134. https://doi.org/10.3390/biomedicines9020134
Eissa N, Elgazzar O, Hussein H, Hendy GN, Bernstein CN, Ghia J-E. Pancreastatin Reduces Alternatively Activated Macrophages, Disrupts the Epithelial Homeostasis and Aggravates Colonic Inflammation. A Descriptive Analysis. Biomedicines. 2021; 9(2):134. https://doi.org/10.3390/biomedicines9020134
Chicago/Turabian StyleEissa, Nour, Omar Elgazzar, Hayam Hussein, Geoffrey N. Hendy, Charles N. Bernstein, and Jean-Eric Ghia. 2021. "Pancreastatin Reduces Alternatively Activated Macrophages, Disrupts the Epithelial Homeostasis and Aggravates Colonic Inflammation. A Descriptive Analysis" Biomedicines 9, no. 2: 134. https://doi.org/10.3390/biomedicines9020134
APA StyleEissa, N., Elgazzar, O., Hussein, H., Hendy, G. N., Bernstein, C. N., & Ghia, J.-E. (2021). Pancreastatin Reduces Alternatively Activated Macrophages, Disrupts the Epithelial Homeostasis and Aggravates Colonic Inflammation. A Descriptive Analysis. Biomedicines, 9(2), 134. https://doi.org/10.3390/biomedicines9020134








