Evaluation of ATOX1 as a Potential Predictive Biomarker for Tetrathiomolybdate Treatment of Breast Cancer Patients with High Risk of Recurrence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Material
2.2. ATOX1 Immunohistochemical Staining and Evaluation
2.3. Statistics
3. Results
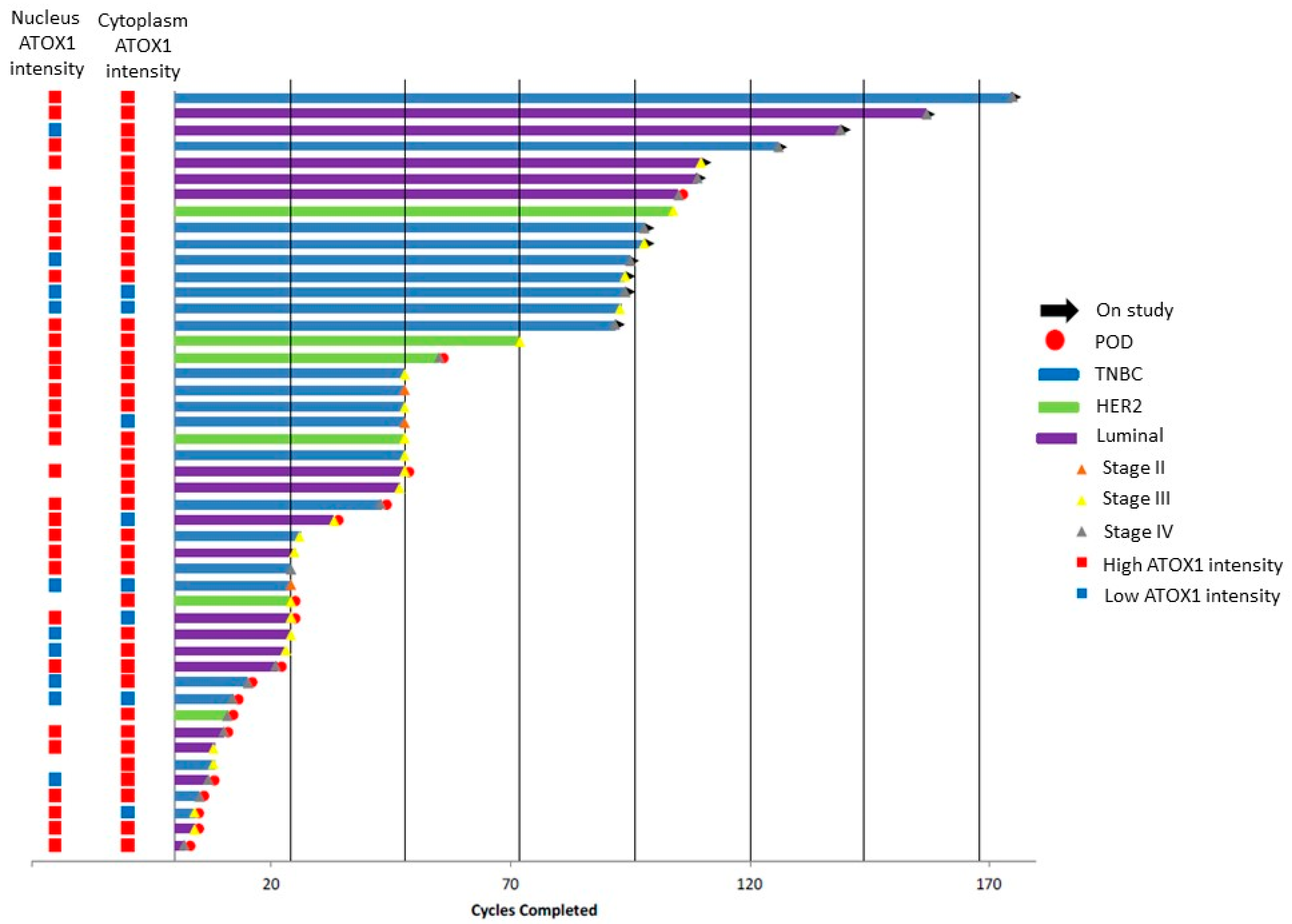
3.1. ATOX1 Is Expressed in Both Nucleus and Cytoplasm of the Tumor Cells and Varies between Breast Tumor Tissue Sections of Patients in the Phase II Clinical Trial for TM
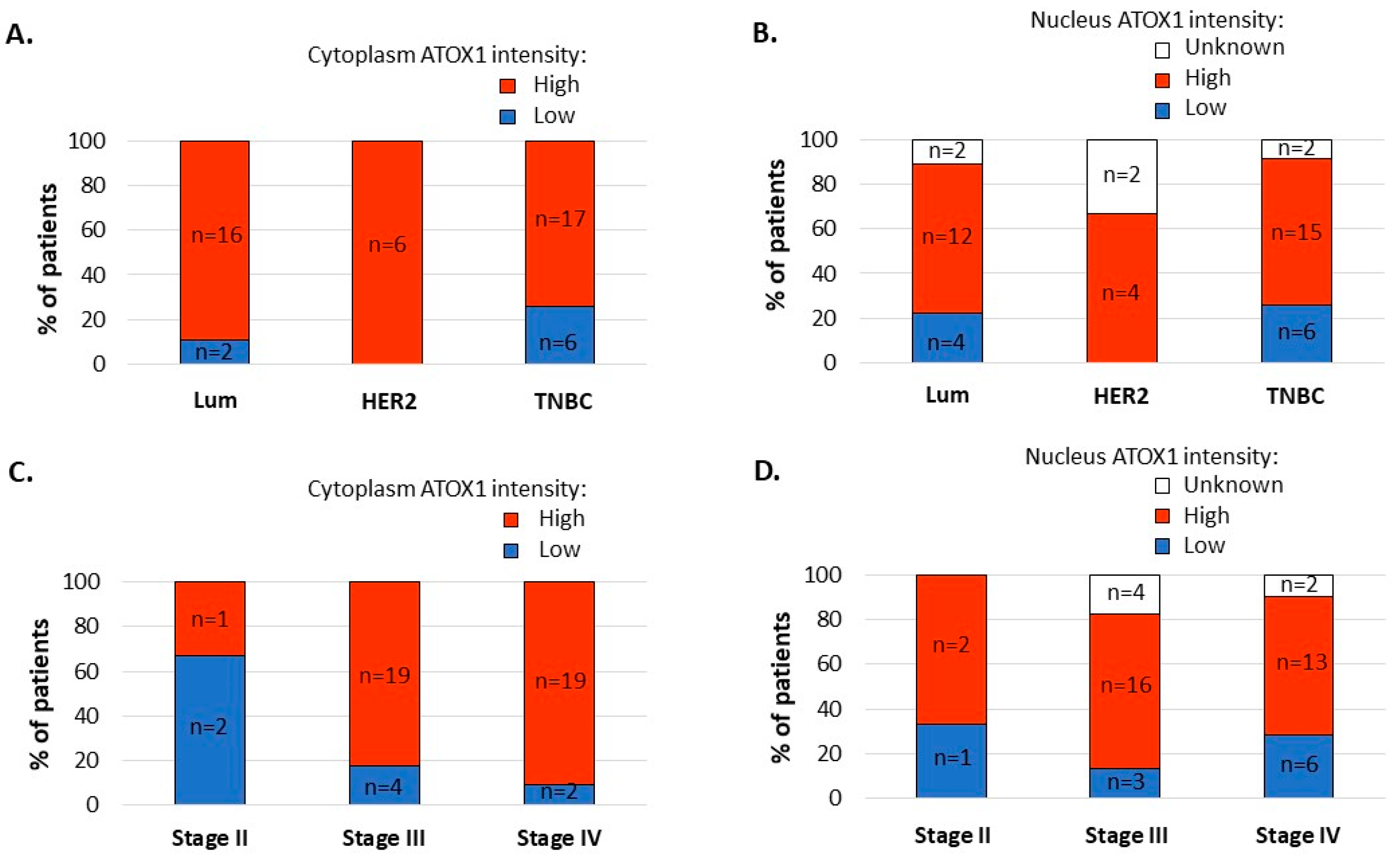
3.2. ATOX1 Expression Levels in the Tumor Cell Cytoplasm, and Not in the Tumor Cell Nuclei, Vary Depending on Subtype and Stage of Disease
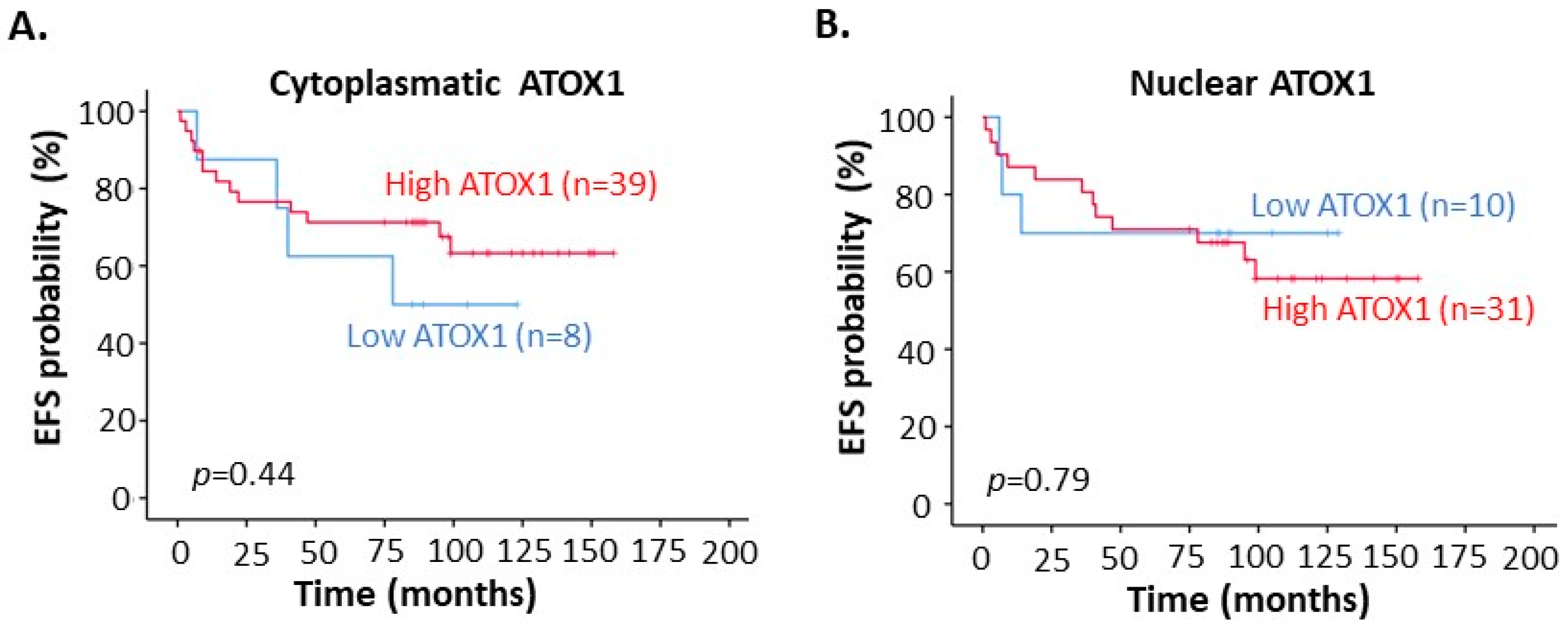
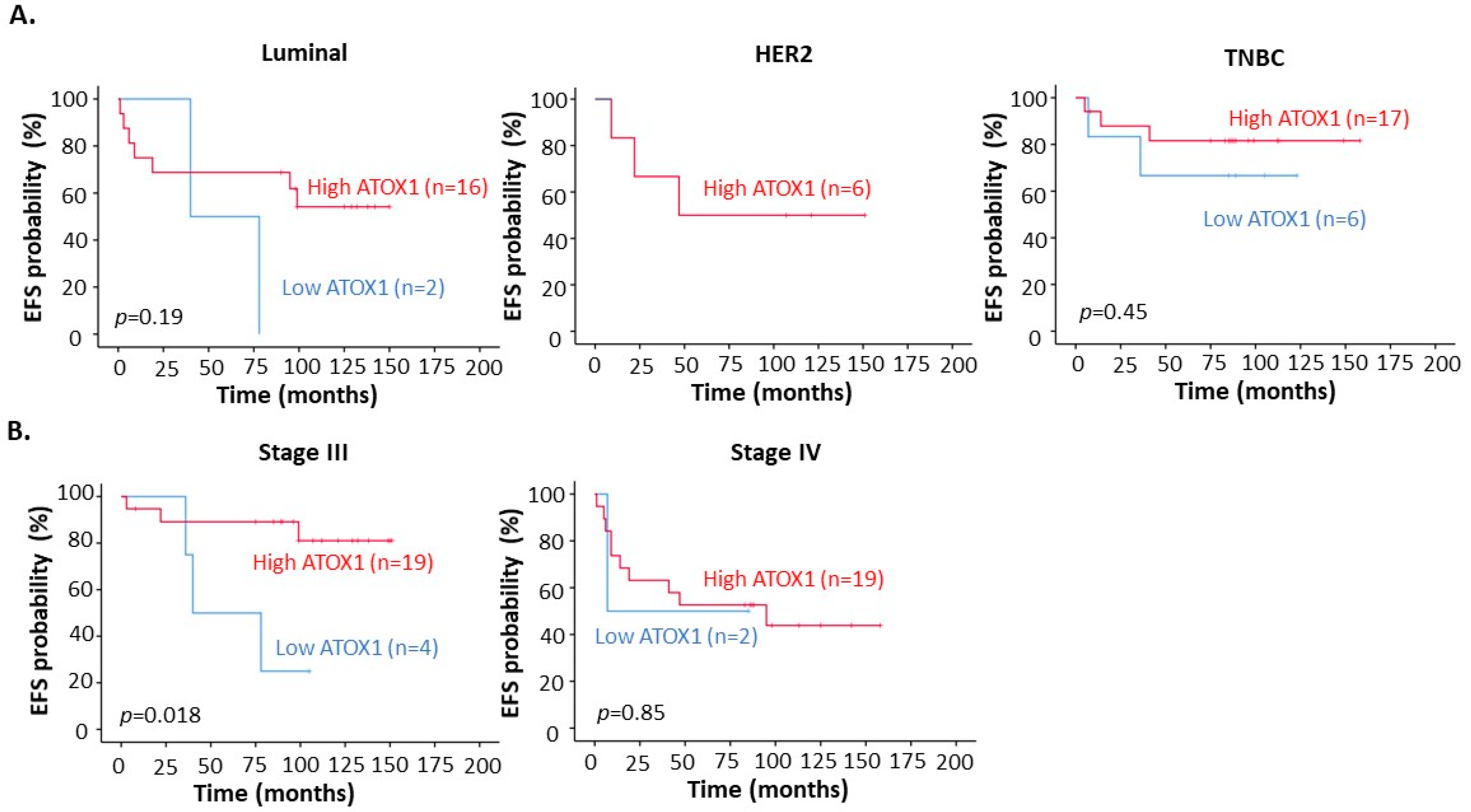
3.3. High ATOX1 Expression in the Tumor Cell Cytoplasm Correlates with a Better EFS upon TM Treatment Especially for TNBC Patients and Patients at Stage III of Disease
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Celauro, E.; Hildesjö, C.; Feizi, O.; Stål, O.; Gonzalez, F.; Wittung-Stafshede, P. Defining the Human Copper Proteome and Analysis of Its Expression Variation in Cancers. Metallomics 2017, 9, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, A.; Das, A.; Krett, N.L.; Guzman, G.; Thomas, A.; Mancinelli., G.; Bauer, J.; Ushio-Fukai, M.; Fukai, T.; Jung, B. Nuclear Translocation of Atox1 Protentiates Activin A-Induced Cell Migration and Colony Formation in Colon Cancer. PLoS ONE 2020, 15, e0227916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Peng, F. Knockdown of Copper Chaperone Antioxidant-1 by RNA Interference Inhibits Copper-Stimulated Proliferation of Non-Small Cell Lung Carcinoma Cells. Oncol Rep. 2013, 30, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Zhang, X.; Wittung-Stafshede, P. Single-Cell Tracking Demonstrates Copper Chaperone ATOX1 to be Required for Breast Cancer Cell Migration. Proc. Natl. Acad. Sci. USA 2020, 117, 2014–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blockhuys, S.; Brady, D.; Wittung-Stafshede, P. Evaluation of Copper Chaperone ATOX1 as Prognostic Biomarker in Breast Cancer. Breast Cancer 2020, 27, 505–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, C.K.; Carey, L.A. Biology, Metastatic Patterns, and Treatment of Patients with Triple-Negative Breast Cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.-A.; Cîmpean, A.M.; Ribatti, D.; Raica, M. Triple Negative Breast Cancer: The Kiss of Death. Oncotarget 2017, 8, 46652. [Google Scholar] [CrossRef] [Green Version]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/types-of-breast-cancer/triple-negative.html (accessed on 10 August 2021).
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blockhuys, S.; Hildesjö, C.; Olsson, H.; Vahdat, L.; Wittung-Stafshede, P. Evaluation of ATOX1 as a Potential Predictive Biomarker for Tetrathiomolybdate Treatment of Breast Cancer Patients with High Risk of Recurrence. Biomedicines 2021, 9, 1887. https://doi.org/10.3390/biomedicines9121887
Blockhuys S, Hildesjö C, Olsson H, Vahdat L, Wittung-Stafshede P. Evaluation of ATOX1 as a Potential Predictive Biomarker for Tetrathiomolybdate Treatment of Breast Cancer Patients with High Risk of Recurrence. Biomedicines. 2021; 9(12):1887. https://doi.org/10.3390/biomedicines9121887
Chicago/Turabian StyleBlockhuys, Stéphanie, Camilla Hildesjö, Hans Olsson, Linda Vahdat, and Pernilla Wittung-Stafshede. 2021. "Evaluation of ATOX1 as a Potential Predictive Biomarker for Tetrathiomolybdate Treatment of Breast Cancer Patients with High Risk of Recurrence" Biomedicines 9, no. 12: 1887. https://doi.org/10.3390/biomedicines9121887
APA StyleBlockhuys, S., Hildesjö, C., Olsson, H., Vahdat, L., & Wittung-Stafshede, P. (2021). Evaluation of ATOX1 as a Potential Predictive Biomarker for Tetrathiomolybdate Treatment of Breast Cancer Patients with High Risk of Recurrence. Biomedicines, 9(12), 1887. https://doi.org/10.3390/biomedicines9121887






