Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature
Abstract
1. Introduction
2. Pathophysiology of Gastric Cancer
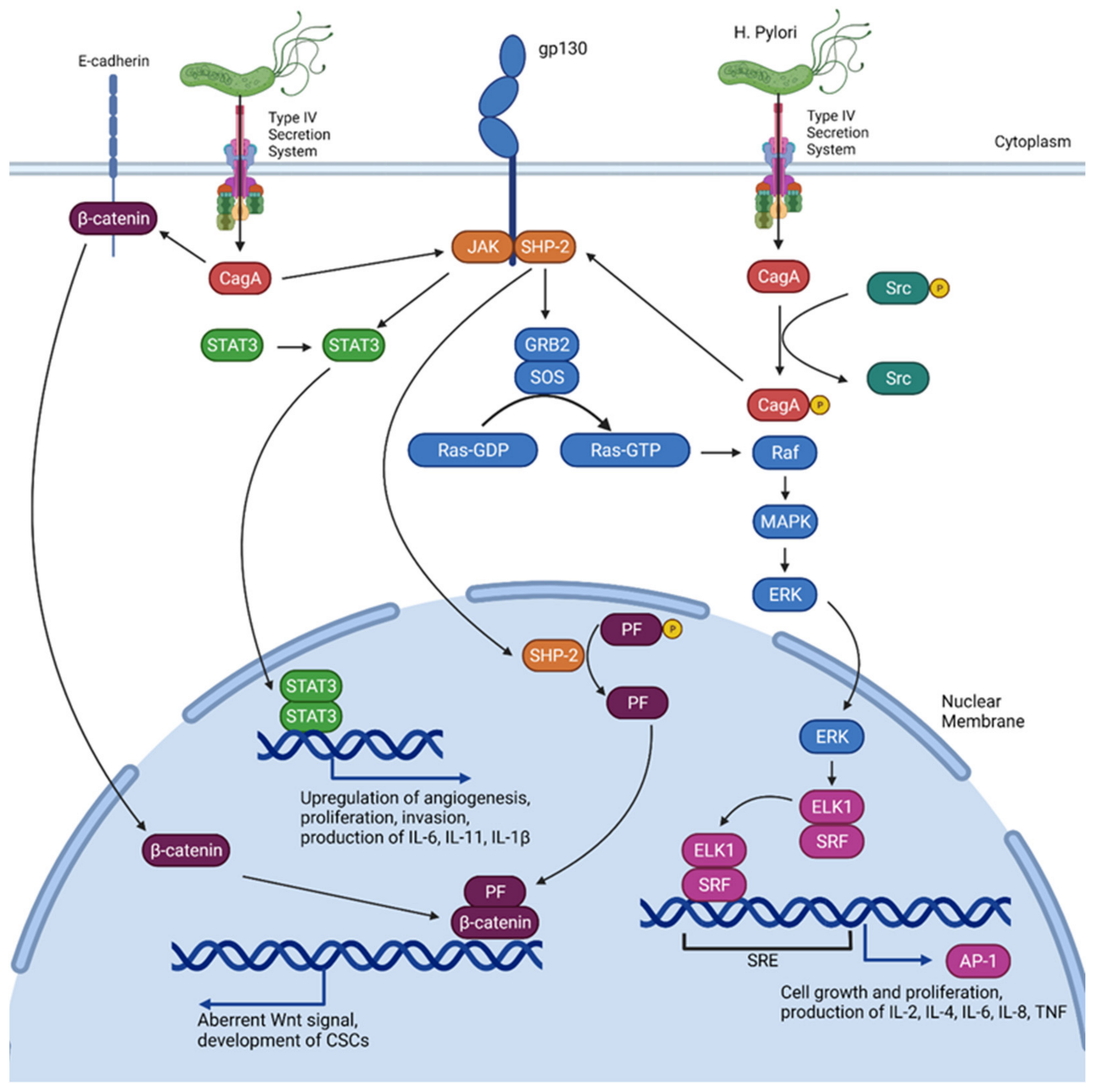
3. Implications of H. Pylori
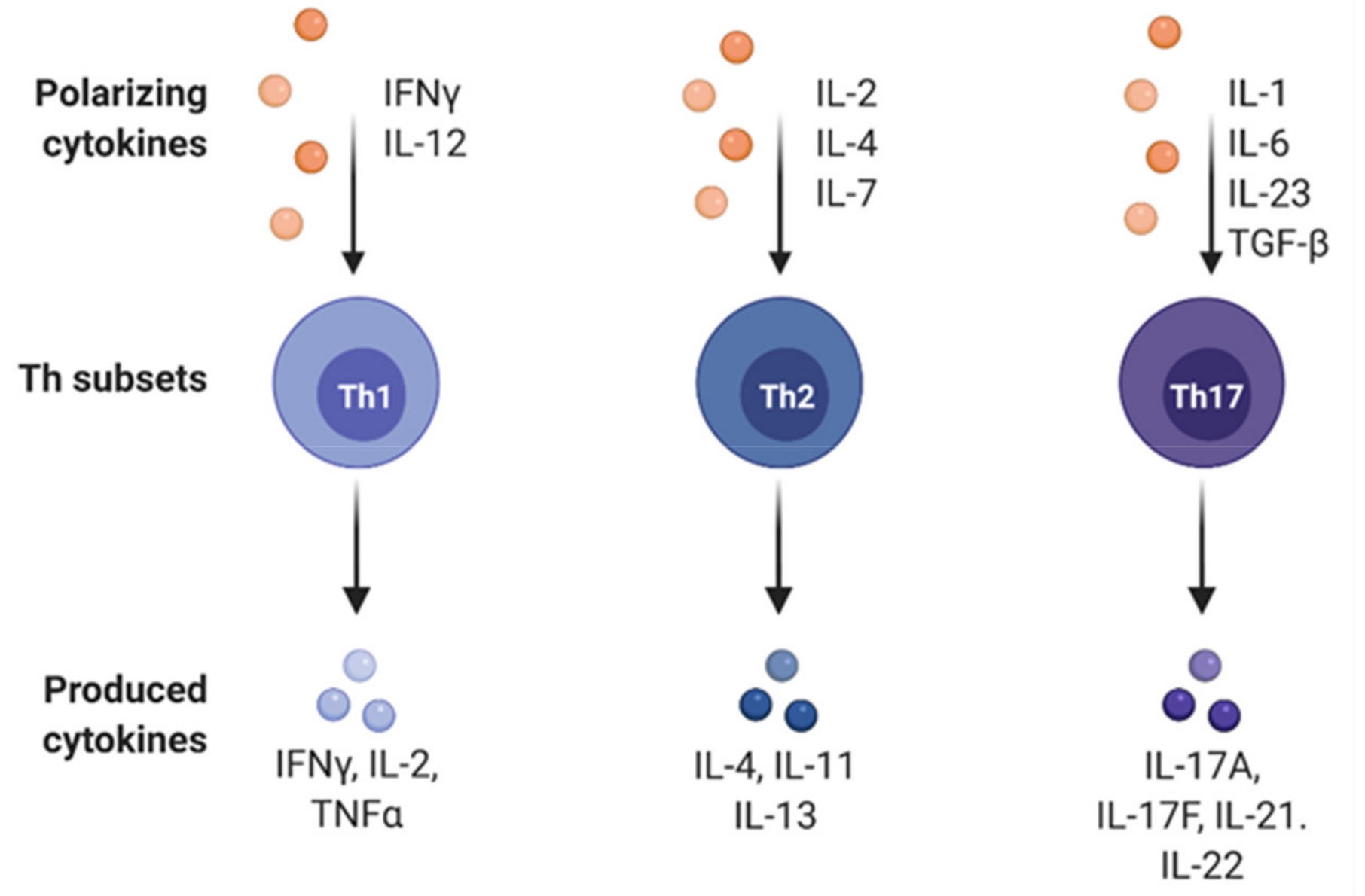
4. Effects of Immune Cell Differentiation in Gastric Cancer
5. Effects of Cytokines on Gastric Cancer Prognosis
5.1. IL-6
5.2. TNF
5.3. IFN-γ
5.4. IL-17A
5.5. IL-10
5.6. IL-8
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, J.; Maciejewski, R.; Polkowski, W. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, S.; Graham, D. Immunotherapy in gastric cancer. World J. Gastroenterol. 2014, 20, 1657. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Chung, H.W. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J. Gastroenterol. 2014, 20, 1667. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori CagA and Gastric Cancer: A Paradigm for Hit-and-Run Carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Ono, S.; Ichikura, T.; Matsumoto, Y.; Yamamoto, J.; Hase, K. Roles of inflammatory cytokines in the progression of gastric cancer: Friends or foes? Gastric Cancer 2010, 13, 212–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gu, W.; Sun, B. Th1/Th2 Cell Differentiation and Molecular Signals; Springer: Dordrecht, The Netherlands, 2014; pp. 15–44. [Google Scholar]
- Bockerstett, K.A.; Dipaolo, R.J. Regulation of gastric carcinogenesis by inflammatory cytokines. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 47–53. [Google Scholar] [CrossRef]
- Madej-Michniewicz, A.; Budkowska, M.; Sałata, D.; Dołęgowska, B.; Starzyńska, T.; Błogowski, W. Evaluation of selected interleukins in patients with different gastric neoplasms: A preliminary report. Sci. Rep. 2015, 5, 14382. [Google Scholar] [CrossRef]
- Amedei, A.; Della Bella, C.; Silvestri, E.; Prisco, D.; D’Elios, M.M. T Cells in gastric cancer: Friends or foes. Clin. Dev. Immunol. 2012, 2012, 1–10. [Google Scholar] [CrossRef][Green Version]
- Berlth, F. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014, 20, 5679. [Google Scholar] [CrossRef]
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef]
- Kaneko, S.; Yoshimura, T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br. J. Cancer 2001, 84, 400–405. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Cai, M.-Y.; Zhang, D.-S.; Wang, Z.-Q.; Wang, D.-S.; Li, Y.-H.; Xu, R.-H. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J. Transl. Med. 2013, 11, 58. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Espinel, J. Treatment modalities for early gastric cancer. World J. Gastrointest. Endosc. 2015, 7, 1062. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Keam, B.; Im, S.-A.; Han, S.-W.; Ham, H.S.; Kim, M.A.; Oh, D.-Y.; Lee, S.-H.; Kim, J.H.; Kim, D.-W.; Kim, T.-Y.; et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer 2008, 8, 148. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Brar, G.; Shah, M.A. The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma. Ther. Adv. Gastroenterol. 2019, 12, 175628481986976. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Gastric Cancer (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 11 November 2021).
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can be Explained by Differences between Helicobacter pylori Strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef]
- Lee, I.O.; Kim, J.H.; Choi, Y.J.; Pillinger, M.H.; Kim, S.-Y.; Blaser, M.J.; Lee, Y.C. Helicobacter pylori CagA Phosphorylation Status Determines the gp130-activated SHP2/ERK and JAK/STAT Signal Transduction Pathways in Gastric Epithelial Cells. J. Biol. Chem. 2010, 285, 16042–16050. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-induced gastric cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Lee, K.S.; Kalantzis, A.; Jackson, C.B.; O’Connor, L.; Murata-Kamiya, N.; Hatakeyama, M.; Judd, L.M.; Giraud, A.S.; Menheniott, T.R. Helicobacter pylori CagA Triggers Expression of the Bactericidal Lectin REG3γ via Gastric STAT3 Activation. PLoS ONE 2012, 7, e30786. [Google Scholar] [CrossRef]
- Bessède, E.; Staedel, C.; Acuña Amador, L.A.; Nguyen, P.H.; Chambonnier, L.; Hatakeyama, M.; Belleannée, G.; Mégraud, F.; Varon, C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial–mesenchymal transition-like changes. Oncogene 2014, 33, 4123–4131. [Google Scholar] [CrossRef]
- Mitsuno, Y. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 2001, 49, 18–22. [Google Scholar] [CrossRef]
- Backert, S.; Naumann, M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010, 18, 479–486. [Google Scholar] [CrossRef]
- Judd, L.M.; Menheniott, T.R.; Ling, H.; Jackson, C.B.; Howlett, M.; Kalantzis, A.; Priebe, W.; Giraud, A.S. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS ONE 2014, 9, e95993. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Judd, L.; Menheniott, T.; Kronborg, I.; Dow, C.; Yeomans, N.; Boussioutas, A.; Robb, L.; Giraud, A. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J. Pathol. 2007, 213, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Howlett, M.; Menheniott, T.R.; Judd, L.M.; Giraud, A.S. Cytokine signalling via gp130 in gastric cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J. Th17 Cell Differentiation: The Long and Winding Road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef]
- Maeda, S.; Yoshida, H.; Ogura, K.; Mitsuno, Y.; Hirata, Y.; Yamaji, Y.; Akanuma, M.; Shiratori, Y.; Omata, M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 2000, 119, 97–108. [Google Scholar] [CrossRef]
- Suzuki, N.; Murata-Kamiya, N.; Yanagiya, K.; Suda, W.; Hattori, M.; Kanda, H.; Bingo, A.; Fujii, Y.; Maeda, S.; Koike, K.; et al. Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci. Rep. 2015, 5, 10024. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.-F. The many roads traveled by Helicobacter pylori to NF-κB activation. Gut Microbes 2010, 1, 109–113. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Meyer, F.; Wilson, K.T.; James, S.P. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect. Immun. 2000, 68, 6265–6272. [Google Scholar] [CrossRef]
- Ubukata, H.; Motohashi, G.; Tabuchi, T.; Nagata, H.; Konishi, S.; Tabuchi, T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 2010, 102, 742–747. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting Edge: Th17 and Regulatory T Cell Dynamics and the Regulation by IL-2 in the Tumor Microenvironment. J. Immunol. 2007, 178, 6730–6733. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, H.; Ikeguchi, M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J. Surg. Res. 2012, 178, 685–691. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, H.; Zhang, H.; Chen, Y.F.; Cao, Y.F.; Li, R.C.; Lin, C.; Wei, Y.C.; Xiang, X.N.; Fang, H.J.; et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2019, 30, 266–273. [Google Scholar] [CrossRef]
- Liu, T.; Peng, L.; Yu, P.; Zhao, Y.; Shi, Y.; Mao, X.; Chen, W.; Cheng, P.; Wang, T.; Chen, N.; et al. Increased Circulating Th22 and Th17 Cells are Associated with Tumor Progression and Patient Survival in Human Gastric Cancer. J. Clin. Immunol. 2012, 32, 1332–1339. [Google Scholar] [CrossRef]
- Zhang, B.; Rong, G.; Wei, H.; Zhang, M.; Bi, J.; Ma, L.; Xue, X.; Wei, G.; Liu, X.; Fang, G. The prevalence of Th17 cells in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2008, 374, 533–537. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.-M.; Choi, K.S.; Son, S.-Y.; Han, S.-U.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef]
- Matsuo, K.; Oka, M.; Murase, K.; Soda, H.; Isomoto, H.; Takeshima, F.; Mizuta, Y.; Murata, I.; Kohno, S. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J. Int. Med Res. 2003, 31, 69–75. [Google Scholar] [CrossRef]
- Huang, S.-P.; Wu, M.-S.; Shun, C.-T.; Wang, H.-P.; Lin, M.-T.; Kuo, M.-L.; Lin, J.-T. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J. Biomed. Sci. 2004, 11, 517–527. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H.; et al. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014, 5, e1295. [Google Scholar] [CrossRef]
- Ham, I.-H.; Lee, D.; Hur, H. Role of Cancer-Associated Fibroblast in Gastric Cancer Progression and Resistance to Treatments. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Simondurairaj, C.; Krishnakumar, R.; Sundaram, S.; Venkatraman, G. Interleukin-6 Receptor (IL-6R) expression in human gastric carcinoma and its clinical significance. Cancer Investig. 2019, 37, 293–298. [Google Scholar] [CrossRef]
- Ashizawa, T.; Okada, R.; Suzuki, Y.; Takagi, M.; Yamazaki, T.; Sumi, T.; Aoki, T.; Ohnuma, S.; Aoki, T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer 2005, 8, 124–131. [Google Scholar] [CrossRef]
- Sánchez-Zauco, N.; Torres, J.; Gómez, A.; Camorlinga-Ponce, M.; Muñoz-Pérez, L.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Giono-Cerezo, S.; Maldonado-Bernal, C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Hatada, T.; Yamamoto, M.; Miyake, T.; Matsunaga, T.; Fukumoto, Y.; Yamada, Y.; Fukuda, K.; Saito, H.; Tatebe, S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009, 12, 95–100. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Mroczko, B.; Gryko, M.; Kędra, B.; Szmitkowski, M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin. Exp. Med. 2011, 11, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Lin, J.T.; Wu, C.Y.; Huang, S.P.; Lin, M.T.; Wu, A.S.H.; Huang, Y.J.; Wu, M.S. Serum Interleukin-6 Level but not Genotype Predicts Survival after Resection in Stages II and III Gastric Carcinoma. Clin. Cancer Res. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.-M.; Goldsmith, D.; Joseph, W.R.; Körner, H.; Sedgwick, J.D.; Baguley, B.C. Induction of Intratumoral Tumor Necrosis Factor (TNF) Synthesis and Hemorrhagic Necrosis by 5,6-Dimethylxanthenone-4-Acetic Acid (DMXAA) in TNF Knockout Mice. Cancer Res. 1999, 59, 3304–3307. [Google Scholar] [PubMed]
- Zhao, C.; Lu, X.; Bu, X.; Zhang, N.; Wang, W. Involvement of tumor necrosis factor-α in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer 2010, 10, 419. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Oshima, H.; Ishikawa, T.; Yoshida, G.J.; Naoi, K.; Maeda, Y.; Naka, K.; Ju, X.; Yamada, Y.; Minamoto, T.; Mukaida, N.; et al. TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 2014, 33, 3820–3829. [Google Scholar] [CrossRef]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Suganuma, M.; Watanabe, T.; Sueoka, E.; Lim, I.K.; Fujiki, H. Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies. Toxins 2021, 13, 181. [Google Scholar] [CrossRef]
- Yang, J.-P.; Hyun, M.-H.; Yoon, J.-M.; Park, M.-J.; Kim, D.; Park, S. Association between TNF-α-308 G/A gene polymorphism and gastric cancer risk: A systematic review and meta-analysis. Cytokine 2014, 70, 104–114. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, S.; Zhang, S.; Min, L.; Wang, Y.; Xie, J.; Hou, Y.; Tian, X.; Cheng, J.; Liu, K.; et al. The relationship between tumor necrosis factor-α polymorphisms and gastric cancer risk: An updated meta-analysis. Biomed. Rep. 2017, 7, 133–142. [Google Scholar] [CrossRef][Green Version]
- Zaidi, M.R.; Merlino, G. The Two Faces of Interferon-γ in Cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 469. [Google Scholar] [CrossRef]
- Tu, S.P.; Quante, M.; Bhagat, G.; Takaishi, S.; Cui, G.; Yang, X.D.; Muthuplani, S.; Shibata, W.; Fox, J.G.; Pritchard, D.M.; et al. IFN-γ Inhibits Gastric Carcinogenesis by Inducing Epithelial Cell Autophagy and T-Cell Apoptosis. Cancer Res. 2011, 71, 4247–4259. [Google Scholar] [CrossRef]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.-F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Allison, C.C.; Ferrand, J.; McLeod, L.; Hassan, M.; Kaparakis-Liaskos, M.; Grubman, A.; Bhathal, P.S.; Dev, A.; Sievert, W.; Jenkins, B.J.; et al. Nucleotide Oligomerization Domain 1 Enhances IFN-γ Signaling in Gastric Epithelial Cells during Helicobacter pylori Infection and Exacerbates Disease Severity. J. Immunol. 2013, 190, 3706–3715. [Google Scholar] [CrossRef]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef]
- Gu, C.; Wu, L.; Li, X. IL-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhou, C.H.; Ma, J.; Jiang, C.; Ji, P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med. Oncol. 2012, 29, 3024–3028. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Zhao, Y.; Ma, P.; Cao, Y.; Liu, C.; Zhang, X.; Wang, W.; Chen, L.; Li, Y. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann. Transl. Med. 2020, 8, 877. [Google Scholar] [CrossRef]
- Karabulut, M.; Usul Afsar, C.; Serimez, M.; Karabulut, S. Serum IL-17 levels can be diagnostic for gastric cancer. Off. J. Balk. Union Oncol. 2019, 24, 1601–1609. [Google Scholar]
- Cheng, G.; Wei, L.; Xiurong, W.; Xiangzhen, L.; Shiguang, Z.; Songbin, F. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell. Mol. Neurobiol. 2009, 29, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Wu, X.; Bian, Z.; Gao, Q. Interleukin 17A Promotes Gastric Cancer Invasiveness via NF-κB Mediated Matrix Metalloproteinases 2 and 9 Expression. PLoS ONE 2014, 9, e96678. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, A.R.; Pendas, A.M.; Sanchez, L.M.; Lopez-Otin, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, T.; Liu, X.; Guo, J.N.; Xie, T.; Ding, Y.; Lin, M.; Yang, H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumor Biol. 2016, 37, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, D.; Tăban, S.; Raica, M.; Sporea, I.; Cornianu, M.; Goldiş, A.; Vernic, C. Immunohistochemical evaluation of the tumor neoangiogenesis as a prognostic factor for gastric cancers. Rom. J. Morphol. Embryol. 2008, 49, 137–148. [Google Scholar]
- Jiang, Y.X.; Yang, S.W.; Li, P.A.; Luo, X.; Li, Z.Y.; Hao, Y.X.; Yu, P.W. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene 2017, 36, 1256–1264. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef]
- Li, T.-J.; Jiang, Y.-M.; Hu, Y.-F.; Huang, L.; Yu, J.; Zhao, L.-Y.; Deng, H.-J.; Mou, T.-Y.; Liu, H.; Yang, Y.; et al. Interleukin-17–Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin. Cancer Res. 2017, 23, 1575–1585. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Q.; Wang, H.; Lu, M.; Kong, H.; Zhang, Y.; Shi, H. Prognostic significance of interleukin-17 in solid tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10515–10536. [Google Scholar]
- Chen, J.-G.; Xia, J.-C.; Liang, X.-T.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.-J.; Wang, Q.-J.; Li, Y.-Q.; Chen, S.-P.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60. [Google Scholar] [CrossRef]
- Iida, T.; Iwahashi, M.; Katsuda, M.; Ishida, K.; Nakamori, M.; Nakamura, M.; Naka, T.; Ojima, T.; Ueda, K.; Hayata, K.; et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol. Rep. 2011, 25, 1271–1277. [Google Scholar] [CrossRef][Green Version]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev.™ Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Zhu, X.; Guo, W.; Zhang, M.; Che, Y.; Tang, L.; Yang, X.; You, Q.; Liu, Z. IL-10 secreted by cancer-associated macrophages regulates proliferation and invasion in gastric cancer cells via c-Met/STAT3 signaling. Oncol. Rep. 2019, 2, 595–604. [Google Scholar] [CrossRef]
- Kindlund, B.; Sjöling, Å.; Yakkala, C.; Adamsson, J.; Janzon, A.; Hansson, L.-E.; Hermansson, M.; Janson, P.; Winqvist, O.; Lundin, S.B. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 2017, 20, 116–125. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Sato, T.; Terai, M.; Tamura, Y.; Alexeev, V.; Mastrangelo, M.J.; Selvan, S.R. Interleukin 10 in the tumor microenvironment: A target for anticancer immunotherapy. Immunol. Res. 2011, 51, 170–182. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, D.; Wu, P.; Wang, Z.; Huang, J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS ONE 2015, 10, e0139598. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Pan, R.; Xu, L.; Ma, Q.; Ying, X.; Zhao, J.; Zhao, H.; Miao, L.; Xu, Y.; Duan, S.; et al. IL10 hypomethylation is associated with the risk of gastric cancer. Oncol. Lett. 2021, 21, 241. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E. Helicobacter pylori and interleukin-8 in gastric cancer. World J. Gastroenterol. 2013, 19, 8192. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pinto-Ribeiro, I.; Wen, X.; Marcos-Pinto, R.; Dinis-Ribeiro, M.; Carneiro, F.; Figueiredo, C. Helicobacter pylori cagA Promoter Region Sequences Influence CagA Expression and Interleukin 8 Secretion. J. Infect. Dis. 2016, 213, 669–673. [Google Scholar] [CrossRef]
- Chen, X.; Jin, R.; Chen, R.; Huang, Z. Complementary action of CXCL1 and CXCL8 in pathogenesis of gastric carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1036–1045. [Google Scholar]
- Gobert, A.P.; Wilson, K.T. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. In Molecular Pathogenesis and Signal Transduction by Helicobacter pylori; Springer International Publishing: New York, NY, USA, 2017; Volume 400, pp. 27–52. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Kitadai, Y.; Haruma, K.; Sumii, K.; Yamamoto, S.; Ue, T.; Yokozaki, H.; Yasui, W.; Ohmoto, Y.; Kajiyama, G.; Fidler, I.J.; et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am. J. Pathol. 1998, 152, 93. [Google Scholar]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFκB through the CBM (Carma3/Bcl10/Malt1) Complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Kido, S.; Kitadai, Y.; Hattori, N.; Haruma, K.; Kido, T.; Ohta, M.; Tanaka, S.; Yoshihara, M.; Sumii, K.; Ohmoto, Y.; et al. Interleukin 8 and vascular endothelial growth factor— prognostic factors in human gastric carcinomas? Eur. J. Cancer 2001, 37, 1482–1487. [Google Scholar] [CrossRef]
- Yeni, M.; Korkut, E.; Aksungur, N.; Kara, S.; Askin, S.; Kartal, M. Determination of Pentraxin-3, Interleukin-8 and Vascular Endothelial Growth Factor Levels in Patients with Gastric Adenocarcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 1507–1512. [Google Scholar] [CrossRef]
- Lin, C.S.; He, P.J.; Hsu, W.T.; Wu, M.S.; Wu, C.J.; Shen, H.W.; Hwang, C.H.; Lai, Y.K.; Tsai, N.M.; Liao, K.W. Helicobacter pylori-derived Heat shock protein 60 enhances angiogenesis via a CXCR2-mediated signaling pathway. Biochem. Biophys. Res. Commun. 2010, 397, 283–289. [Google Scholar] [CrossRef]
- Ju, D.; Sun, D.; Xiu, L.; Meng, X.; Zhang, C.; Wei, P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med. Oncol. 2012, 29, 91–99. [Google Scholar] [CrossRef]
- Chen, L.; Min, L.; Wang, X.; Zhao, J.; Chen, H.; Qin, J.; Chen, W.; Shen, Z.; Tang, Z.; Gan, Q.; et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling Loop. Cancer Res. 2015, 75, 3832–3841. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764. [Google Scholar] [CrossRef]
- Hu, W.; Wang, J.; Luo, G.; Luo, B.; Wu, C.; Wang, W.; Xiao, Y.; Li, J. Proteomics-based analysis of differentially expressed proteins in the CXCR1-knockdown gastric carcinoma MKN45 cell line and its parental cell. Acta Biochim. Biophys. Sin. 2013, 45, 857–866. [Google Scholar] [CrossRef]
- Rajagopalan, L.; Rajarathnam, K. Ligand Selectivity and Affinity of Chemokine Receptor CXCR1. J. Biol. Chem. 2004, 279, 30000–30008. [Google Scholar] [CrossRef] [PubMed]

| Cytokine | Overall Effect | Sources within GC TME | Mechanisms | Effect on Prognosis |
|---|---|---|---|---|
| IL-6 | Pro-tumour | Tumour-associated fibroblasts, tumour cells [51,52,54] | IL-6 serum levels and IL-6R levels in GC tissue have been linked to GC severity and poorer OS. A systematic review by Vainer et al. [64] Found that all six papers which assessed clinical characteristics of GC and IL-6 levels linked decreased OS to increased IL-6. Ashizawa et al. [60] Demonstrated GC survival rates of 43% and 87% in patients with high and low serum levels of IL-6, respectively. | |
| OS | Pro- and anti-tumour | Epithelial Cells, stromal cells [67] |
| No studies which investigated the relationship between TNF levels and GC patient prognosis were found. |
| IFN-γ | Pro- and anti-tumour | CD4+ Th1 and CD8+ cytotoxic T-cells, NK cells [77] | N Sánchez-Zauco. et al. [61] showed that high plasma/serum levels of IFN-γ are associated with GC incidence rate. No studies were found to investigate the relationship between IFN-γ levels and GC patient prognosis. | |
| IL-17A | Pro-tumour | CD4+ Th17 and CD8+ cytotoxic T-cells, NK cells, neutrophils [86,87,88] |
| Mixed evidence: Iida et al. [99] (n = 82) linked increased IL-17 mRNA to increased tumour depth and lymph node involvement, but a meta-analysis by Zeng et al. [97] concluded that there was no statistical significance between IL-17 and worsened prognosis in GC. |
| IL-10 | Pro- and anti-tumour | CD4+ Th1 and Th2 cells, Treg cells, TAMs [101,102,103] | No studies which investigated the relationship between levels of IL-10 and GC prognosis/outcome were found. | |
| IL-8 | Pro-tumour | Macrophages, neutrophils [113], tumour cells [109,114], epithelial cells [111] | Limited evidence available. Kido et al. showed decreased OS in GC patients with high levels of IL-8 vs. low IL-8 (n = 56), but results were not statistically significant [117]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.; Chua, W.; Ng, W.; Roberts, T.L. Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines 2021, 9, 1916. https://doi.org/10.3390/biomedicines9121916
Yang E, Chua W, Ng W, Roberts TL. Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines. 2021; 9(12):1916. https://doi.org/10.3390/biomedicines9121916
Chicago/Turabian StyleYang, Elton, Wei Chua, Weng Ng, and Tara Laurine Roberts. 2021. "Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature" Biomedicines 9, no. 12: 1916. https://doi.org/10.3390/biomedicines9121916
APA StyleYang, E., Chua, W., Ng, W., & Roberts, T. L. (2021). Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines, 9(12), 1916. https://doi.org/10.3390/biomedicines9121916






