Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It?
Abstract
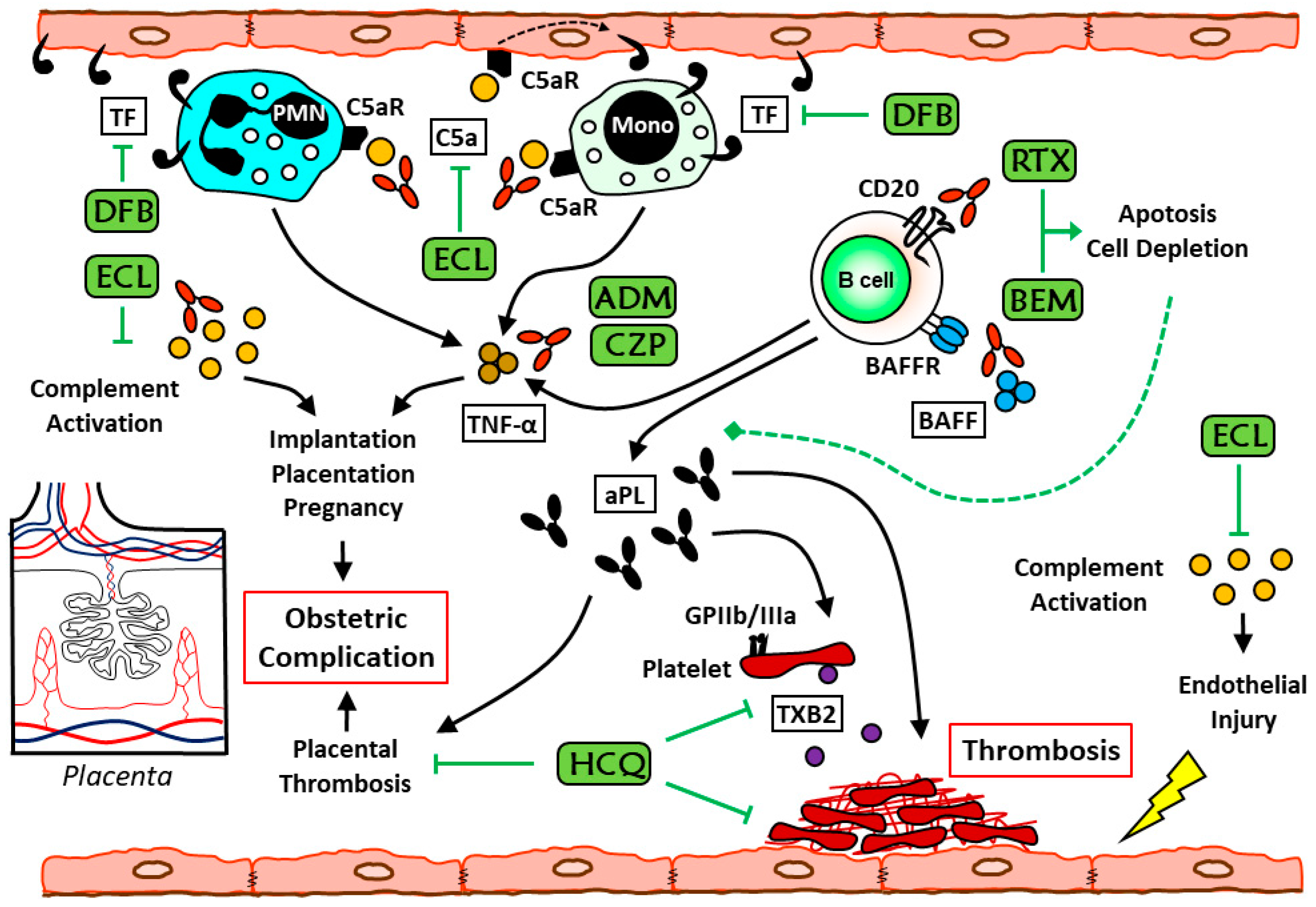
1. Introduction
2. Etiopathogenesis
2.1. Dendritic Cells in APS
2.2. T Cells in APS
2.3. B Cells in APS
2.4. Monocytes in APS
2.5. Neutrophils in APS
2.6. Complement in APS
3. The Current Role of Immunosuppressant Drugs in Antiphospholipid Syndrome
3.1. Hydroxychloroquine
3.2. Rituximab
3.3. Tumor Necrosis Factor-α Blockers
3.4. Eculizumab
3.5. Olendalizumab
3.6. Belimumab
3.7. Other Immunosuppressants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCL | anticardiolipin antibodies |
| Anti-β2GP I | anti-β2-glycoprotein I antibodies |
| aPL | antiphospholipid antibodies |
| APS | antiphospholipid syndrome |
| BAFF | B cell activating factor |
| BLyS | soluble B lymphocyte stimulator |
| CAPS | catastrophic antiphospholipid syndrome |
| DCs | dendritic cells |
| EULAR | European League Against Rheumatism |
| HCQ | hydroxychloroquine |
| INR | international normalized ratio |
| IVIGs | intravenous immunoglobulins |
| LA | lupus anticoagulant |
| LMWH | low-molecular-weight heparin |
| mTORC | mammalian target of rapamycin complex |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PI3K | phosphatidyl-inositol 3-kinase |
| SAPS | secondary antiphospholipid syndrome |
| SIRS | systemic inflammatory response syndrome |
| SLE | systemic lupus erythematosus |
| TEs | thromboembolic events |
| Th | T-helper |
| TLR4 | Toll-like receptors 4 |
| TNF-α | tumor necrosis factor α |
References
- Cervera, R. Antiphospholipid syndrome. Thromb. Res. 2017, 151 (Suppl. 1), S43–S47. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, S.; Park, C.-J.; Chi, H.-S. Clinical Application of Revised Laboratory Classification Criteria for Antiphospholipid Antibody Syndrome: Is the Follow-Up Interval of 12 Weeks Instead of 6 Weeks Significantly Useful? BioMed Res. Int. 2016, 2016, 2641526. [Google Scholar] [CrossRef] [PubMed]
- Unlu, O.; Zuily, S.; Erkan, D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur. J. Rheumatol. 2016, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Asherson, R.A.; Acevedo, M.L.; Gómez-Puerta, J.A.; Espinosa, G.; De La Red, G.; Gil, V.; Ramos-Casals, M.; García-Carrasco, M.; Ingelmo, M.; et al. Antiphospholipid syndrome associated with infections: Clinical and microbiological characteristics of 100 patients. Ann. Rheum. Dis. 2004, 63, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puerta, J.A.; Cervera, R.; Aldea-Parés, A.; Aguiló, S.; Bucciarelli, S.; Ramos-Casals, M.; Ingelmo, M.; Asherson, R.A.; Font, J. Antiphospholipid Antibodies Associated with Malignancies: Clinical and Pathological Characteristics of 120 Patients. Semin. Arthritis Rheum. 2006, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mezhov, V.; Segan, J.D.; Tran, H.A.; Cicuttini, F.M. Antiphospholipid syndrome: A clinical review. Med. J. Aust. 2019, 211, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Piette, J.-C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheumatol. 2002, 46, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Cervera, R.; Asherson, R.A. Catastrophic antiphospholipid syndrome and sepsis. A common link? J. Rheumatol. 2007, 34, 923–926. [Google Scholar]
- Strakhan, M.; Hurtado-Sbordoni, M.; Galeas, N.; Bakirhan, K.; Alexis, K.; Elrafei, T. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: A case report and review of literature. Case Rep. Hematol. 2014, 2014, 704371. [Google Scholar] [CrossRef]
- Erre, G.L.; Pardini, S.; Faedda, R.; Passiu, G. Effect of rituximab on clinical and laboratory features of antiphospholipid syndrome: A case report and a review of literature. Lupus 2008, 17, 50–55. [Google Scholar] [CrossRef]
- Cervera, R.; Rodríguez-Pintó, I.; Espinosa, G. Catastrophic antiphospholipid syndrome: Task force report summary. Lupus 2014, 23, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puerta, J.A.; Espinosa, G.; Cervera, R. Catastrophic antiphospholipid syndrome: Diagnosis and management in pregnancy. Clin. Lab. Med. 2013, 33, 391–400. [Google Scholar]
- Asherson, R.A.; Cervera, R.; De Groot, P.G.; Erkan, D.; Boffa, M.-C.; Piette, J.-C.; Khamashta, M.A.; Shoenfeld, Y. Catastrophic Antiphospholipid Syndrome Registry Project Group Catastrophic antiphospholipid syndrome: International consensus statement on classification criteria and treatment guidelines. Lupus 2003, 12, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Pappalardo, F.; Murdaca, G.; Indiveri, F.; Puppo, F. The antiphospholipid syndrome: From pathophysiology to treatment. Clin. Exp. Med. 2017, 17, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Al Marzooqi, A.; Leone, A.; Al Saleh, J.; Khamashta, M.; Leone, A. Current Status and future prospects for the treatment of Antiphospholipid Syndrome. Expert Rev. Clin. Immunol. 2016, 12, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Gresele, P.; Barcellona, D.; Erba, N.; Testa, S.; Marongiu, F.; Bison, E.; Denas, G.; et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010, 8, 237–242. [Google Scholar] [CrossRef]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Testa, S.; Fierro, T.; Marongiu, F.; de Micheli, V.; Gresele, P.; Tonello, M.; Ghirarduzzi, A. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: A multicenter prospective study. Blood 2011, 118, 4714–4718. [Google Scholar] [CrossRef]
- Kelchtermans, H.; Pelkmans, L.; De Laat, B.; Devreese, K.M. IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: A critical review of their association with thrombosis. J. Thromb. Haemost. 2016, 14, 1530–1548. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Cuadrado, M.J.; Ruiz-Arruza, I.; Brey, R.; Crowther, M.; Derksen, R.; Erkan, D.; Krilis, S.; Machin, S.; Pengo, V.; et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: Report of a Task Force at the 13th International Congress on Antiphospholipid Antibodies. Lupus 2011, 20, 206–218. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Laffan, M. Pathogenesis and management of antiphospholipid syndrome. Br. J. Haematol. 2017, 178, 181–195. [Google Scholar] [CrossRef]
- Bates, S.M. Consultative Hematology: The Pregnant Patient Pregnancy Loss. Hematology 2010, 2010, 166–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danza, A.; Ruiz-Irastorza, G.; Khamashta, M. Antiphospohlipid syndrome in obstetrics. Best Pr. Res. Clin. Obstet. Gynaecol. 2012, 26, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Limper, M.; Scirè, C.A.; Talarico, R.; Amoura, Z.; Avcin, T.; Basile, M.; Burmester, G.; Carli, L.; Cervera, R.; Costedoat-Chalumeau, N.; et al. Antiphospholipid syndrome: State of the art on clinical practice guidelines. RMD Open 2018, 4, e000785. [Google Scholar] [CrossRef] [PubMed]
- Scoble, T.; Wijetilleka, S.; Khamashta, M.A. Management of refractory anti-phospholipid syndrome. Autoimmun. Rev. 2011, 10, 669–673. [Google Scholar] [CrossRef]
- Simchen, M.J.; Dulitzki, M.; Rofe, G.; Shani, H.; Langevitz, P.; Schiff, E.; Pauzner, R. High positive antibody titers and adverse pregnancy outcome in women with antiphospholipid syndrome. Acta Obstet. Gynecol. Scand. 2011, 90, 1428–1433. [Google Scholar] [CrossRef]
- Espinosa, G.; Cervera, R. Current treatment of antiphospholipid syndrome: Lights and shadows. Nat. Rev. Rheumatol. 2015, 11, 586–596. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Ferrer-Oliveras, R.; EUROAPS Study Group. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): A preliminary first year report. Lupus 2012, 21, 766–768. [Google Scholar] [CrossRef]
- de Jesus, G.R.; Santos, F.C.d.; Oliveira, C.S.; Mendes-Silva, W.; de Jesus, N.R.; Levy, R.A. Management of obstetric antiphospholipid syndrome. Curr. Rheumatol. Rep. 2012, 14, 79–86. [Google Scholar] [CrossRef]
- Alijotas-Reig, J. Treatment of refractory obstetric antiphospholipid syndrome: The state of the art and new trends in the therapeutic management. Lupus 2013, 22, 6–17. [Google Scholar] [CrossRef]
- Hoogen, L.L.V.D.; Van Roon, J.A.; Radstake, T.R.; Fritsch-Stork, R.D.; Derksen, R.H. Delineating the deranged immune system in the antiphospholipid syndrome. Autoimmun. Rev. 2016, 15, 50–60. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- De Laat, H.B.; Derksen, R.H.; De Groot, P.G. β2-Glycoprotein I, the playmaker of the antiphospholipid syndrome. Clin. Immunol. 2004, 112, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Agar, C.; van Os, G.M.; Morgelin, M.; Sprenger, R.R.; Marquart, J.A.; Urbanus, R.T.; Derksen, R.H.W.M.; Meijers, J.C.M.; de Groot, P.G. Beta2-glycoprotein I can exist in 2 conformations: Implications for our understanding of the antiphospholipid syndrome. Blood 2010, 116, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- de Laat, B.; Derksen, R.H.; van Lummel, M.; Pennings, M.T.; de Groot, P.G. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood 2006, 107, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Borghi, M.O.; Raschi, E.; Tedesco, F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat. Rev. Rheumatol. 2011, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bondanza, A.; Rovere, P.; Zimmermann, V.S.; Balestrieri, G.; Tincani, A.; Sabbadini, M.G.; Manfredi, A.A. Requirement for dendritic cells in the establishment of anti-phospholipid antibodies. Autoimmunity 2007, 40, 302–306. [Google Scholar] [CrossRef]
- Kuwana, M.; Matsuura, E.; Kobayashi, K.; Okazaki, Y.; Kaburaki, J.; Ikeda, Y.; Kawakami, Y. Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood 2005, 105, 1552–1557. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Seta, N.; Kaburaki, J.; Kobayashi, K.; Matsuura, E.; Kuwana, M. Excessive exposure to anionic surfaces maintains autoantibody response to beta(2)-glycoprotein I in patients with antiphospholipid syndrome. Blood 2007, 110, 4312–4318. [Google Scholar] [CrossRef][Green Version]
- Rovere, P.; Sabbadini, M.G.; Vallinoto, C.; Fascio, U.; Recigno, M.; Crosti, M.; Ricciardi-Castagnoli, P.; Balestrieri, G.; Tincani, A.; Manfredi, A.A. Dendritic cell presentation of antigens from apoptotic cells in a proinflammatory context: Role of opsonizing anti-beta2-glycoprotein I antibodies. Arthritis Rheumatol. 1999, 42, 1412–1420. [Google Scholar] [CrossRef]
- Torres-Aguilar, H.; Blank, M.; Kivity, S.; Misgav, M.; Luboshitz, J.; Pierangeli, S.S.; Shoenfeld, Y. Tolerogenic dendritic cells inhibit antiphospholipid syndrome derived effector/memory CD4(+) T cell response to beta2GPI. Ann. Rheum. Dis. 2012, 71, 120–128. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Jakiela, B.; Iwaniec, T.; Plutecka, H.; Celinska-Lowenhoff, M.; Dziedzina, S.; Musial, J. Signs of impaired immunoregulation and enhanced effector T-cell responses in the primary antiphospholipid syndrome. Lupus 2015, 25, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ben, E.R.R.D.; Prado, C.H.D.; Baptista, T.S.A.; Bauer, M.E.; Staub, H.L. Decreased Levels of Circulating CD4+CD25+Foxp3+ Regulatory T Cells in Patients with Primary Antiphospholipid Syndrome. J. Clin. Immunol. 2013, 33, 876–879. [Google Scholar]
- Álvarez, L.; Riancho-Zarrabeitia, L.; Calvo-Alén, J.; López-Hoyos, M.; Martínez-Taboada, V.M. Peripheral B-Cell Subset Distribution in Primary Antiphospholipid Syndrome. Int. J. Mol. Sci. 2018, 19, 589. [Google Scholar] [CrossRef]
- Schneider, P.; Mackay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.-L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef]
- Hahne, M.; Kataoka, T.; Schröter, M.; Hofmann, K.; Irmler, M.; Bodmer, J.-L.; Schneider, P.; Bornand, T.; Holler, N.; French, L.E.; et al. APRIL, a New Ligand of the Tumor Necrosis Factor Family, Stimulates Tumor Cell Growth. J. Exp. Med. 1998, 188, 1185–1190. [Google Scholar] [CrossRef]
- Rawlings, D.J.; Metzler, G.; Wray-Dutra, M.; Jackson, S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017, 17, 421–436. [Google Scholar] [CrossRef]
- PKahn, P.; Ramanujam, M.; Bethunaickan, R.; Huang, W.; Tao, H.; Madaio, M.P.; Factor, S.M.; Davidson, A. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008, 58, 2824–2834. [Google Scholar]
- Erkan, D.; Vega, J.; Ramon, G.; Kozora, E.; Lockshin, M.D. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheumatol. 2013, 65, 464–471. [Google Scholar] [CrossRef]
- Lopez-Pedrera, C.; Buendia, P.; Cuadrado, M.J.; Siendones, E.; Aguirre, M.A.; Barbarroja, N.; Montiel-Duarte, C.; Torres, A.; Khamashta, M.; Velasco, F. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheumatol. 2006, 54, 301–311. [Google Scholar]
- Sorice, M.; Longo, A.; Capozzi, A.; Garofalo, T.; Misasi, R.; Alessandri, C.; Conti, F.; Buttari, B.; Riganò, R.; Ortona, E.; et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheumatol. 2007, 56, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Blbulyan, A.; Martirosyan, A.; Petrek, M.; Navratilova, Z.; Manukyan, G. Antiphospholipid syndrome and monocytes: New aspects. Georgian Med. News 2017, 2017, 12–17. [Google Scholar]
- Martirosyan, A.; Petrek, M.; Navratilova, Z.; Blbulyan, A.; Boyajyan, A.; Manukyan, G. Differential Regulation of Proinflammatory Mediators following LPS- and ATP-Induced Activation of Monocytes from Patients with Antiphospholipid Syndrome. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Teruel, R.; Pérez-Sánchez, C.; Corral, J.; Herranz, M.T.; Pérez-Andreu, V.; Saiz, E.; García-Barberá, N.; Martínez-Martínez, I.; Roldán, V.; Vicente, V.; et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef]
- Wirestam, L.; Arve, S.; Linge, P.; Bengtsson, A.A. Neutrophils—Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Marini, O.; Tecchio, C.; Cassatella, M.A. Human neutrophils in the saga of cellular heterogeneity: Insights and open questions. Immunol. Rev. 2016, 273, 48–60. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef]
- Montrucchio, G.; Lupia, E.; De Martino, A.; Silvestro, L.; Savu, S.R.; Cacace, G.; De Filippi, P.; Emanuelli, G.; Camussi, G. Plasmin Promotes an Endothelium-Dependent Adhesion of Neutrophils. Circulation 1996, 93, 2152–2160. [Google Scholar] [CrossRef]
- Ames, P.R.; Alves, J.D.; Gentile, F. Coagulation and complement in antiphospholipid syndrome. Thromb. Res. 2017, 158, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Brodsky, R.A.; McCrae, K.R. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.; Noone, D.G.; Khan, M.A.; Pluthero, F.G.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. Complement Activation Induces Neutrophil Adhesion and Neutrophil-Platelet Aggregate Formation on Vascular Endothelial Cells. Kidney Int. Rep. 2017, 2, 66–75. [Google Scholar] [CrossRef]
- Redecha, P.; Franzke, C.-W.; Ruf, W.; Mackman, N.; Girardi, G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J. Clin. Investig. 2008, 118, 3453–3461. [Google Scholar] [CrossRef]
- Gropp, K.; Weber, N.; Reuter, M.; Micklisch, S.; Kopka, I.; Hallstrom, T.; Skerka, C. β(2)-glycoprotein I, the major target in antiphospholipid syndrome, is a special human complement regulator. Blood 2011, 118, 2774–2783. [Google Scholar] [CrossRef]
- Arfors, L.; Lefvert, A.K. Enrichment of antibodies against phospholipids in circulating immune complexes (CIC) in the anti-phospholipid syndrome (APLS). Clin. Exp. Immunol. 1997, 108, 47–51. [Google Scholar] [CrossRef]
- Akhter, E.; Burlingame, R.W.; Seaman, A.L.; Magder, L.; Petri, M. Anti-C1q antibodies have higher correlation with flares of lupus nephritis than other serum markers. Lupus 2011, 20, 1267–1274. [Google Scholar] [CrossRef]
- Oku, K.; Amengual, O.; Hisada, R.; Ohmura, K.; Nakagawa, I.; Watanabe, T.; Bohgaki, T.; Horita, T.; Yasuda, S.; Atsumi, T. Autoantibodies against a complement component 1 q subcomponent contribute to complement activation and recurrent thrombosis/pregnancy morbidity in anti-phospholipid syndrome. Rheumatology 2016, 55, 1403–1411. [Google Scholar] [CrossRef]
- Davis, W.D.; Brey, R.L. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin. Exp. Rheumatol. 1992, 10, 455–460. [Google Scholar]
- Erdozain, J.G.; Ruiz-Irastorza, G.; Egurbide, M.V.; Aguirre, C. Sustained response to rituximab of autoimmune hemolytic anemia associated with antiphospholipid syndrome. Haematologica 2004, 89, ECR34. [Google Scholar] [PubMed]
- Trappe, R.; Loew, A.; Thuss-Patience, P.; Dorken, B.; Riess, H. Successful treatment of thrombocytopenia in primary antiphospholipid antibody syndrome with the anti-CD20 antibody rituximab--monitoring of antiphospholipid and anti-GP antibodies: A case report. Ann. Hematol. 2006, 85, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.B.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2020, 72, 529–556. [Google Scholar] [CrossRef] [PubMed]
- Yelnik, C.M.; Laskin, C.A.; Porter, T.F.; Branch, D.W.; Buyon, J.P.; Guerra, M.M.; Lockshin, M.D.; Petri, M.; Merrill, J.T.; Sammaritano, L.R.; et al. Lupus anticoagulant is the main predictor of adverse pregnancy outcomes in aPL-positive patients: Validation of PROMISSE study results. Lupus Sci. Med. 2016, 3, e000131. [Google Scholar] [CrossRef]
- Pons, I.; Aldea-Parés, A.; Cervera, R. Efficacy and safety of rituximab in the treatment of primary antiphospholipid syndrome: Analysis of 24 cases from the bibliography review. Med. Clin. 2015, 144, 97–104. [Google Scholar] [CrossRef]
- Schmidt-Tanguy, A.; Voswinkel, J.; Henrion, D.; Subra, J.F.; Loufrani, L.; Rohmer, V.; Ifrah, N.; Belizna, C. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J. Thromb. Haemost. 2013, 11, 1927–1929. [Google Scholar] [CrossRef]
- Johansson, E.; Forsberg, K.; Johnsson, H. Clinical and experimental evaluation of the thromboprophylactic effect of hydroxychloroquine sulfate after total hip replacement. Haemostasis 1981, 10, 89–96. [Google Scholar] [CrossRef]
- Mekinian, A.; Lazzaroni, M.G.; Kuzenko, A.; Alijotas-Reig, J.; Ruffatti, A.; Levy, P.; Canti, V.; Bremme, K.; Bezanahary, H.; Bertero, T.; et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun. Rev. 2015, 14, 498–502. [Google Scholar] [CrossRef]
- Sciascia, S.; Hunt, B.J.; Talavera-Garcia, E.; Lliso, G.; Khamashta, M.A.A.; Cuadrado, M.J. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am. J. Obstet. Gynecol. 2016, 214, 273.e1–273.e8. [Google Scholar] [CrossRef]
- Belizna, C.; Pregnolato, F.; Abad, S.; Alijotas-Reig, J.; Amital, H.; Amoura, Z.; Andreoli, L.; Andres, E.; Aouba, A.; Bilgen, S.A.; et al. HIBISCUS: Hydroxychloroquine for the secondary prevention of thrombotic and obstetrical events in primary antiphospholipid syndrome. Autoimmun. Rev. 2018, 17, 1153–1168. [Google Scholar] [CrossRef]
- De Carolis, S.; Botta, A.; Salvi, S.; Di Pasquo, E.; Del Sordo, G.; Garufi, C.; Lanzone, A.; De Carolis, M. Is there any role for the hydroxychloroquine (HCQ) in refractory obstetrical antiphospholipid syndrome (APS) treatment? Autoimmun. Rev. 2015, 14, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Mar, N.; Kosowicz, R.; Hook, K. Recurrent thrombosis prevention with intravenous immunoglobulin and hydroxychloroquine during pregnancy in a patient with history of catastrophic antiphospholipid syndrome and pregnancy loss. J. Thromb. Thrombolysis 2014, 38, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Tonello, M.; Hoxha, A.; Sciascia, S.; Cuadrado, M.J.; Latino, J.O.; Udry, S.; Reshetnyak, T.; Costedoat-Chalumeau, N.; Morel, N.; Marozio, L.; et al. Effect of Additional Treatments Combined with Conventional Therapies in Pregnant Patients with High-Risk Antiphospholipid Syndrome: A Multicentre Study. Thromb. Haemost. 2018, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Ferrer-Oliveras, R.; Llurba, E.; Gris, J.M. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Fazal, S.; Kaplan, R.B.; Spero, J.; Costa, R. Successful plasma exchange combined with rituximab therapy in aggressive APS-related cutaneous necrosis. Clin. Rheumatol. 2010, 32 (Suppl. 1), 79–82. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Ehrchen, J.; Görge, T. Severe antiphospholipid antibody syndrome–response to plasmapheresis and rituximab. J. Dermatol. Treat. 2017, 28, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, A.; Glimm, H.; Lubbert, M.; Grullich, C. Successful treatment of life-threatening Evans syndrome due to antiphospholipid antibody syndrome by rituximab-based regimen: A case with long-term follow-up. Lupus 2008, 17, 757–760. [Google Scholar] [CrossRef]
- Asherson, R.A.; Espinosa, G.; Menahem, S.; Yinh, J.; Bucciarelli, S.; Bosch, X.; Cervera, R. Relapsing catastrophic antiphospholipid syndrome: Report of three cases. Semin. Arthritis Rheum. 2008, 37, 366–372. [Google Scholar] [CrossRef]
- Rubenstein, E.; Arkfeld, D.G.; Metyas, S.; Shinada, S.; Ehresmann, S.; Liebman, H.A. Rituximab treatment for resistant antiphospholipid syndrome. J. Rheumatol. 2006, 33, 355–357. [Google Scholar]
- Berman, H.; Rodriguez-Pinto, I.; Cervera, R.; Morel, N.; Costedoat-Chalumeau, N.; Erkan, D.; Shoenfeld, Y.; Espinosa, G. Rituximab use in the catastrophic antiphospholipid syndrome: Descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun. Rev. 2013, 12, 1085–1090. [Google Scholar] [CrossRef]
- Dogru, A.; Ugan, Y.; Sahin, M.; Karahan, N.; Tunc, S.E. Catastrophic antiphospholipid syndrome treated with rituximab: A case report. Eur. J. Rheumatol. 2017, 4, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Wig, S.; Chan, M.; Thachil, J.; Bruce, I.; Barnes, T. A case of relapsing and refractory catastrophic anti-phospholipid syndrome successfully managed with eculizumab, a complement 5 inhibitor. Rheumatol. 2015, 55, 382–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagata, M.; Kaneko, K.; Kohno, C.; Mishima, S.; Okazaki, Y.; Murashima, A. A case of successful pregnancy following multidrug treatment including rituximab and intravenous immunoglobulin for primary antiphospholipid antibody syndrome refractory to conventional treatment. Mod. Rheumatol. Case Rep. 2020, 4, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Yazici, A.; Yazirli, B.; Erkan, D. Belimumab in primary antiphospholipid syndrome. Lupus 2016, 26, 1123–1124. [Google Scholar] [CrossRef]
- Meroni, P.L.; Macor, P.; Durigutto, P.; De Maso, L.; Gerosa, M.; Ferraresso, M.; Borghi, M.O.; Mollnes, T.E.; Tedesco, F. Complement activation in antiphospholipid syndrome and its inhibition to prevent rethrombosis after arterial surgery. Blood 2016, 127, 365–367. [Google Scholar] [CrossRef]
- Zikos, T.A.; Sokolove, J.; Ahuja, N.; Berube, C. Eculizumab Induces Sustained Remission in a Patient With Refractory Primary Catastrophic Antiphospholipid Syndrome. JCR J. Clin. Rheumatol. 2015, 21, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Barratt-Due, A.; Fløisand, Y.; Orrem, H.L.; Kvam, A.K.; Holme, P.A.; Bergseth, G.; Tjønnfjord, G.E.; Mollnes, T.E. Complement activation is a crucial pathogenic factor in catastrophic antiphospholipid syndrome. Rheumatology 2016, 55, 1337–1339. [Google Scholar] [CrossRef]
- Tinti, M.G.; Carnevale, V.; Inglese, M.; Molinaro, F.; Bernal, M.; Migliore, A.; de Cata, A. Eculizumab in refractory catastrophic antiphospholipid syndrome: A case report and systematic review of the literature. Clin. Exp. Med. 2019, 19, 281–288. [Google Scholar] [CrossRef]
- Guillot, M.; Rafat, C.; Buob, D.; Coppo, P.; Jamme, M.; Rondeau, E.; Fain, O.; Mekinian, A. Eculizumab for catastrophic antiphospholipid syndrome—A case report and literature review. Rheumatology 2018, 57, 2055–2057. [Google Scholar] [CrossRef]
- Lonze, B.E.; Singer, A.L.; Montgomery, R.A. Eculizumab and Renal Transplantation in a Patient with CAPS. N. Engl. J. Med. 2010, 362, 1744–1745. [Google Scholar] [CrossRef]
- Lonze, B.E.; Zachary, A.A.; Magro, C.M.; Desai, N.M.; Orandi, B.J.; Dagher, N.N.; Singer, A.L.; Carter-Monroe, N.; Nazarian, S.M.; Segev, D.L.; et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am. J. Transpl. 2014, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, A.; Skattum, L.; Bergseth, G.; Lorentzen, B.; Floisand, Y.; Bosnes, V.; Mollnes, T.E.; Barratt-Due, A. Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: A case report. Medicine 2017, 96, e6338. [Google Scholar] [CrossRef] [PubMed]
- Canaud, G.; Bienaime, F.; Tabarin, F.; Bataillon, G.; Seilhean, D.; Noel, L.H.; Dragon-Durey, M.; Snanoudj, R.; Friedlander, G.; Halbwachs-Mecarelli, L.; et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N. Engl. J. Med. 2014, 371, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ramirez, M.; Gonzalez-Pacheco, H.; Amezcua-Guerra, L.M. Stents Coated with Mammalian Target of Rapamycin Inhibitors (mTOR) Appear to Be the Best Choice in Patients with Antiphospholipid Syndrome and Myocardial Infarction. J. Clin. Rheumatol. 2016, 22, 281. [Google Scholar] [CrossRef]
- Burcoglu-O’Ral, A.; Erkan, D.; Asherson, R. Treatment of catastrophic antiphospholipid syndrome with defibrotide, a proposed vascular endothelial cell modulator. J. Rheumatol. 2002, 29, 2006–2011. [Google Scholar]
- Dobrowolski, C.; Erkan, D. Treatment of antiphospholipid syndrome beyond anticoagulation. Clin. Immunol. 2019, 206, 53–62. [Google Scholar] [CrossRef]
- Sperber, K.; Quraishi, H.; Kalb, T.H.; Panja, A.; Stecher, V.; Mayer, L. Selective regulation of cytokine secretion by hydroxychloroquine: Inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J. Rheumatol. 1993, 20, 803–808. [Google Scholar]
- Rand, J.H.; Wu, X.-X.; Quinn, A.S.; Ashton, A.W.; Chen, P.P.; Hathcock, J.J.; Andree, H.A.M.; Taatjes, D.J. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: Evidence for a novel effect for an old antimalarial drug. Blood 2010, 115, 2292–2299. [Google Scholar] [CrossRef]
- Espinola, R.G.; Pierangeli, S.S.; Gharavi, A.E.; Harris, E.N.; Ghara, A.E. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb. Haemost. 2002, 87, 518–522. [Google Scholar] [CrossRef]
- Schreiber, K.; Breen, K.; Parmar, K.; Rand, J.H.; Wu, X.-X.; Hunt, B.J. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology 2018, 57, 120–124. [Google Scholar] [CrossRef]
- Wu, T.K.; Tsapogas, M.J.; Jordan, F.R. Prophylaxis of deep venous thrombosis by hydroxychloroquine sulfate and heparin. Surg. Gynecol. Obstet. 1977, 145, 714–718. [Google Scholar] [PubMed]
- Marchetti, T.; Ruffatti, A.; Wuillemin-Rambosson, C.G.; De Moerloose, P.; Cohen, M.-B. Hydroxychloroquine restores trophoblast fusion affected by antiphospholipid antibodies. J. Thromb. Haemost. 2014, 12, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-F.; Lim, W. What is the role of hydroxychloroquine in reducing thrombotic risk in patients with antiphospholipid antibodies? Hematology 2016, 2016, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Berghen, N.; Vulsteke, J.-B.; Westhovens, R.; Lenaerts, J.; De Langhe, E. Rituximab in systemic autoimmune rheumatic diseases: Indications and practical use. Acta Clin. Belg. 2019, 74, 272–279. [Google Scholar] [CrossRef]
- Engel, P.; Gómez-Puerta, J.A.; Ramos-Casals, M.; Lozano, F.; Bosch, X. Therapeutic Targeting of B Cells for Rheumatic Autoimmune Diseases. Pharmacol. Rev. 2011, 63, 127–156. [Google Scholar] [CrossRef]
- Veneri, D.; Ambrosetti, A.; Franchini, M.; Mosna, F.; Poli, G.; Pizzolo, G. Remission of severe antiphospholipid syndrome associated with non-Hodgkin’s B-cell lymphoma after combined treatment with rituximab and chemotherapy. Haematologica 2005, 90 (Suppl. ECR37), e104–e105. [Google Scholar]
- Harner, K.C.; Jackson, L.W.; Drabick, J.J. Normalization of anticardiolipin antibodies following rituximab therapy for marginal zone lymphoma in a patient with Sjogren’s syndrome. Rheumatology 2004, 43, 1309–1310. [Google Scholar] [CrossRef][Green Version]
- Weide, R.; Heymanns, J.; Pandorf, A.; Köppler, H. Successful long-term treatment of systemic lupus erythematosus with rituximab maintenance therapy. Lupus 2003, 12, 779–782. [Google Scholar] [CrossRef]
- Adachi, Y.; Inaba, M.; Sugihara, A.; Koshiji, M.; Sugiura, K.; Amoh, Y.; Mori, S.; Kamiya, T.; Genba, H.; Ikehara, S. Effects of administration of monoclonal antibodies (anti-CD4 or anti-CD8) on the development of autoimmune diseases in (NZW x BXSB)F1 mice. Immunobiology 1998, 198, 451–464. [Google Scholar] [CrossRef]
- Ames, P.R.J.; Tommasino, C.; Fossati, G.; Scenna, G.; Brancaccio, V.; Ferrara, F. Limited effect of rituximab on thrombocytopaenia and anticardiolipin antibodies in a patient with primary antiphospholipid syndrome. Ann. Hematol. 2006, 86, 227–228. [Google Scholar] [CrossRef]
- Skorpen, C.G.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; Silva, J.A.P.D.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Marin, J.; Beaulieu, M.C. Rituximab induction therapy for de novo ANCA associated vasculitis in pregnancy: A case report. BMC Nephrol. 2018, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Khalid, A.; Daw, H.; Maroo, P. Management of Primary Mediastinal B-Cell Lymphoma in Pregnancy. Cureus 2018, 10, e2215. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Ahn, K.H.; Hong, S.C.; Park, Y.; Kim, B.S.; Lee, E.H. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy for diffuse large B-cell lymphoma in pregnancy may be associated with preterm birth. Obstet. Gynecol. Sci. 2014, 57, 526–529. [Google Scholar] [CrossRef]
- Mandal, P.K.; Dolai, T.K.; Bagchi, B.; Ghosh, M.K.; Bose, S.; Bhattacharyya, M. B Cell Suppression in Newborn Following Treatment of Pregnant Diffuse Large B-cell Lymphoma Patient with Rituximab Containing Regimen. Indian J. Pediatr. 2014, 81, 1092–1094. [Google Scholar] [CrossRef]
- Padberg, S.; Mick, I.; Frenzel, C.; Greil, R.; Hilberath, J.; Schaefer, C. Transient congenital dilated cardiomyopathy after maternal R-CHOP chemotherapy during pregnancy. Reprod. Toxicol. 2017, 71, 146–149. [Google Scholar] [CrossRef]
- Perez, C.A.; Amin, J.; Aguina, L.M.; Cioffi-Lavina, M.; Santos, E.S. Primary Mediastinal Large B-Cell Lymphoma during Pregnancy. Case Rep. Hematol. 2012, 2012, 197347. [Google Scholar] [CrossRef]
- Sprenger-Mahr, H.; Zitt, E.; Soleiman, A.; Lhotta, K. Successful pregnancy in a patient with pulmonary renal syndrome double-positive for anti-GBM antibodies and p-ANCA. Clin. Nephrol. 2019, 91, 101–106. [Google Scholar] [CrossRef]
- Friedrichs, B.; Tiemann, M.; Salwender, H.; Verpoort, K.; Wenger, M.K.; Schmitz, N. The effects of rituximab treatment during pregnancy on a neonate. Haematologica 2006, 91, 1426–1427. [Google Scholar]
- Gall, B.; Yee, A.; Berry, B.; Bircham, D.; Hayashi, A.; Dansereau, J.; Hart, J. Rituximab for management of refractory pregnancy-associated immune thrombocytopenic purpura. J. Obstet. Gynaecol. Can. 2010, 32, 1167–1171. [Google Scholar] [CrossRef]
- Ostensen, M.; Lockshin, M.; Doria, A.; Valesini, G.; Meroni, P.; Gordon, C.; Brucato, A.; Tincani, A. Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology 2008, 47 (Suppl. 3), iii28–iii31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berman, J.; Girardi, G.; Salmon, J.E. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J. Immunol. 2005, 174, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.; Förger, F.; Hwang, C.; Thorp, J.; Dolhain, R.J.; Van Tubergen, A.; Shaughnessy, L.; Simpson, J.; Teil, M.; Toublanc, N.; et al. Minimal to no transfer of certolizumab pegol into breast milk: Results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann. Rheum. Dis. 2017, 76, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Förger, F.; Abraham, B.; Flynn, A.D.; Molto, A.; Flipo, R.-M.; Van Tubergen, A.; Shaughnessy, L.; Simpson, J.; Teil, M.; et al. Lack of placental transfer of certolizumab pegol during pregnancy: Results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann. Rheum. Dis. 2018, 77, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Perez-De-Lis, M.; Retamozo, S.; Flores-Chavez, A.; Kostov, B.; Perez-Alvarez, R.; Brito-Zeron, P. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opin. Drug Saf. 2017, 16, 1255–1271. [Google Scholar] [CrossRef]
- Hoogen, L.L.V.D.; Van Laar, J.M. Targeted therapies in systemic sclerosis, myositis, antiphospholipid syndrome, and Sjögren’s syndrome. Best Pr. Res. Clin. Rheumatol. 2020, 34, 101485. [Google Scholar] [CrossRef]
- Trouw, L.A.; Pickering, M.C.; Blom, A.M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 538–547. [Google Scholar] [CrossRef]
- Jayne, D.R.W.; Bruchfeld, A.N.; Harper, L.; Schaier, M.; Venning, M.C.; Hamilton, P.; Burst, V.; Grundmann, F.; Jadoul, M.; Szombati, I.; et al. Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2017, 28, 2756–2767. [Google Scholar] [CrossRef]
- Vergunst, C.E.; Gerlag, D.M.; Dinant, H.; Schulz, L.; Vinkenoog, M.; Smeets, T.J.M.; Sanders, M.E.; Reedquist, K.A.; Tak, P.P. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology 2007, 46, 1773–1778. [Google Scholar] [CrossRef]
- Cicconi, V.; Carloni, E.; Franceschi, F.; Nocente, R.; Silveri, N.G.; Manna, R.; Servidei, S.; Bentivoglio, A.R.; Gasbarrini, A.; Gasbarrini, G. Disappearance of antiphospholipid antibodies syndrome after Helicobacter pylori eradication. Am. J. Med. 2001, 111, 163–164. [Google Scholar] [CrossRef]
- Kronbichler, A.; Frank, R.; Kirschfink, M.; Szilagyi, A.; Csuka, D.; Prohaszka, Z.; Schratzberger, P.; Lhotta, K.; Mayer, G. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: A case report. Medicine 2014, 93, e143. [Google Scholar] [CrossRef] [PubMed]
- Shapira, I.; Andrade, D.; Allen, S.L.; Salmon, J.E. Brief report: Induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheumatol. 2012, 64, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Geethakumari, P.R.; Mille, P.; Gulati, R.; Nagalla, S. Complement inhibition with eculizumab for thrombotic microangiopathy rescues a living-donor kidney transplant in a patient with antiphospholipid antibody syndrome. Transfus. Apher. Sci. 2017, 56, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Bakhtar, O.; Thajudeen, B.; Braunhut, B.L.; Yost, S.E.; Bracamonte, E.R.; Sussman, A.N.; Kaplan, B. A case of thrombotic microangiopathy associated with antiphospholipid antibody syndrome successfully treated with eculizumab. Transplantation 2014, 98, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Canaud, G.; Kamar, N.; Anglicheau, D.; Esposito, L.; Rabant, M.; Noël, L.-H.; Guilbeau-Frugier, C.; Sberro-Soussan, R.; Del Bello, A.; Martinez, F.; et al. Eculizumab Improves Posttransplant Thrombotic Microangiopathy Due to Antiphospholipid Syndrome Recurrence but Fails to Prevent Chronic Vascular Changes. Arab. Archaeol. Epigr. 2013, 13, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Sarno, L.; Tufano, A.; Maruotti, G.M.; Martinelli, P.; Balletta, M.M.; Russo, D. Eculizumab in pregnancy: A narrative overview. J. Nephrol. 2019, 32, 17–25. [Google Scholar] [CrossRef]
- Blair, H.A.; Duggan, S.T. Belimumab: A Review in Systemic Lupus Erythematosus. Drugs 2018, 78, 355–366. [Google Scholar] [CrossRef]
- Hoogen, L.L.V.D.; Palla, G.; Bekker, C.P.J.; Fritsch-Stork, R.D.E.; Radstake, T.R.D.J.; Roon, J.A.G.V. Increased B-cell activating factor (BAFF)/B-lymphocyte stimulator (BLyS) in primary antiphospholipid syndrome is associated with higher adjusted global antiphospholipid syndrome scores. RMD Open 2018, 4, e000693. [Google Scholar] [CrossRef]
- Emmi, G.; Bettiol, A.; Palterer, B.; Silvestri, E.; Vitiello, G.; Parronchi, P.; Prisco, D. Belimumab reduces antiphospholipid antibodies in SLE patients independently of hydroxychloroquine treatment. Autoimmun. Rev. 2019, 18, 312–314. [Google Scholar] [CrossRef]
- Carmi, O.; Berla, M.; Shoenfeld, Y.; Levy, Y. Diagnosis and management of catastrophic antiphospholipid syndrome. Expert Rev. Hematol. 2017, 10, 365–374. [Google Scholar] [CrossRef]
- Cervera, R. Update on the Diagnosis, Treatment, and Prognosis of the Catastrophic Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2010, 12, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, U.D.; Erkan, D.; Bucciarelli, S.; Espinosa, G.; Asherson, R. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J. Rheumatol. 2007, 34, 346–352. [Google Scholar] [PubMed]
- Pescador, R.; Porta, R.; Ferro, L. An integrated view of the activities of defibrotide. Semin. Thromb. Hemost. 1996, 22 (Suppl. 1), 71–75. [Google Scholar] [PubMed]
| Drug | Reference | Total Patients Treated (N) | Indication | Outcome Number of Patients = N (%) |
|---|---|---|---|---|
| HCQ | [76] | 20 | TEs prevention | No TEs 100% (N = 20) |
| [77] | 18 | TEs prevention | No TEs 88% (N = 16) | |
| [78] | 14 | ROC | Live birth 78% (N = 11) | |
| [79] | 31 | ROC | Live birth 67% (N = 20) | |
| [80] | 170 | TEs and OC prevention | NA | |
| [81] | 1 | ROC | Live birth 100% (N = 1) | |
| [82] | 1 | ROC | Live birth 100% (N = 1) | |
| [83] | 94 | ROC | Live birth 87.2% (N = 82) | |
| Adalimumab | [84] | 16 | ROC | Live birth 62% (N = 10) |
| Certolizumab | [84] | 2 | ROC | Live birth 100% (N = 2) |
| NCT03152058 | Recruiting | ROC | NA | |
| Rituximab | [50] | 19 | APS-NCM | Recovery 45% (N = 7) |
| [75] | 24 | APS-NCM | Recovery 45% (N = 11) | |
| [72] | 1 | APS-NCM | Recovery 100% (N = 1) | |
| [71] | 1 | APS-NCM | Recovery 100% (N = 1) | |
| [85] | 1 | APS-NCM | Recovery 100% (N = 1) | |
| [86] | 1 | APS-NCM | Recovery 100% (N = 1) | |
| [87] | 1 | APS-NCM | Recovery 100% (N = 1) | |
| [88] | 1 | APS-NCM | Partial response 100% (N = 1) | |
| [89] | 3 | APS-NCM/CAPS | Recovery 100% (N = 3) | |
| [10] | 1 | TEs/LNH | No TEs 100% (N = 1) | |
| [90] | 20 | CAPS | Recovery 65% (N = 13) | |
| [91] | 1 | CAPS | Recovery 65% (N = 13) | |
| [92] | 1 | CAPS | No improvement 100% (N = 1) | |
| [88] | 3 | CAPS | Recovery 100% (N = 2) | |
| [93] | 1 | APS-NCM/ROC | Live birth 100% (N = 1) | |
| Belimumab | [94] | 2 | APS-NCM | No flares 100% (N = 2) |
| Eculizumab | [95] | 1 | TEs prevention | No TEs 100% (N = 1) |
| [92] | 1 | CAPS | Recovery 100% (N = 1) | |
| [96] | 1 | CAPS | Recovery 100% (N = 1) | |
| [9] | 1 | CAPS | Recovery 100% (N = 1) | |
| [97] | 1 | CAPS | Recovery 100% (N = 1) | |
| [98] | 1 | CAPS | Recovery 100% (N = 1) | |
| [99] | 1 | CAPS | Recovery 100% (N = 1) | |
| [100] | 1 | Prevent CAPS after RT | Better graft survival | |
| [101] | 3 | Prevent CAPS after RT | Better graft survival | |
| NCT01029587 | 1 | Prevent CAPS after RT | NA | |
| [102] | 1 | ROC/Prevent CAPS | No flares 100% (N = 1) | |
| Olendalizumab | NCT02128269 | 9 | APS-NCM | NA |
| Sirolimus | [103] | 10 | APSN and RT | Better graft survival |
| [104] | 1 | PCI in APS | No in-stent stenosis 100% (N = 1) | |
| Defibrotide | [105] | 1 | CAPS | Recovery 100% (N = 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mormile, I.; Granata, F.; Punziano, A.; de Paulis, A.; Rossi, F.W. Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It? Biomedicines 2021, 9, 132. https://doi.org/10.3390/biomedicines9020132
Mormile I, Granata F, Punziano A, de Paulis A, Rossi FW. Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It? Biomedicines. 2021; 9(2):132. https://doi.org/10.3390/biomedicines9020132
Chicago/Turabian StyleMormile, Ilaria, Francescopaolo Granata, Alessandra Punziano, Amato de Paulis, and Francesca Wanda Rossi. 2021. "Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It?" Biomedicines 9, no. 2: 132. https://doi.org/10.3390/biomedicines9020132
APA StyleMormile, I., Granata, F., Punziano, A., de Paulis, A., & Rossi, F. W. (2021). Immunosuppressive Treatment in Antiphospholipid Syndrome: Is It Worth It? Biomedicines, 9(2), 132. https://doi.org/10.3390/biomedicines9020132







